Bee Product Royal Jelly Reduces Oxidative Stress in Healthy MRC-5 Cells and Upregulates GSTP1 Expression †
Abstract
1. Introduction
2. Materials and Methods
3. Results
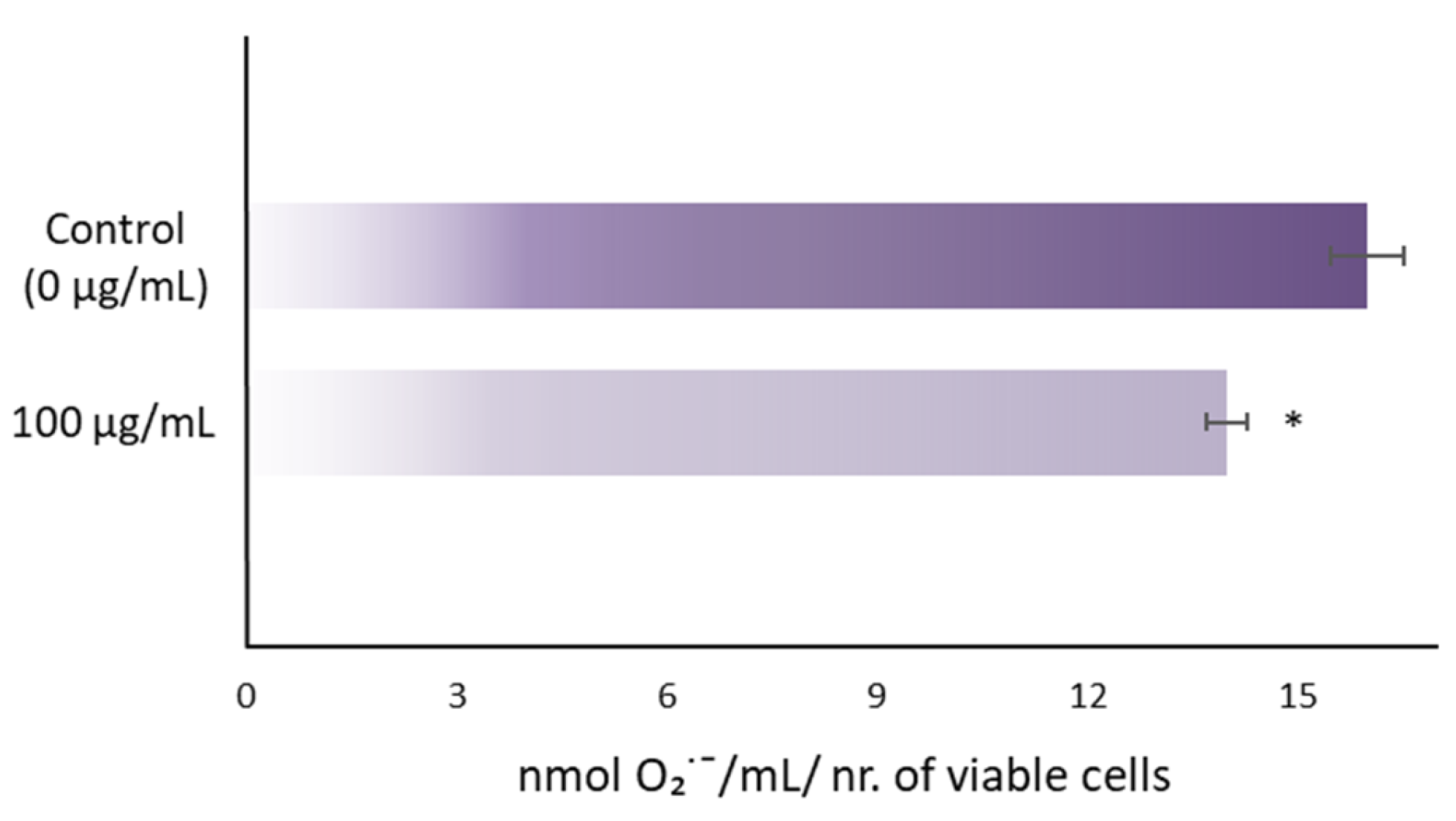
3.1. RJ Reduces O2∙− Concentration
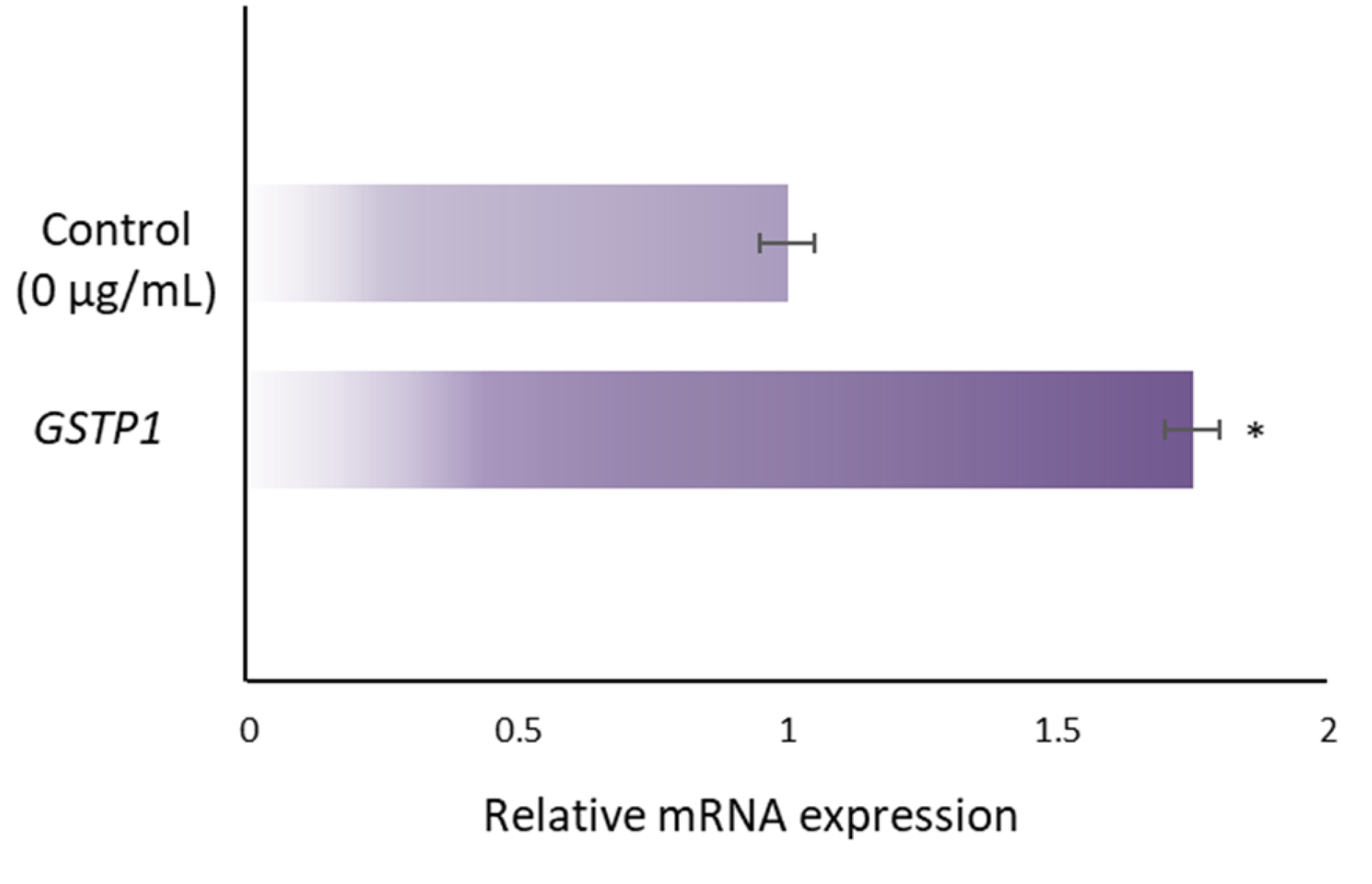
3.2. RJ Upregulates Expression of GSTP1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.M.; Richardson, D.R. The good samaritan glutathione-S-transferase P1: An evolving relationship in nitric oxide metabolism mediated by the direct interactions between multiple effector molecules. Redox Biol. 2023, 59, 102568. [Google Scholar] [CrossRef] [PubMed]
- Letelier, M.E.; Molina-Berríos, A.; Cortés-Troncoso, J.; Jara-Sandoval, J.A.; Müller, A.; Aracena-Parks, P. Comparative effects of superoxide anion and hydrogen peroxide on microsomal and cytosolic glutathione S-transferase activities of rat liver. Biol. Trace Elem. Res. 2010, 134, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 17, 477. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sakai, H. Anti-cancer and protective effects of royal jelly for therapy-induced toxicities in malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef] [PubMed]
- Pavel, C.; Marghitas, L.A.; Bobis, O.; Desmirean, D.S.; Sapcaliu, A.; Radoi, I.; Madas, M.N. Biological activities of royal jelly–review. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 108–118. [Google Scholar]
- Orsolić, N. Royal jelly: Component efficiency, analysis, and standardisation. Arh. Hyg. Rada Toxicol. 2013, 64, 445–460. [Google Scholar]
