NH4F Modified Al-SBA-15 Materials for Esterification of Valeric Acid to Alkyl Valerates †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization Techniques
2.3. Catalytic Activity
3. Results and Discussion
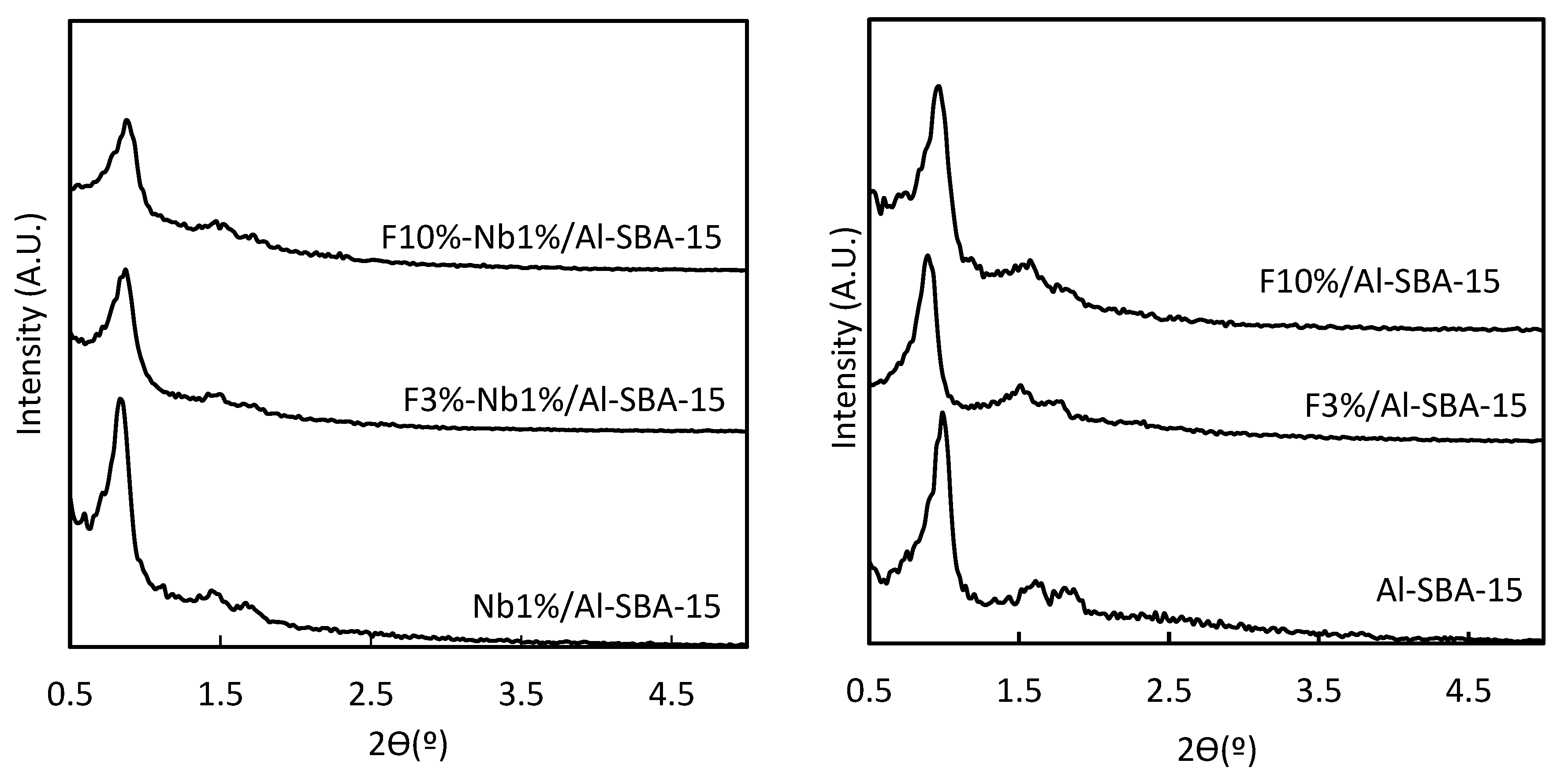
3.1. X-ray Diffraction
3.2. DRIFT Spectra
3.3. Textural Properties
3.4. Acidic Properties
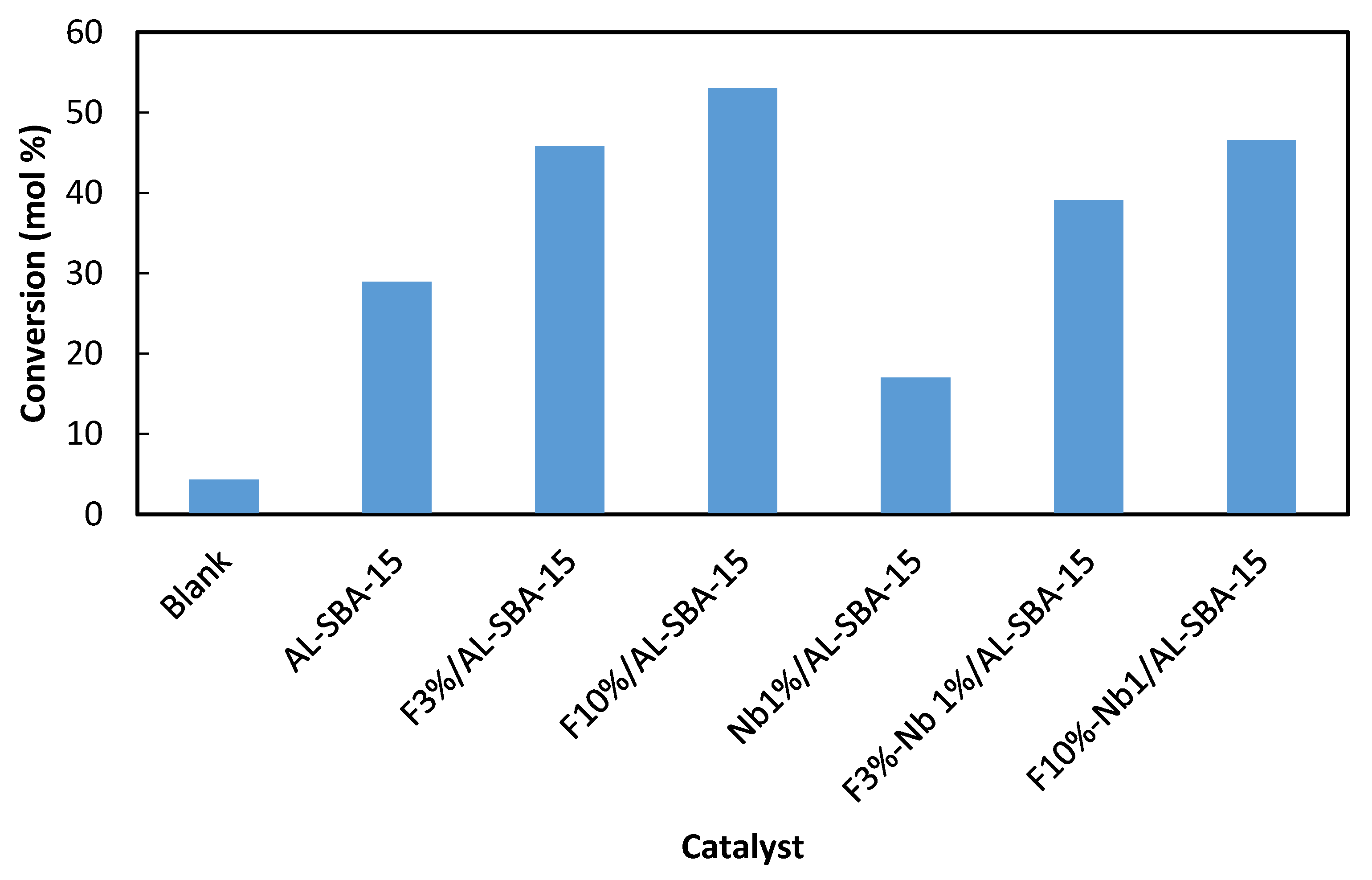
3.5. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Naika, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Laia, X.; Lee, A.F.; Wilson, K.; Rehan, M. A magnetically separable SO4/Fe-Al-TiO2 solid acid catalyst for biodiesel production from waste cooking oil. Appl. Catal. B 2018, 234, 268–278. [Google Scholar] [CrossRef]
- Palkovits, R. Pentenoic Acid Pathways for Cellulosic Biofuels. Angew. Chem. Int. Ed. 2010, 49, 4336–4338. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Lee, J. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 2010, 101, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.M.; Albayati, T.M.; Abbas, A.S.; Alismaeel, Z.T. Biodiesel production by esterification of oleic acid over zeolite Y prepared from kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef]
- López, D.E.; Goodwin, J.G., Jr.; Bruce, D.A.; Furuta, S. Esterification and transesterification using modified-zirconia catalysts. Appl. Catal. A 2008, 339, 76–83. [Google Scholar] [CrossRef]
- Rade, L.L.; Lemos, C.O.T.; Barrozo, M.A.S.; Ribas, R.M.; Monteiro, R.S.; Hori, C.E. Optimization of continuous esterification of oleic acid with ethanol over niobic acid. Renew. Energy 2018, 115, 208–216. [Google Scholar] [CrossRef]
