Chemiresistive Properties of a Novel Composite Comprised of ITO-Nanoparticles and 1,8-Diaminooctane †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
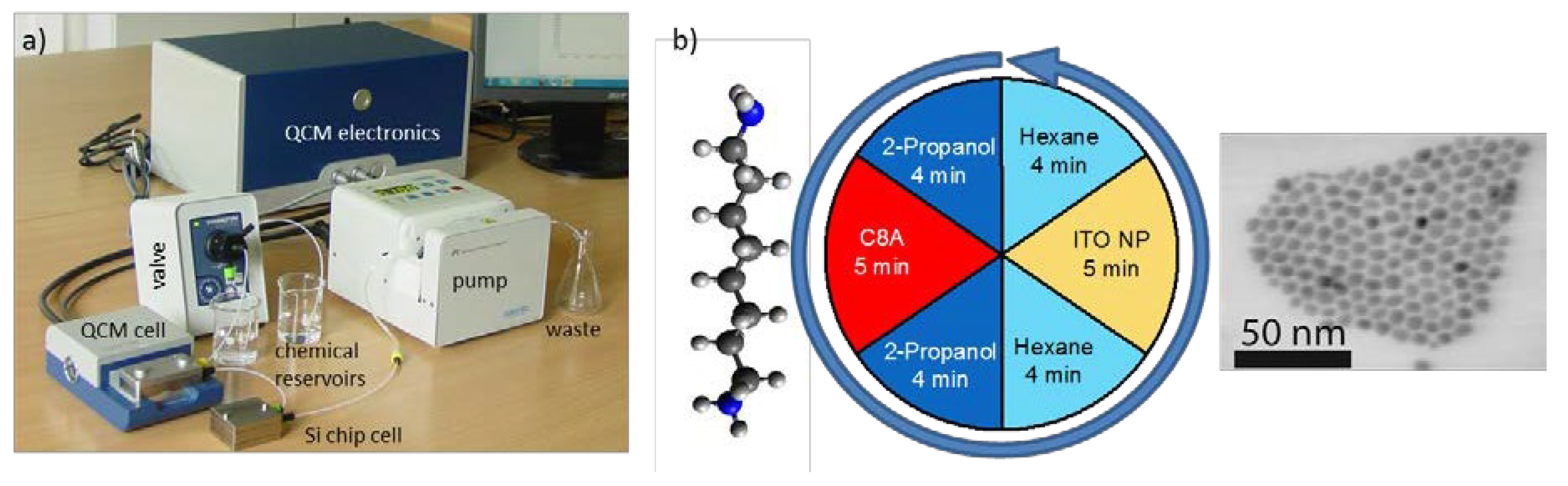
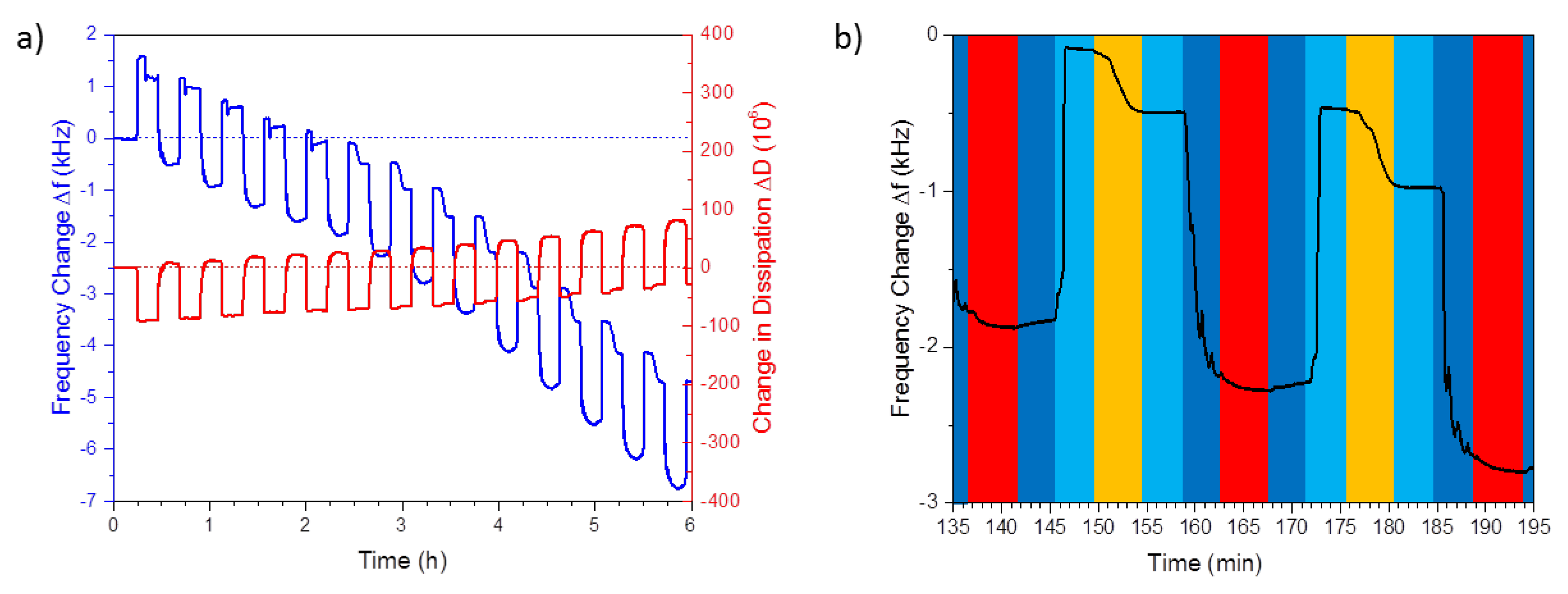
3.1. Layer-by-Layer Self-Assembly
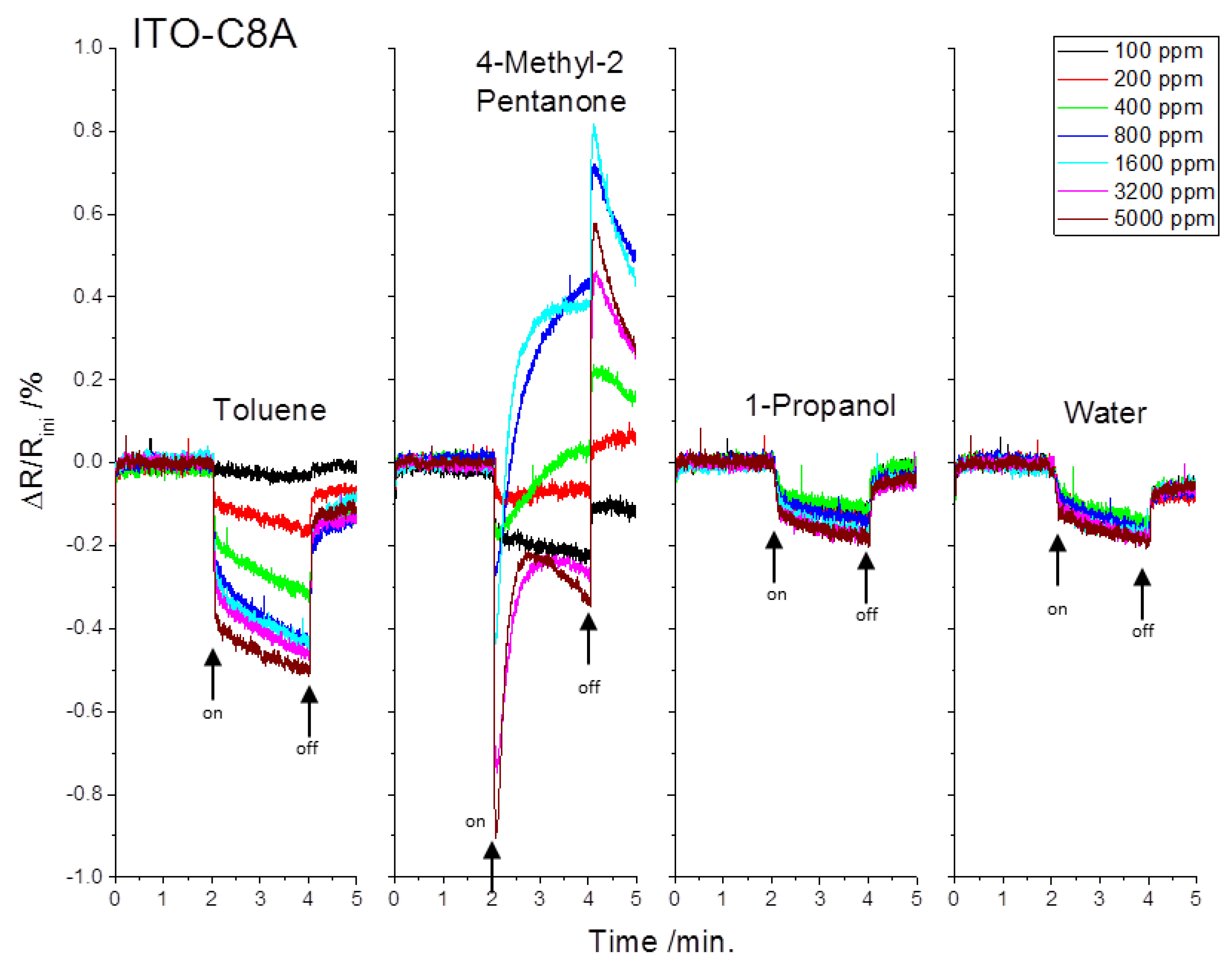
3.2. Chemiresistive Sensing
4. Conclusions and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Joseph, Y.; Besnard, I.; Rosenberger, M.; Guse, B.; Nothofer, H.G.; Wessels, J.M.; Wild, U.; Knop-Gericke, A.; Su, D.; Schlögl, R.; et al. Self-Assembled Gold Nanoparticle/Alkanedithiol Films: Preparation, Electron Microscopy, XPS-Analysis, Charge Transport, and Vapor-Sensing Properties. J. Phys. Chem. B 2003, 107, 7406–7413. [Google Scholar] [CrossRef]
- Samadi Khoshkhoo, M.; Joseph, Y.; Maiti, Y.; Schreiber, F.; Chassé, T.; Scheele, M. Tunable Charge Transport in Hybrid Superlattices of Indium Tin Oxide Nanocrystals and Metal Phthalocyanines—Towards Sensing Applications. Adv. Mater. Interfaces 2018, 5, 1701623. [Google Scholar] [CrossRef]