- Sugiyama, T.; Takahashi, K.; Mori, H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Ćupurdija, M.; Nikodijević, D.; Milutinović, M.; Cvetković, D.; Rakobradović, J.; Marković, S. Effects of royal jelly on energy status and expression of apoptosis and biotransformation genes in normal fibroblast and colon cancer cells. Kragujevac J. Sci. 2018, 40, 175–192. [Google Scholar] [CrossRef]
- Šeklić, D.S.; Stanković, M.S.; Milutinović, M.G.; Topuzović, M.D.; Štajn, A.Š.; Marković, S.D. Cytotoxic, antimigratory, pro-and antioxidative activites of extracts medical mushrooms on colon cancer cell lines. Arch. Biol. Sci. 2016, 68, 93–105. [Google Scholar] [CrossRef]
- Jovanović, M.M.; Šeklić, D.S.; Rakobradović, J.D.; Planojević, N.S.; Vuković, N.L.; Vukić, M.D.; Marković, S.D. Royal jelly and trans-10-hydroxy-2-decenoic acid inhibit migration and invasion of colorectal carcinoma cells. Food Technol. Biotechnol. 2022, 60, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Nutrition, Health; Chapter 2. Bee Product Science; Elesevier: Amsterdam, The Netherland, 2016. [Google Scholar]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxidative Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef] [PubMed]
- Khoob, M.S.; Hosseini, S.M.; Kazemi, S. In vitro and in vivo antioxidant and anticancer potentials of royal jelly for dimethylhydrazine-induced colorectal cancer in wistar rats. Oxidative Med. Cell. Longev. 2022, 2022, 9506026. [Google Scholar]
