A Highly Sensitive Impedimetric Metamitron Microsensor Based on All-Solid-State Membrane Using a New Ion-Pair Complex, [3,3′-Co(1,2-closo-C2B9H11)2]−[C10H11ON4]+ †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Ion-Pair Complex [C10H11ON4]+[3,3′-Co(1,2-closo-C2B9H11)2]−
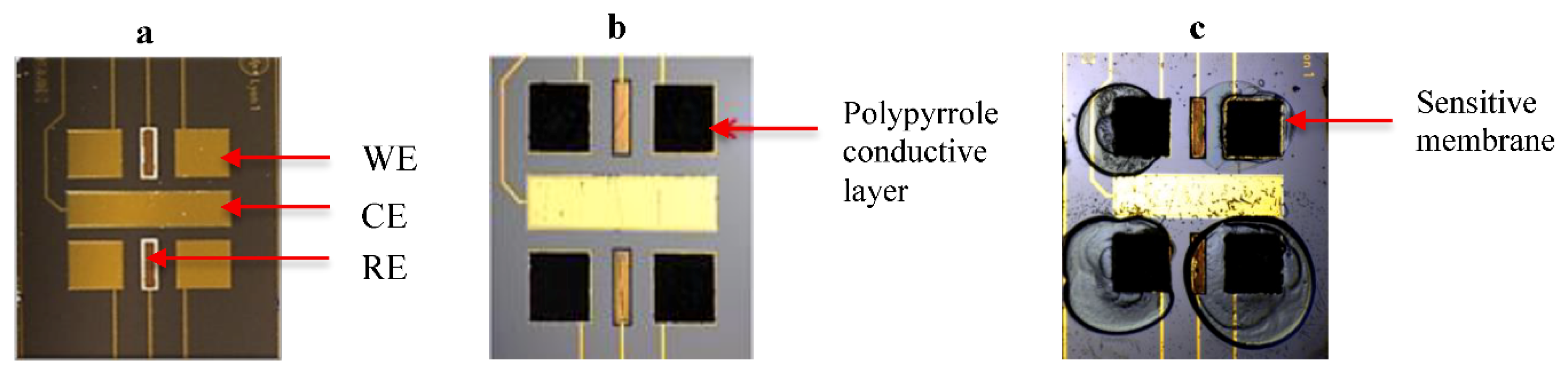
2.2. Surface Modification
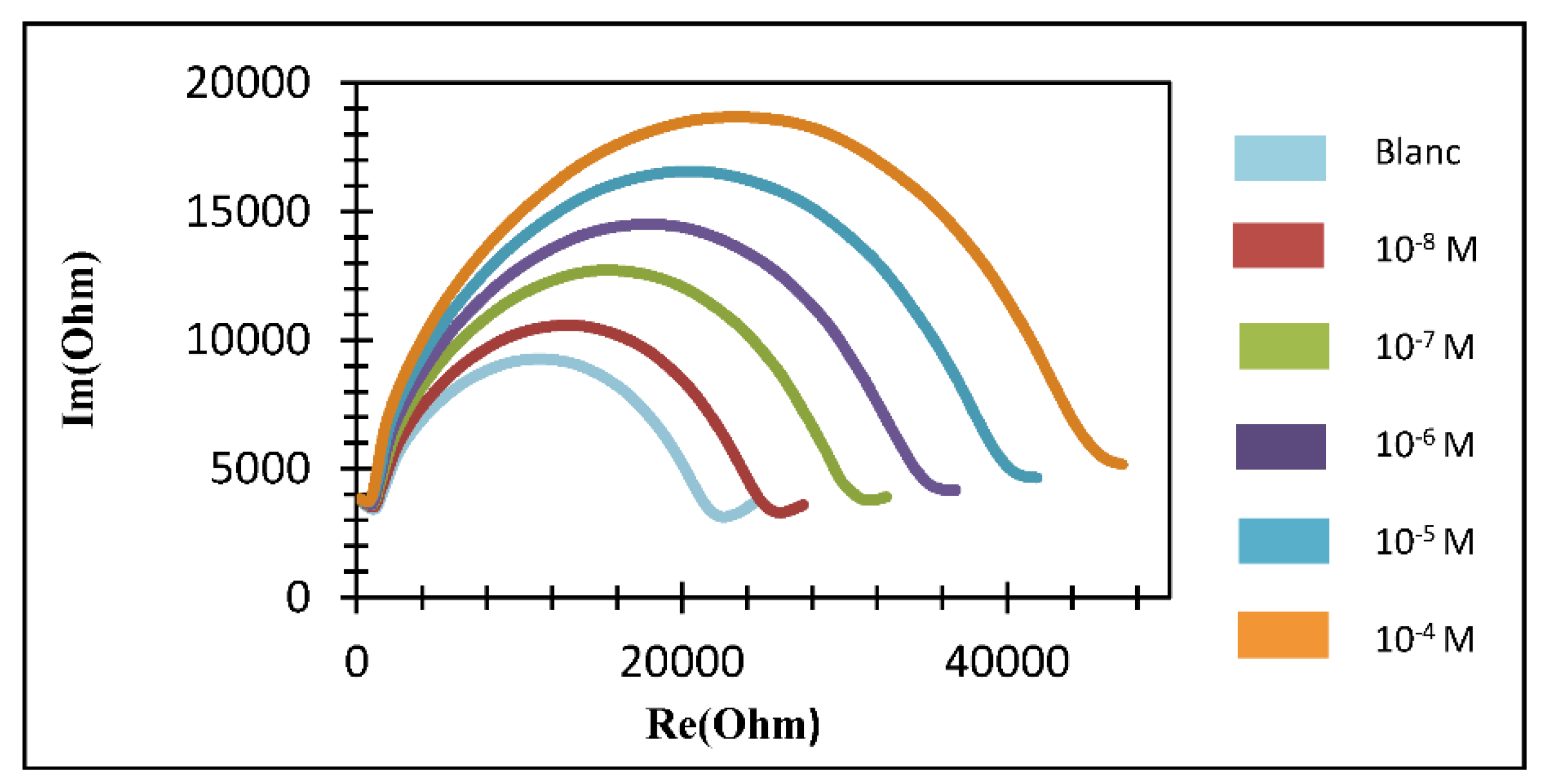
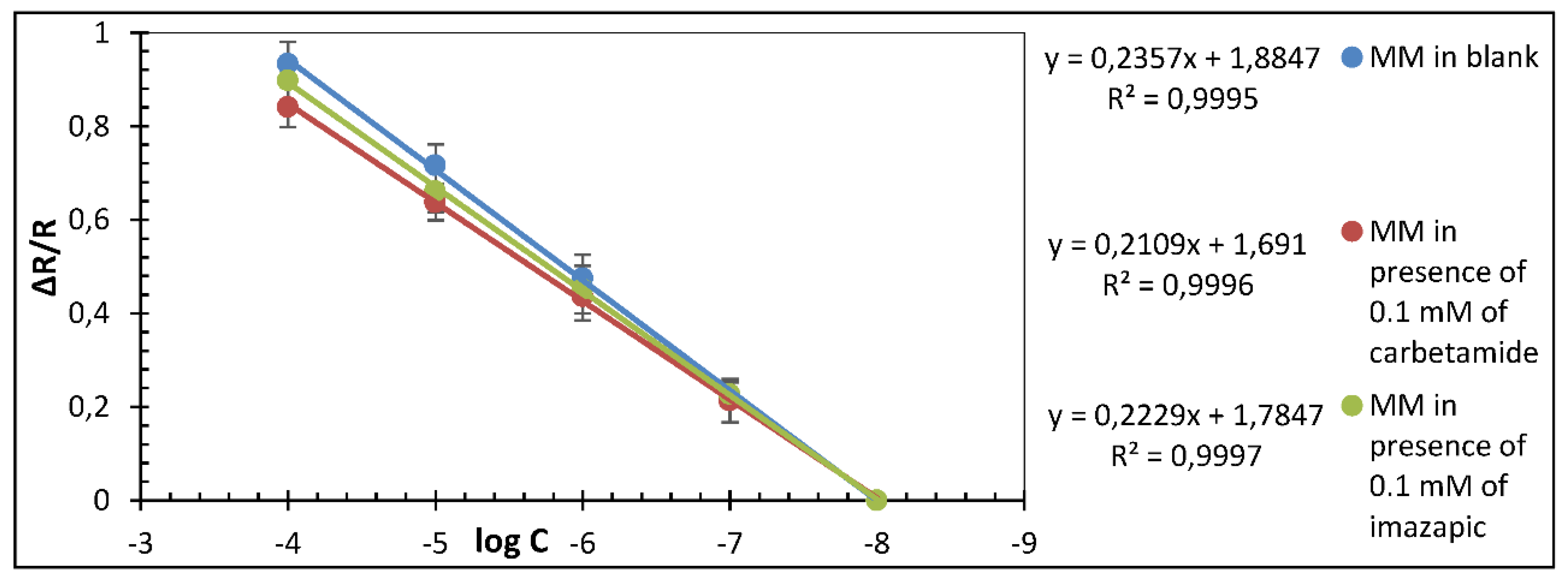
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hayes, T.; Haston, K.; Tsui, M.; Hoang, A.; Haeffele, C. Feminization of male frogs in the wild. Water-borne herbicide threatens amphibian populations in parts of the United States. Nature 2002, 419, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.T.; Letcher, R.J.; Heneweer, M. Effects of Chloro-s-Triazine Herbicides and Metabolites on Aromatase Activity in Various Human Cell Lines and on Vitellogenin Production in Male carp hepatocytes. Environ. Health Perspect. 2001, 109, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tandon, S.; Sand, N.K. Determination and method validation of metamitron in soil by RP-HPLC. Bull. Environ. Contam. Toxicol. 