- Maiti, S.; Maiti, S.; Joseph, Y.; Wolf, A.; Brütting, W.; Dorfs, D.; Schreiber, F.; Scheele, M. Electronically Coupled, Two-Dimensional Assembly of Cu1.1S Nanodiscs for Selective Vapor Sensing Applications. J. Phys. Chem. C 2018, 122, 23720–23727. [Google Scholar] [CrossRef]
- Gilstrap, R.A.; Capozzi, C.J.; Carson, C.G.; Gerhardt, R.A.; Summers, C.J. Synthesis of a Nonagglomerated Indium Tin Oxide Nanoparticle Dispersion. Adv. Mater. 2008, 20, 4163–4166. [Google Scholar] [CrossRef]
- Daskal, Y.; Tauchnitz, T.; Güth, F.; Dittrich, R.; Joseph, Y. Assembly Behavior of Organically Interlinked Gold Nanoparticle Composite Films: A Quartz Crystal Microbalance Investigation. Langmuir 2017, 33, 11869–11877. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoshkhoo, M.S.; Rabe, S.; Scheele, M.; Joseph, Y. Chemiresistive Properties of a Novel Composite Comprised of ITO-Nanoparticles and 1,8-Diaminooctane. Proceedings 2018, 2, 1516. https://doi.org/10.3390/proceedings2131516
Khoshkhoo MS, Rabe S, Scheele M, Joseph Y. Chemiresistive Properties of a Novel Composite Comprised of ITO-Nanoparticles and 1,8-Diaminooctane. Proceedings. 2018; 2(13):1516. https://doi.org/10.3390/proceedings2131516
Chicago/Turabian StyleKhoshkhoo, Mahdi Samadi, Susann Rabe, Marcus Scheele, and Yvonne Joseph. 2018. "Chemiresistive Properties of a Novel Composite Comprised of ITO-Nanoparticles and 1,8-Diaminooctane" Proceedings 2, no. 13: 1516. https://doi.org/10.3390/proceedings2131516
APA StyleKhoshkhoo, M. S., Rabe, S., Scheele, M., & Joseph, Y. (2018). Chemiresistive Properties of a Novel Composite Comprised of ITO-Nanoparticles and 1,8-Diaminooctane. Proceedings, 2(13), 1516. https://doi.org/10.3390/proceedings2131516




