Redox Active Self-Assembled Monolayer Functioning as a pH Actuator †
Abstract
:1. Introduction
2. Materials and Methods
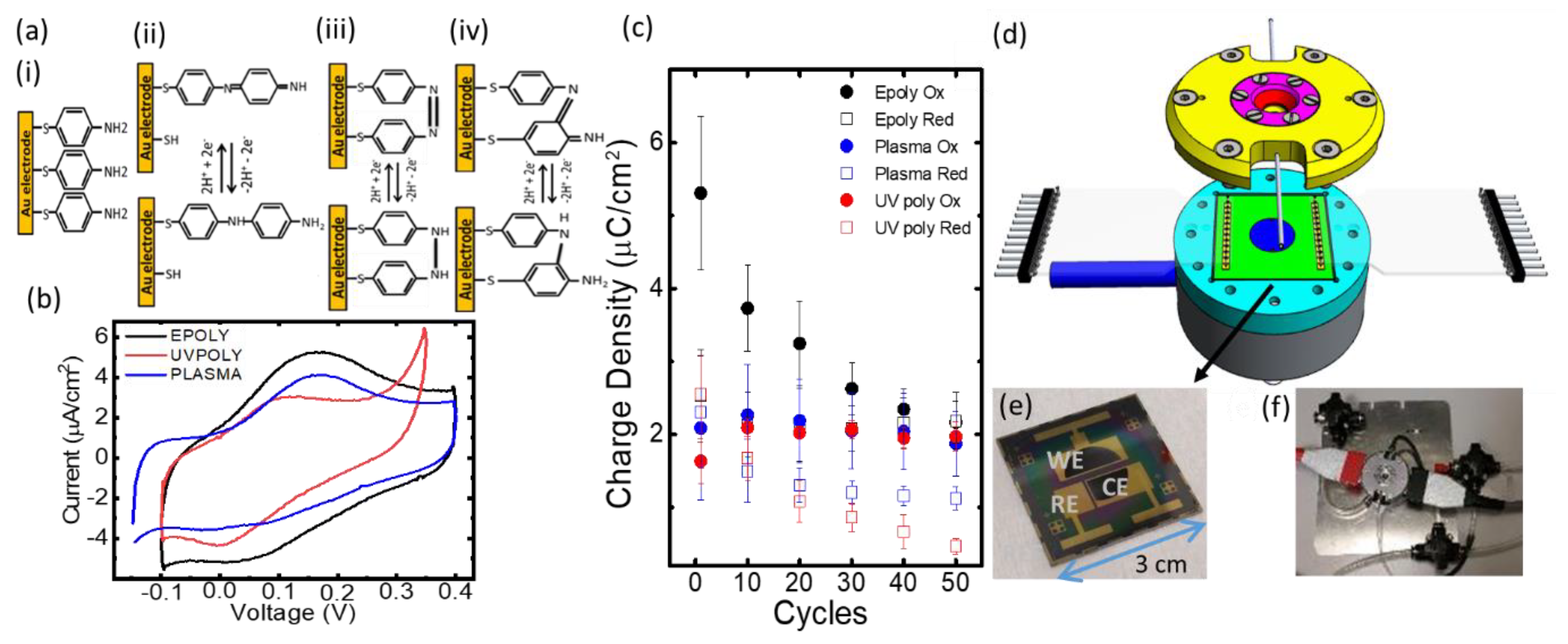
2.1. Sample Preparation and Study of Polymerisation Methods of ATP
2.2. Design of the Electrochemical Chip
2.3. Electropolymerization and pH Control on the Platform
3. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Penner, R.M. Electrochemistry on tap. Nat. Chem. 2010, 2, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, D.; Lamblin, G.; Thomann, J.S.; Guillot, J.; Duday, D.; van den Berg, A.; Olthuis, W.; Pascual Garcia, C. Influence of polymerisation on the reversibility of low-energy proton exchange reactions by Para-Aminothiolphenol. Sci. Rep. 2017, 7, 15401. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, D.; Lamblin, G.; Thomann, J.S.; van den Berg, A.; Olthuis, W.; Pascual Garcia, C. Electrochemical control of pH in nano-litre volumes. Nano Lett. 2018, 18, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, D.; Gerard, M.; Girod, S.; Frari, D.D.; Olthuis, W.; Pascual Garcia, C. Redox active polymer a pH actuator on a Re-sealable microfluidic platform. J. Mater. Sci. Eng. 2018, 7, 456. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakrishnan, D.; Olthuis, W.; García, C.P. Redox Active Self-Assembled Monolayer Functioning as a pH Actuator. Proceedings 2018, 2, 1073. https://doi.org/10.3390/proceedings2131073
Balakrishnan D, Olthuis W, García CP. Redox Active Self-Assembled Monolayer Functioning as a pH Actuator. Proceedings. 2018; 2(13):1073. https://doi.org/10.3390/proceedings2131073
Chicago/Turabian StyleBalakrishnan, Divya, Wouter Olthuis, and César Pascual García. 2018. "Redox Active Self-Assembled Monolayer Functioning as a pH Actuator" Proceedings 2, no. 13: 1073. https://doi.org/10.3390/proceedings2131073
APA StyleBalakrishnan, D., Olthuis, W., & García, C. P. (2018). Redox Active Self-Assembled Monolayer Functioning as a pH Actuator. Proceedings, 2(13), 1073. https://doi.org/10.3390/proceedings2131073





