Abstract
In this paper we present a results of fabrication of 3D printed micropot with integrated cantilever-like force sensor. Different thickness cantilevers was used. Presented micropot was use for characterization of grow Lepidium sativium.
1. Introduction
In modern agriculture, knowledge of growing potential of plant is one of the key factors to achieve high efficiency of harvest. There are various methods of testing plants in pre-cultivation stage [1,2]. One of the them is method presented by Hida et al. where pressure generated by growing root is measured [3]. Typical this kind of device are made from silicon or glass. Structure of the growing microchannel is micromachined in substrates. Next the substrates are bonded together to obtain microfluidic structure. Microengineering of the sensor involves expensive materials (i.e., silicon, borosilicate glass), hazardous liquids (i.e., KOH, HF) and many photolithographic steps. Important issue is also that these technics are time consuming. Also problematic is fabrication of connectors enabling plug of consumer grade sensors (temperature, flow etc.) and flow actuators like pumping system. On the other side polymer sensors (made of PDMS or thermoplastics) require fabrication an expensive forming mold. After forming a structure of channels it is necessary to connect them with other parts of the chip. It limits geometry of the microfluidic structure and complicated technology of the sensor. Therefore new technologies of microfluidics fabrication are investigated. One of them is 3D printing. We present a fully 3D printed microfluidic pot with integrated cantilever-type force sensors with optical method of root and stalk growing force measurements. I3dp is precision 3D printing technic enables to fabricate microfluidic systems [4] especially DNA chips [5], modular [6] and integrated [7] fluidic systems. In spite of 3D printing application, in comparison to [3] our solution is modular one with the second cantilever for stalk grow measurement as well as a microfluidic channel for continuous supplying of a growing medium (Figure 1).
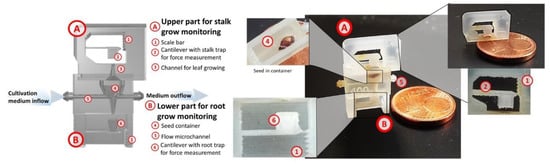
Figure 1.
3D printed micropot-computer design (left image) with described main components and a physical realization (right).
2. Material and Method
2.1. Fabrication and Beam Characterisation
Inkjet 3D printing system from 3D Systems Projet 3510 (USA) was utilized with XHD printing mode. Single layer thickness was 16 μm and planar (X-Y) resolution was 750 dpi. mPot was printed in parallel direction with final surface rouges of 0.388 μm [8]. The upot was designed in two parts: lower for root and upper for leaf grow measurement. Visijet M3 build material and S300 wax support material were used for fabrication. Those technic is able to produce microfluidics channels with smallest dimension near 200 mm [9]. After printing process the support material was removed from structure in post processing treatment carried out a mineral oil bath, detergent cleaning, and ultrasonic cleaning.
The 3D printed cantilever-type force sensors were 4 mm long and 2 mm wide. Different thicknesses of the cantilevers (200–600 μm) were mechanically characterized (Figure 2) by Bondtester Dage 4000 Plus (Nordson Dage, Erkrath, Germany). Results of fully characterization of microbeam printed by inkjet 3D were described by authors in [10].
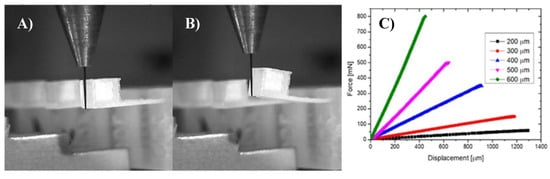
Figure 2.
Characterization of microcantilevers: (A) non deflected, (B) fully deflected, (C) cantilever deflection vs bending force (cantilever length 4 mm and 2 mm wide).
2.2. Image Analyze and Characterisation Set-up
Root/stalk driving force measurement (Figure 3) is based on the image processing of the deflected cantilevers. Images are recorded by a CCD camera and analyzed by dedicated software (Figure 4). Correlation coefficient detection method for image analyzing implemented in dedicated software for lab-on-a-chip image analyze were used [11]. Obtained time laps were taken and proceed to video format. Example of obtained photo for stalk and leaf grow were shown on Figure 3.

Figure 3.
Example image of growing stalk (upper part) and leaf (lower part) utilized for image analyze of growing seed.
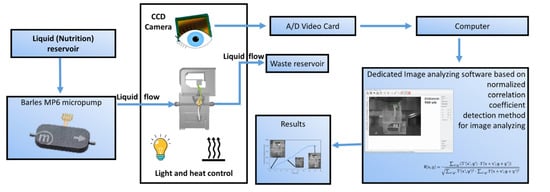
Figure 3.
Scheme of the system for micrpot planting with main components and image analyze method.
Mechanical parameters of printed cantilevers were implemented to the developed software to determine the driving force depending on sensor deflection. The micropot was supplied with tap water and nutrient (BIOHUMUS, natural nutrient, EKODARPOL) using piezoelectric micropumps mp6-series (Bartels, Germany) through 500 μm microchannel with 2 mL/h flow at 22 °C temperature under halogen lamp illumination.). Planting system scheme is shown on Figure 3.