- Cebrian-Garcia, S.; Balu, A.M.; Luque, R. Ultrasound-Assisted Esterification of Valeric Acid to Alkyl Valerates Promoted by Biosilicified Lipases. Front. Chem. 2018, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Corradini, M.C.C.; Costa, B.M.; Bressani, A.P.P.; Garcia, K.C.A.; Pereira, E.B.; Mendes, A.A. Improvement of the enzymatic synthesis of ethyl valerate by esterification reaction in a solvent system. Prep. Biochem. Biotech. 2017, 47, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Wilson, K.; Lee, A.F.; dos Santos, V.C.; Bacilla, A.C.; Mantovani, K.M.; Nakagaki, S. Nb2O5/SBA-15 catalyzed propanoic acid esterification. Appl. Catal. B 2017, 205, 498–504. [Google Scholar] [CrossRef]
- García-Sancho, C.; Saboya, R.M.A.; Cecilia, J.A.; Sales, A.V.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Influence of pore size and loading for Nb2O5/SBA-15 catalysts on synthetic ester production from free fatty acids of castor oil. J. Mol. Catal. 2017, 436, 267–275. [Google Scholar] [CrossRef]
- Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. NH4F effect in post-synthesis treatment of Al-MCM-41 mesoporous materials. Microporous Mesoporous Mater. 2005, 84, 11–20. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Seiler, M.; Buchholz, A.; Hunger, M. Improved Brønsted Acidity of Mesoporous [Al]MCM-41 Material Treated with Ammonium Fluoride. J. Phys. Chem. B 2002, 106, 3202–3208. [Google Scholar] [CrossRef]
- Jarry, B.; Launay, F.; Nogier, J.P.; Montouillout, V.; Gengembre, L.; Bonardet, J.L. Characterisation, acidity and catalytic activity of Ga–SBA-15 materials prepared following different synthesis procedures. Appl. Catal. A 2006, 309, 177–186. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A.; Calvino, J.J.; Rodriguez-Luque, M.P. Synthesis of acidic Al-MCM-48: Influence of the Si/Al ratio, degree of the surfactant hydroxyl exchange, and post-treatment in NH4F solution. J. Catal. 2005, 230, 327–338. [Google Scholar] [CrossRef]