- Lv, N.; Huang, C.; Huang, H.; Dong, Z.; Chen, X.; Lu, C.; Zhang, Y. Overexpression of glutathione S-transferases in human diseases: Drug targets and therapeutic implications. Antioxidants 2023, 12, 1970. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Du, L.; Yu, W.; Wang, Y.; Ma, N.; Qu, B. GSTP1 as a novel target in radiation induced lung injury. J. Transl. Med. 2021, 19, 297. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Huang, X.; Niu, B.; Guo, X.; Lei, X.; Qu, B. Targeting GSTP1-dependent ferroptosis in lung cancer radiotherapy: Existing evidence and future directions. Front Mol. Biosci. 2022, 9, 1102158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, M.; Šeklić, D. Bee Product Royal Jelly Reduces Oxidative Stress in Healthy MRC-5 Cells and Upregulates GSTP1 Expression. Proceedings 2025, 119, 3. https://doi.org/10.3390/proceedings2025119003
Jovanović M, Šeklić D. Bee Product Royal Jelly Reduces Oxidative Stress in Healthy MRC-5 Cells and Upregulates GSTP1 Expression. Proceedings. 2025; 119(1):3. https://doi.org/10.3390/proceedings2025119003
Chicago/Turabian StyleJovanović, Milena, and Dragana Šeklić. 2025. "Bee Product Royal Jelly Reduces Oxidative Stress in Healthy MRC-5 Cells and Upregulates GSTP1 Expression" Proceedings 119, no. 1: 3. https://doi.org/10.3390/proceedings2025119003
APA StyleJovanović, M., & Šeklić, D. (2025). Bee Product Royal Jelly Reduces Oxidative Stress in Healthy MRC-5 Cells and Upregulates GSTP1 Expression. Proceedings, 119(1), 3. https://doi.org/10.3390/proceedings2025119003