2014, 92, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.G.; Baraket, A.; Errachid, A. A Highly Sensitive Potentiometric Amphetamine Microsensor Based on All-Solid-State Membrane Using a New Ion-Pair Complex, [3,3′-Co(1,2-closo-C2B9H11)2]−[C9H13NH]+. Sens. Actuators B Chem. 2017, 2–5. [Google Scholar] [CrossRef]
- Saxberg, B.E.H.; Kowalski, B.R. Generalized standard addition method. Anal. Chem. 1979, 51, 1031–1038. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of Potentiometric Ion Sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayroud, Z.; Gallardo-Gonzalez, J.; Baraket, A.; Hangouet, M.; Alcácer, A.; Streklas, A.; Bausells, J.; Errachid, A.; Zine, N. A Highly Sensitive Impedimetric Metamitron Microsensor Based on All-Solid-State Membrane Using a New Ion-Pair Complex, [3,3′-Co(1,2-closo-C2B9H11)2]−[C10H11ON4]+ . Proceedings 2018, 2, 1093. https://doi.org/10.3390/proceedings2131093
Ayroud Z, Gallardo-Gonzalez J, Baraket A, Hangouet M, Alcácer A, Streklas A, Bausells J, Errachid A, Zine N. A Highly Sensitive Impedimetric Metamitron Microsensor Based on All-Solid-State Membrane Using a New Ion-Pair Complex, [3,3′-Co(1,2-closo-C2B9H11)2]−[C10H11ON4]+ . Proceedings. 2018; 2(13):1093. https://doi.org/10.3390/proceedings2131093
Chicago/Turabian StyleAyroud, Zakaria, Juan Gallardo-Gonzalez, Abdoullatif Baraket, Marie Hangouet, Albert Alcácer, Angelos Streklas, Joan Bausells, Abdelhamid Errachid, and Nadia Zine. 2018. "A Highly Sensitive Impedimetric Metamitron Microsensor Based on All-Solid-State Membrane Using a New Ion-Pair Complex, [3,3′-Co(1,2-closo-C2B9H11)2]−[C10H11ON4]+ " Proceedings 2, no. 13: 1093. https://doi.org/10.3390/proceedings2131093
APA StyleAyroud, Z., Gallardo-Gonzalez, J., Baraket, A., Hangouet, M., Alcácer, A., Streklas, A., Bausells, J., Errachid, A., & Zine, N. (2018). A Highly Sensitive Impedimetric Metamitron Microsensor Based on All-Solid-State Membrane Using a New Ion-Pair Complex, [3,3′-Co(1,2-closo-C2B9H11)2]−[C10H11ON4]+ . Proceedings, 2(13), 1093. https://doi.org/10.3390/proceedings2131093