3. Results
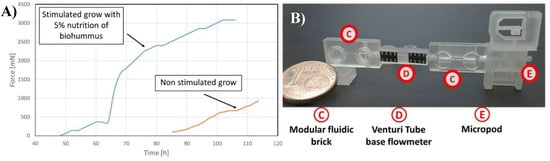
Described micropot was characterized and tested. Lepidium sativium was used as a model plant. Additional the influence of stimulant agents on plant growing potential was verified. Solution of nutrition was added during the growing process and significant growth time acceleration was noticed. We successfully measured the maximum driving force equal to 95 mN N for the root for the plant supplied with tap water (Figure 5). It was noticed that stalk exhibits higher driving force (more than 0.9 N) than root. Influence on the growing potential in the presence of the nutrients was measured and it was clearly visible that the time of stalk and root development is reduced and the growing force is increased.
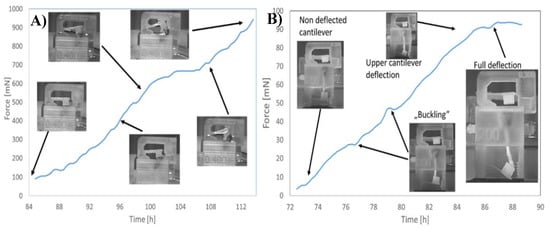
Figure 4.
Determined force generated by leaf (A) and root (B) in function of cultivation time.
In the second experiment influence of nutrients was investigated. Seed of Lepidium sativium were supplied by 5% solution of biohummus in tap water solution. Temperature for bought experiments was 22 °C and halogen light irradiations was involved. Comparison of growing stalk and root shows that adding nitride to flowing solution improve plant grow, needs less time to start observing cantilever deflection (less than 50 h in comparison to 85 h). Also observed force pressured to cantilever is match bigger in competition to non-stimulated seed. Differences between stimulated and non-stimulated seed grow is show on Figure 5. In this experiment upot working as a part of modular microfluidic [6] system with venturii microflow meter[] as a flow sensor.
4. Summary
In this paper we present for the first time an inkjet 3D printed micropot for determination of seed potential measurement. The microdevice enables determination of growing potential of the plant by measurement of the driving force of root and stalk. The device consists of two cantilever- type sensors for the forces monitoring. Deflection of the cantilevers is determined by optical analysis of the captured micropot images and then deflection is converted to force value. Successful monitoring of the root and stalk growing forces in different cultivation medium was obtained.
Author Contributions
K.A. Desing, fabrication and supervising the work. B.K. mesuarent of potencial grow. R.W. Chef of the laboratory
Funding
This works was financed by Wroclaw university of Science and Technology grant for young scientist number 0402/0051/17 and Wroclaw statuary grant number 0401/0019/17
Conflicts of Interest
The authors declare no conflict of interest.
References
- Basu, P.; Pal, A. A new tool for analysis of root growth in the spatio-temporal continuum. New Phytol. 2012, 195, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Judd, L.A.; Jackson, B.E.; Fonteno, W.C. Advancements in Root Growth Measurement Technologies and Observation Capabilities for Container-Grown Plants. Plants 2015, 4, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Hida, H.; Ozoe, K.; Iguro, D.; Kanno, I.; Nogaguchi, M. Methods for physical characterization of growing plants roots. In Proceedings of the 21st international conference on miniaturized systems for chemistry and life science (MicroTAS), Gyeongju, Korea, 25–29 October 2015; pp. 185–186. [Google Scholar]
- Walczak, R.; Adamski, K.; Pokrzywnicka, A.; Kubicki, W. Inkjet 3D Printing-Studies on Applicability for Lab-on-a-chip Technique. Procedia Eng. Tom 2016, 168, 1362–1365. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K.; Kubicki, W. Inkjet 3D printed chip for capillary gel electrophoresis. Sens. Actuators B Chem. 2018, 261C, 474–480. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K.; Kubicki, W. Configurable on-Chip Gel Electrophoresis in Inkjet 3D Printed Microfluidic Modules. Proceedings 2017, 1, 520. [Google Scholar] [CrossRef]
- Adamski, K.; Adamski, J.; Dziuban, J.; Walczak, R. Inkjet 3D Printed Miniature Water Turbine Energy Harvester-Flow Meter for Distributed Measurement Systems. MDPI Proc. 2017, 1, 578. [Google Scholar]
- Walczak, R.; Adamski, K.; Lizanets, D. Inkjet 3D printed check microvalve. J. Micromech. Microeng. 2017, 27, 047002. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K. Inkjet 3D printing of microfluidic structures—On the selection of the printer towards printing your own microfluidic chips J. Micromech. Microeng. 2015, 25, 8. [Google Scholar] [CrossRef]
- Kawa, B.; Adamski, K.; Walczak, R.; Lizanets, D. Mechanical Characterization of Inkjet 3D Printed Microcantilevers. In Proceedings of the XV International Scientific Conference on Optoelectronic and Electronic Sensors COE, Warsaw, Poland, 17–20 Jun 2018. [Google Scholar]
- Lizanets, D.; Walczak, R. Cell detection and tracking in lab-on-a-chip devices by image processing. Opt. Appl. 2018. [Google Scholar] [CrossRef]
- Adamski, K.; Kawa, B.; Walczak, R. 3D Printed Flowmeter Based on Venturi Effect with Integrated Pressure Sensors, Eurosensors 2018. MDPI Proc. 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).