1. Introduction
The world’s heavy reliance on the combustion of fossil fuels to meet energy demands has led to a sharp increase in atmospheric carbon dioxide (CO
2) levels. Fossil fuel consumption is projected to reach 56% by 2040 [
1]. As CO
2 is a major contributor to the greenhouse effect, concerns about reducing its emissions have grown significantly. One of the most explored solutions in recent years is carbon capture and storage (CCS), which involves trapping CO
2 emissions from industrial sources, transporting them, and storing in underground geological formations [
2]. However, due to high costs and potential long-term environmental risks, global attention is shifting toward a more promising alternative: carbon capture and utilization (CCU) [
1]. Within this context, CO
2 methanation has emerged as an increasingly attractive approach. The exothermic Sabatier reaction, Equation (1), is accompanied by the endothermic reverse water gas shift reaction (RWGS), Equation (2) [
3].
Using the Sabatier reaction, synthetic natural gas (SNG), primarily methane (CH
4), can be produced from CO
2 and hydrogen (H
2). When hydrogen is sourced renewably via electrolysis, this pathway offers a compelling route to combat global warming [
4]. SNG stands out for its high energy content and lower CO
2 emissions compared to traditional fossil fuels [
5]. Moreover, it benefits from compatibility with the existing extensive natural gas infrastructure, enabling seamless integration into current energy systems [
4].
This study focused on the influence of temperature, pressure, and the H2/CO2 molar ratio on reaction yield, the formation of by-products such as carbon oxides, and the overall energy balance. The objective is to identify the operating conditions that maximize methane production and minimize the formation of undesired compounds, thereby contributing to the optimization of the methanation process.
2. Materials and Methods
Considering the methanation reaction of CO
2, assuming a perfect gas mixture, the equilibrium constant is expressed as follows in Equation (3) [
6]:
where
are the molar fractions of the compounds. P
T is the total pressure and P
0 the reference pressure (1 bar).
Following the same reasoning, for the RWGS reaction, the equilibrium constant can be given by Equation (4):
The equilibrium constant can also be determined using the Gibbs free energy method, by Equations (5) and (6):
This method allows the molar fraction of each component present in the reaction products to be calculated from the state of thermodynamic equilibrium.
3. Results
3.1. Effect of Pressure and Temperature
As shown in
Figure 1a, CH
4 formation is favored at low temperatures, below 450 °C, due to the exothermic nature of the CO
2 methanation reaction. Additionally, high pressures also promote CH
4 formation and, consequently, improve CO
2 conversion efficiency, since the methanation reaction involves a decrease in the total number of moles from reactants to products.
Figure 1b illustrates the negative effect of the RWGS reaction, which, being endothermic, is favored by increasing reaction temperatures. This leads to the formation of CO and contributes to catalyst deactivation through carbon deposition [
3]. At 1 bar and 400 °C, the molar fraction of CH
4 reaches 0.26, increasing to 0.30 at 10 bar. Conversely, at 550 °C and 1 bar, methane decreases to 0.13, while CO increases to 0.035 due to the dominance of the RWGS reaction.
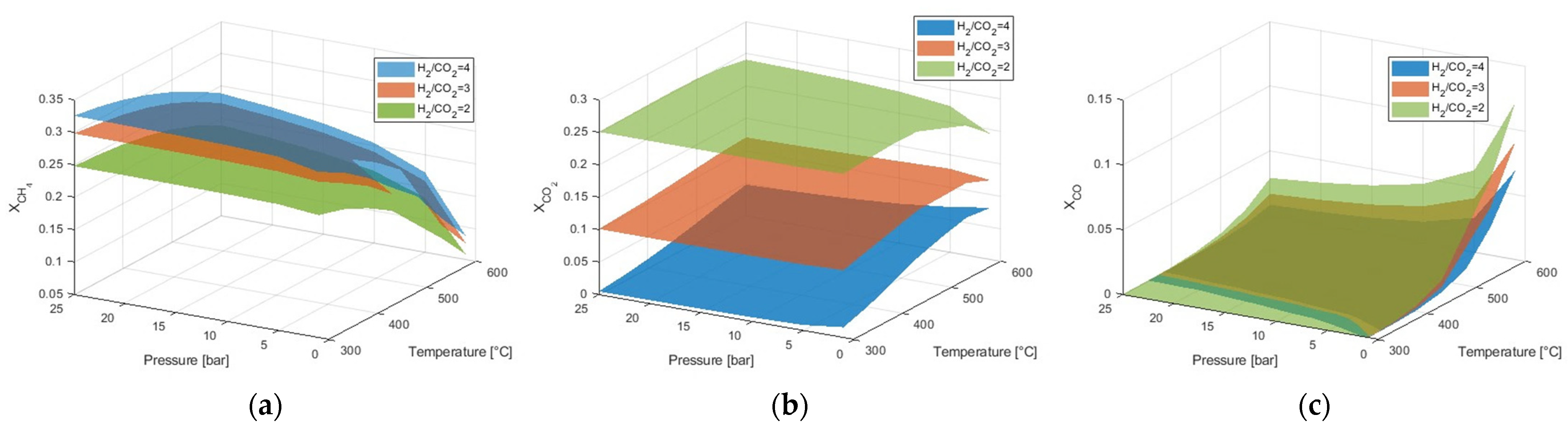
3.2. Effect of H2/CO2 Ratio
Since H
2 is an expensive component, the highest H
2/CO
2 ratio studied was 4, which corresponds to the stoichiometry of the CO
2 methanation reaction [
7].
