From Waste to Value: Phenolic Content and Antioxidant Potential in Cistus ladanifer Residues via Solid–Liquid and Subcritical Water Extraction †
Abstract
1. Introduction
2. Materials and Methods
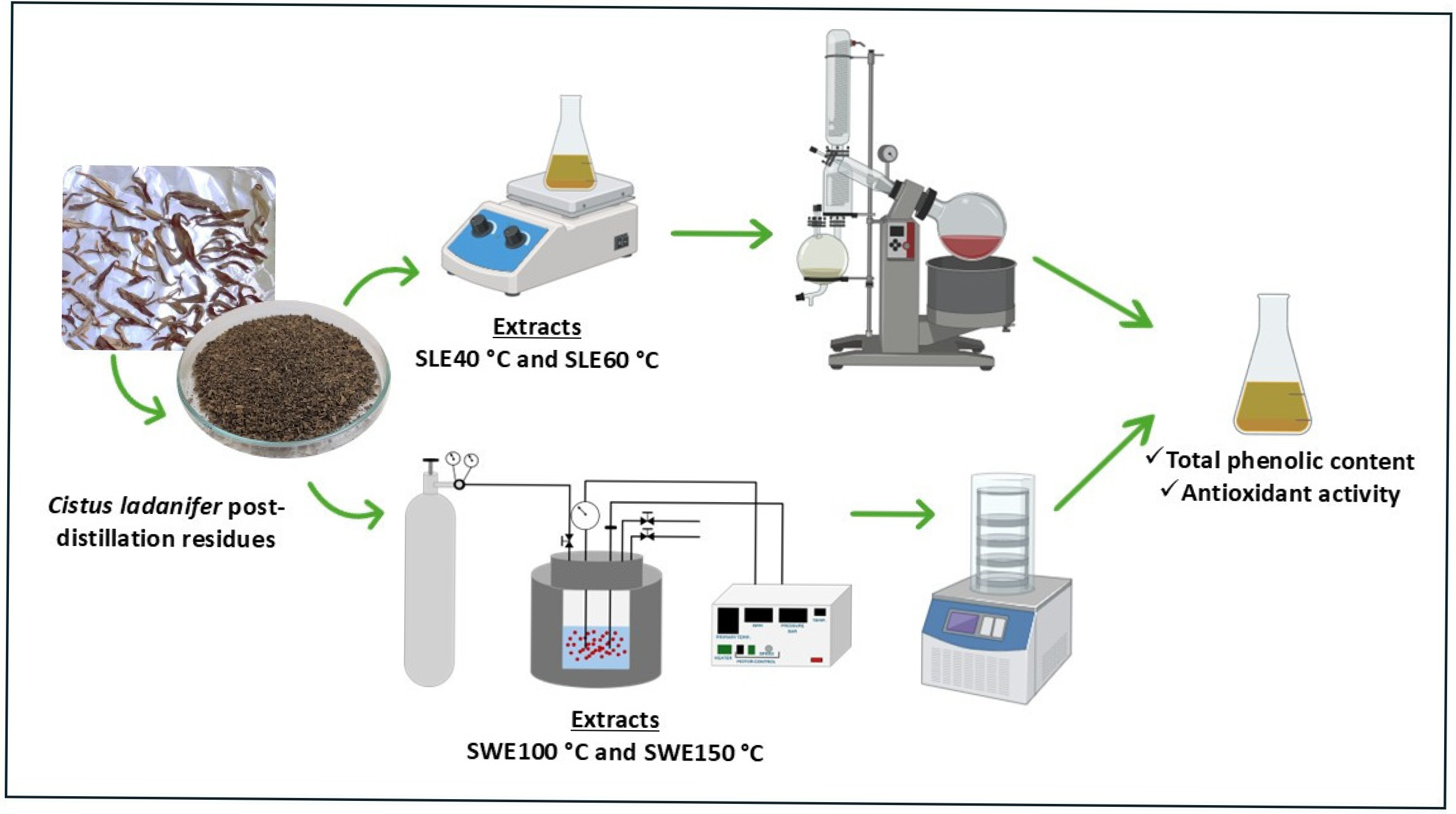
2.1. Samples and Extractions
2.2. Total Phenolic Content (TPC) and Antioxidant Activity
2.3. Statistical Analysis
3. Results
Total Phenolic Content and Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrajón-Catalán, E.; Tomás-Menor, L.; Morales-Soto, A.; Bruñá, N.M.; López, D.S.; Segura-Carretero, A.; Micol, V. Rockroses (Cistus sp.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2015; pp. 649–658. [Google Scholar] [CrossRef]
- El Karkouri, J.; Sbai, H.; El Aggadi, S.; Bouyahya, A.; Zengin, G.; Harhar, H.; Zair, T. Phytochemical analysis, antioxidant, toxicity, and antihyperglycemic effects of Cistus ladaniferus L., extracts. Microchem. J. 2024, 207, 111766. [Google Scholar] [CrossRef]
- Abdelfattah, B.; Mrabet, A.; Simou, A.; Khaddor, M. Exploring the Bioactive Potential of Cistus ladanifer Leaves from Northern Morocco (Tangier). Ecol. Eng. Environ. Technol. 2024, 25, 316–330. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Das, S.; Chaudhari, A.K.; Dubey, N.K. Cistus ladanifer L. essential oil as a plant based preservative against molds infesting oil seeds, aflatoxin B1 secretion, oxidative deterioration and methylglyoxal biosynthesis. LWT 2018, 92, 395–403. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid solid-liquid dynamic extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Gallina, L.; Cravotto, C.; Capaldi, G.; Grillo, G.; Cravotto, G. Plant Extraction in Water: Towards Highly Efficient Industrial Applications. Processes 2022, 10, 2233. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Fernandes, F.; Delerue-Matos, C.; Grosso, C. Valorization of Spent Coffee Grounds: Comparing Phenolic Content and Antioxidant Activity in Solid-Liquid vs. Subcritical Water Extraction Methods. Biol. Life Sci. Forum 2025, 40, 23. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Fernandes, F.; Gorissen, K.; Delerue-Matos, C.; Grosso, C. Valorisation of Agro-Food By-Products for the Extraction of Phenolic Compounds. Biol. Life Sci. Forum 2022, 18, 61. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Macedo, C.; Silva, A.M.; Ferreira, A.S.; Moreira, M.M.; Delerue-Matos, C.; Rodrigues, F. Microwave- and Ultrasound-Assisted Extraction of Cucurbita pepo Seeds: A Comparison Study of Antioxidant Activity, Phenolic Profile, and In-Vitro Cells Effects. Appl. Sci. 2022, 12, 1763. