Determination of Antioxidant Activity of Vitamin C by Voltammetric Methods †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
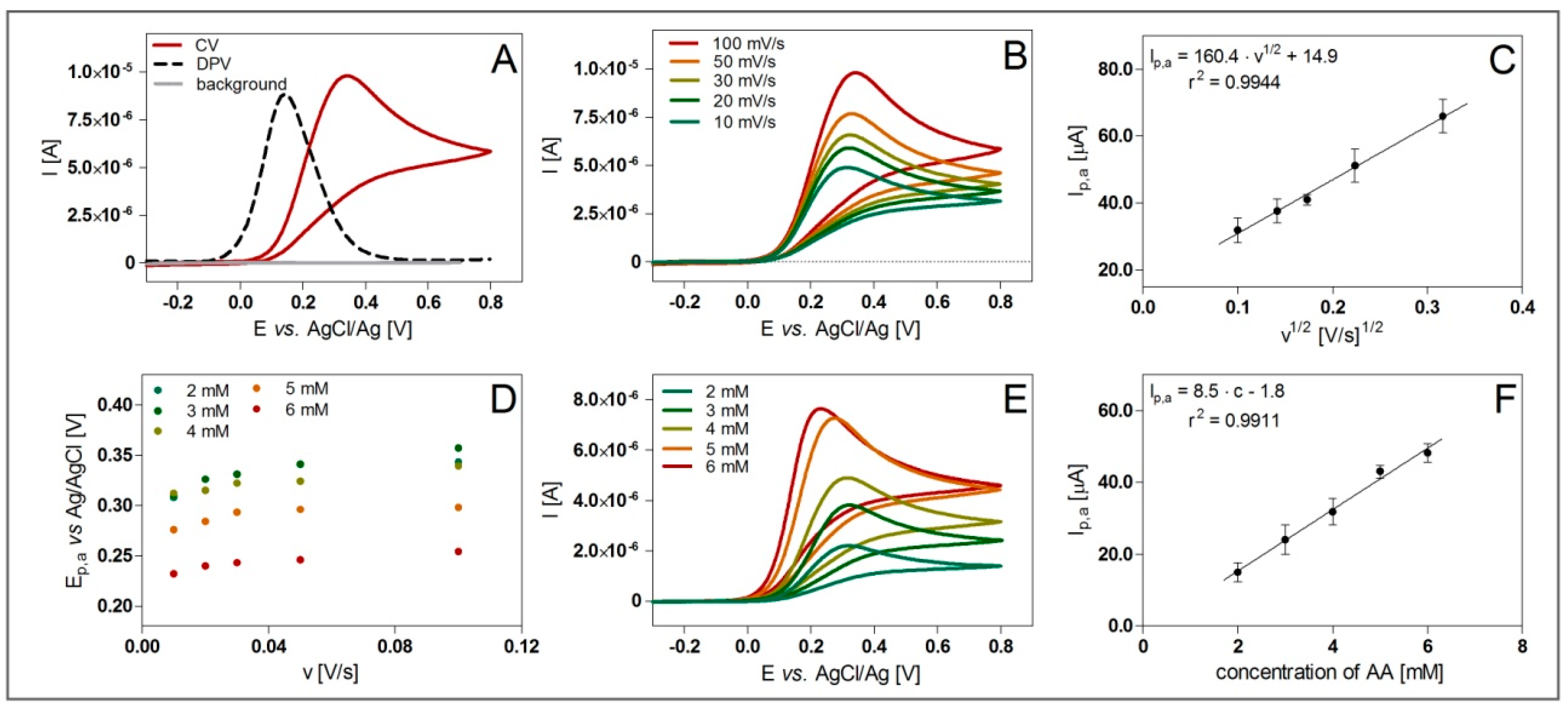
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Meng, L.; Gelb, A.W.; Alexander, B.S.; Cerussi, A.E.; Tromberg, B.J.; Yu, Z.; Mantulin, W.W. Impact of phenylephrine administration on cerebral tissue oxygen saturation and blood volume is modulated by carbon dioxide in anaesthetized patients. Br. J. Anaesth. 2012, 108, 815–822. [Google Scholar] [CrossRef]
- Roleira, F.M.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic phenolic antioxidants: correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. The relationship between standard reduction potentials of catechins andbiological activities involved in redox control. Redox Biol. 2018, 17, 355–366. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L. R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 236, 503, 709. [Google Scholar]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry: Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993; p. 427. [Google Scholar]
- Kissinger, P.T.; Heineman, W.H. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed.Marcel Dekker: New York, NY, USA, 1996; p. 224. [Google Scholar]
- Timbola, A.K.; Souza, C.D.; Giacomelli, C.; Spinelli, A. Electrochemical oxidation of quercetin in hydro-alkoholic solution. J. Braz. Chem. Soc. 2006, 37, 617–624. [Google Scholar]
- Harrison, J.A.; Khan, Z.A. The oxidation of hydrazine on platinum in acid solution. J. Electroanal. Chem. Interfacial Electrochem. 1970, 28, 131–138. [Google Scholar] [CrossRef]
- Yaghoubian, H.; Beitollah, H.; Soltani-Nejad, V.; Mohadesi, A.; Afzali, D.; Zamani, H. Simultaneous voltammetric determination of epinephrine and acetaminophene at the surface of modified carbon nantube paste electrode. Int. J. Electrochem. Sci. 2011, 6, 1307–1316. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suliborska, K.; Baranowska, M.; Bartoszek, A.; Chrzanowski, W.; Namieśnik, J. Determination of Antioxidant Activity of Vitamin C by Voltammetric Methods. Proceedings 2019, 11, 23. https://doi.org/10.3390/proceedings2019011023
Suliborska K, Baranowska M, Bartoszek A, Chrzanowski W, Namieśnik J. Determination of Antioxidant Activity of Vitamin C by Voltammetric Methods. Proceedings. 2019; 11(1):23. https://doi.org/10.3390/proceedings2019011023
Chicago/Turabian StyleSuliborska, Klaudia, Monika Baranowska, Agnieszka Bartoszek, Wojciech Chrzanowski, and Jacek Namieśnik. 2019. "Determination of Antioxidant Activity of Vitamin C by Voltammetric Methods" Proceedings 11, no. 1: 23. https://doi.org/10.3390/proceedings2019011023
APA StyleSuliborska, K., Baranowska, M., Bartoszek, A., Chrzanowski, W., & Namieśnik, J. (2019). Determination of Antioxidant Activity of Vitamin C by Voltammetric Methods. Proceedings, 11(1), 23. https://doi.org/10.3390/proceedings2019011023