Figure 2a shows that the molar fraction of CH
4 is unfavorable for H
2/CO
2 ratios below the stoichiometric value, with a corresponding increase in the molar fraction of CO
2 in the reaction products at equilibrium (
Figure 2b). A lower H
2/CO
2 ratio also intensifies CO formation (
Figure 2c). However, when optimal temperature and pressure conditions for maximum CH
4 selectivity are considered, the H
2/CO
2 ratio does not significantly affect the molar fraction of CO in the reaction products. When the H
2/CO
2 ratio decreases from 4 to 2 at 400 °C and 10 bar, the molar fraction of CH
4 drops from 0.30 to 0.24, with a corresponding increase in the molar fractions of CO
2 and CO.
4. Discussion
The results confirm that the CO2 methanation reaction is highly dependent on operating conditions, particularly temperature and pressure. Temperatures above 450 °C significantly reduce CO2 conversion efficiency, consistent with the exothermic nature of the reaction. Under these conditions, the RWGS reaction becomes more favorable, leading to increased CO formation.
Additionally, the H2/CO2 ratio plays an important role in chemical equilibrium. Ratios lower than the stoichiometric value (4/1) result in lower methane production and higher concentrations of residual CO2 and CO in the products.
These results are in good agreement with previous studies. Fan and Tahir [
1] reported that CO
2 methanation is most efficient at low temperatures (150–450 °C), high pressures, and an H
2/CO
2 ratio of 4, which favor CH
4 formation and suppress CO production. Miguel et al. [
7] reached similar conclusions in their thermodynamic analysis. Overall, our findings confirm that methane yield is maximized under comparable operating conditions.
5. Conclusions
This study confirmed that CO2 methanation is most efficient at temperatures below 450 °C and high-pressure conditions, using an H2/CO2 ratio close to the stoichiometric value. The reaction shows great potential as a solution for CO2 valorization, particularly when hydrogen is produced from renewable sources such as water electrolysis powered by solar or wind energy. Moreover, the methane produced can be readily injected into existing natural gas networks without the need for major infrastructure modifications, representing a significant advantage in terms of integration and cost. These findings highlight the potential of the methanation process as a key technology for the energy transition and the reduction in greenhouse gas emissions.
Author Contributions
Conceptualization, A.B.; methodology, A.B.; investigation, C.A., M.M. and A.B.; resources, A.B.; data curation, C.A., M.M. and A.B.; writing—original draft preparation, C.A. and M.M.; writing—review and editing, C.A., M.M. and A.B.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was developed under the project A-MoVeR—“Mobilizing Agenda for the Development of Products & Systems towards an Intelligent and Green Mobility”, operation n.º 02/C05-i01.01/2022.PC646908627-00000069, approved under the terms of the call n.º 02/C05-i01/2022—Mobilizing Agendas for Business Innovation, financed by European funds pro-vided to Portugal by the Recovery and Resilience Plan (RRP), in the scope of the European Recovery and Resilience Facility (RRF), framed in the Next Generation UE, for the period from 2021–2026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fan, W.K.; Tahir, M. Investigating the Product Distribution Behaviour of CO2 Methanation through Thermodynamic Optimized Experimental Approach Using Micro/Nano Structured Titania Catalyst. Energy Convers. Manag. 2022, 254, 115240. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Catalytic Thermal Conversion of CO2 into Fuels: Perspective and Challenges. Renew. Sustain. Energy Rev. 2019, 115, 109333. [Google Scholar] [CrossRef]
- Sun, D.; Khan, F.M.; Simakov, D.S.A. Heat Removal and Catalyst Deactivation in a Sabatier Reactor for Chemical Fixation of CO2: Simulation-Based Analysis. Chem. Eng. J. 2017, 329, 165–177. [Google Scholar] [CrossRef]
- Solis-Garcia, A.; Zepeda, T.A.; Fierro-Gonzalez, J.C. Spectroscopic Evidence of Surface Species during CO2 Methanation Catalyzed by Supported Metals: A Review. Catal. Today 2022, 394, 2–12. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. The Role of Alkali and Alkaline Earth Metals in the CO2 Methanation Reaction and the Combined Capture and Methanation of CO2. Catalysts 2020, 10, 812. [Google Scholar] [CrossRef]
- Ghaib, K.; Nitz, K.; Ben-Fares, F. Chemical Methanation of CO2: A Review. ChemBioEng Rev. 2016, 3, 266–275. [Google Scholar] [CrossRef]
- Miguel, C.V.; Soria, M.A.; Mendes, A.; Madeira, L.M. Direct CO2 Hydrogenation to Methane or Methanol from Post-Combustion Exhaust Streams—A Thermodynamic Study. J. Nat. Gas. Sci. Eng. 2015, 22, 1–8. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).