- Franco, A.; De, S.; Balu, A.M.; Romero, A.A.; Luque, R. Selective Oxidation of Isoeugenol to Vanillin over Mechanochemically Synthesized Aluminosilicate Supported Transition Metal Catalysts. Chemistryselect 2017, 2, 9546–9551. [Google Scholar] [CrossRef]

| Materials | SBET (m2g−1) | D (nm) | VBJH (cm3g−1) | Total Acidity (μmol Py/g) | Brönsted Acidity (μmol DMPy/g) | Lewis Acidity (μmol/g) |
|---|---|---|---|---|---|---|
| Al-SBA-15 | 812 | 8.1 | 1.85 | 129 | 55 | 74 |
| F3%/Al-SBA-15 | 555 | 8.8 | 0.88 | 115 | 58 | 57 |
| F10%/Al-SBA-15 | 291 | 10.1 | 0.89 | 133 | 74 | 59 |
| Nb1%/Al-SBA-15 | 685 | 8.0 | 0.82 | 120 | 61 | 59 |
| F3%-Nb1%/Al-SBA-15 | 382 | 8.8 | 0.64 | 95 | 43 | 52 |
| F10%-Nb1%/Al-SBA-15 | 322 | 9.0 | 0.64 | 129 | 55 | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Sánchez, M.; Franco, A.; Pineda, A.; Balu, A.; Romero, A.; Luque, R. NH4F Modified Al-SBA-15 Materials for Esterification of Valeric Acid to Alkyl Valerates. Proceedings 2019, 3, 4. https://doi.org/10.3390/IOCN_2018-1-05481
Blanco-Sánchez M, Franco A, Pineda A, Balu A, Romero A, Luque R. NH4F Modified Al-SBA-15 Materials for Esterification of Valeric Acid to Alkyl Valerates. Proceedings. 2019; 3(1):4. https://doi.org/10.3390/IOCN_2018-1-05481
Chicago/Turabian StyleBlanco-Sánchez, Miguel, Ana Franco, Antonio Pineda, Alina Balu, Antonio Romero, and Rafael Luque. 2019. "NH4F Modified Al-SBA-15 Materials for Esterification of Valeric Acid to Alkyl Valerates" Proceedings 3, no. 1: 4. https://doi.org/10.3390/IOCN_2018-1-05481
APA StyleBlanco-Sánchez, M., Franco, A., Pineda, A., Balu, A., Romero, A., & Luque, R. (2019). NH4F Modified Al-SBA-15 Materials for Esterification of Valeric Acid to Alkyl Valerates. Proceedings, 3(1), 4. https://doi.org/10.3390/IOCN_2018-1-05481