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from forest biomass II. Bioactive compounds from the steam-distillation by-products of Cupressus lusitanica Mill. and Cistus ladanifer L. wastes. Ind. Crops Prod. 2020, 158, 112991. [Google Scholar] [CrossRef]
- Bouothmany, K.; Bourhia, M.; Aoussar, N.; Attaleb, M.; Salamatullah, A.M.; Nafidi, H.A.; Mellouki, F.; El Mzibri, M.; Aboul-Soud, M.A.; Benbacer, L. Leaf Extracts of Cistus ladanifer Exhibit Potent Antioxidant and Antiproliferative Activities against Liver, Prostate and Breast Cancer Cells. Appl. Sci. 2022, 12, 8603. [Google Scholar] [CrossRef]
| Sample | TPC (mg GAEs/g dw) | DPPH• (mg TEs/g dw) | ABTS•+ (mg TEs/g dw) | FRAP (mg AAEs/g dw) |
|---|---|---|---|---|
| SLE40 °C | 175.24 ± 21.82 b | 334.27 ± 36.06 b | 438.07 ± 77.22 c | 10.91 ± 2.03 c |
| SLE60 °C | 146.53 ± 11.68 b | 381.02 ± 85.18 b | 453.80 ± 41.23 c | 11.64 ± 2.96 c |
| SWE100 °C | 276.37 ± 20.59 a | 532.17 ± 66.38 a | 594.08 ± 33.57 a | 170.26 ± 25.36 a |
| SWE150 °C | 256.05 ± 32.61 a | 475.73 ± 50.42 a | 520.42 ± 17.50 b | 132.39 ± 27.96 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, F.; Delerue-Matos, C.; Grosso, C. From Waste to Value: Phenolic Content and Antioxidant Potential in Cistus ladanifer Residues via Solid–Liquid and Subcritical Water Extraction. Proceedings 2025, 119, 5. https://doi.org/10.3390/proceedings2025119005
Fernandes F, Delerue-Matos C, Grosso C. From Waste to Value: Phenolic Content and Antioxidant Potential in Cistus ladanifer Residues via Solid–Liquid and Subcritical Water Extraction. Proceedings. 2025; 119(1):5. https://doi.org/10.3390/proceedings2025119005
Chicago/Turabian StyleFernandes, Filipe, Cristina Delerue-Matos, and Clara Grosso. 2025. "From Waste to Value: Phenolic Content and Antioxidant Potential in Cistus ladanifer Residues via Solid–Liquid and Subcritical Water Extraction" Proceedings 119, no. 1: 5. https://doi.org/10.3390/proceedings2025119005
APA StyleFernandes, F., Delerue-Matos, C., & Grosso, C. (2025). From Waste to Value: Phenolic Content and Antioxidant Potential in Cistus ladanifer Residues via Solid–Liquid and Subcritical Water Extraction. Proceedings, 119(1), 5. https://doi.org/10.3390/proceedings2025119005








