Abstract
This study demonstrates the electrorotation of single microalgae cells captured in the center of a set of planar microelectrodes within a microfluidic device, and compares the rotational speeds of single microalgae cells obtained in experiments and theoretical calculations. Negative dielectrophoresis force and rotational electric field are generated by the microelectrode set to position and rotate single microalgae cells. The rotational speed of single microalgae cells in the microfluidic device is calculated automatically using an image-processing algorithm. The electrorotation spectra of microalgae cells enduring different periods of nitrogen starvation agree with the simulation results for low- and high-lipid content cells. These results show the great potential of using electrorotation in quantifying the dielectric characteristics of microalgae cells.
1. Introduction
Microalgae has been considered as effective producers of various energy and food commodities such as lipids, carbohydrates, and proteins [1]. The production of biodiesel from microalgal lipids has been studied intensively in the past decade; however, the progress of the enhancement of cellular lipid abundance is relatively slow due to the lack of rapid and accurate methods for quantifying cellular lipids. Conventional methods for cellular lipid quantification such as weighting and chromatography are time consuming and involve multiple steps of treatments. Although the fluorescence intensity is commonly applied on qualitative comparisons, quantitative analysis remains challenging. Therefore, this study aims to develop a rapid, in-situ, and single-step quantification method for microalgae single cells based on the dielectric characteristics.
The dielectric characterization of living cells can be achieved either by bioimpedance measurement [2] or by electrorotation analysis [3]. The latter method has the advantage of being insensitive to the volume of the analyte, which makes it more adapted to the single cell measurement [4]. In that case, the cell is exposed to a rotating electric field and experiences a mechanical rotation depending on the contrast between the dielectric properties of itself and the surrounding medium.
The cell is trapped at the center of the electrode set with a stationary electric field, and induced into rotation owing to a superimposed rotating electrical field [5]. The rotational velocity of the cell expresses:
where ε0 and εm are the permittivity of vacuum and the relative permittivity of medium, respectively. E is the strength (root-mean-squared value) of the electric field, η is the dynamic viscosity of the medium, and fCM is the Clausius-Mossotti factor which depends on the dielectric properties of the cell:
where σ is the conductivity, and the subscript c and m denote the cell and the medium, respectively.
The complex permittivity of the cell is a function of the dielectric properties of its components (conductivity and permittivity of the wall, membrane, cytoplasm, and lipid content). The complex permittivity of the cell (εc) is modeled here thanks to the mixing theory [6] as a multi-shell system, consisting on an inner core of lipids, surrounded within the cytoplasm, encapsulated by the membrane [3].
2. Materials and Methods
2.1. Device Fabrication
We design a microfluidic device with the four-planar-electrode configuration so that single microalgae cell can be trapped at the center of the microelectrode set by the negative dielectrophoresis force (Figure 1). The microfluidic device, including the 4 electrodes, was fabricated using conventional cleanroom technology. A 2 inches quartz wafer was used as the substrate on which chromium and gold was successively deposited and patterned to form the electrodes using UV photolithography. On the top of this electrode layer, a 30-μm thick photoresist layer of Microchem© SU-8 was then patterned in order to form the microfluidic channel confining the microalgae to be analysed. Both the electrode and microfluidic layers are visible in Figure 1b.
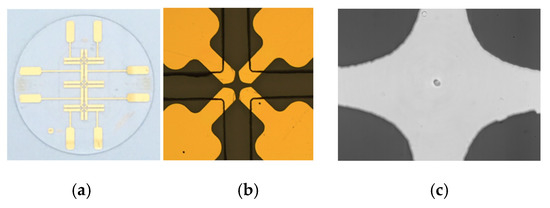
Figure 1.
Microfluidic device for characterizing electrorotation of a microalgae cell: (a) the microelectrode design; (b) a close-up of the microelectrodes (gold) and the microchannels (black lines); (c) single microalgae cell (Scenedesmus abundans) captured at the center of the microelectrode set.
2.2. Experimental Setup
The final biochip (seen on Figure 1a) was then positioned on a Printed Circuit Board for the connection of electrical signals. One of the AC generators (33250A, Agilent Technologies, Inc., Colorado Springs, CO, USA) provides the 1 V, 100 kHz voltages for the stationary electric field used for cell trapping, while the other generator (AFG 3102, Tektronix Inc., USA) provides the 3 V voltages with a frequency up to 100 MHz to produce the rotating electric field.
The rotational speed of the microalgae cell is then obtained by image processing of the sequence taken by a high-speed camera (Phantom v9.1, Vision Research, Inc., Wayne, NJ, USA) with a frame rate of 100 fps and an exposure time of 3 ms. The camera is attached to a microscope (Axio Scope.A1, Carl Zeiss Microscopy GmbH Inc., München, Germany) using a 50× objective. First, binarization is applied to every frame in the image sequence. After identifying the microalgae cell, signal noise is removed by the threshold filtering. As seen in Figure 2, the variation of the longitudinal length of the cell with time is then detected. Fast Fourier Transform is applied to obtain the frequency from the fluctuation in the time domain, from which the rotational speed is determined. The uncertainty in the measurement of the rotational speed is less than 3% and the analyzing time takes less than 5 min, which makes it possible to acquire the whole spectrum for the cell.
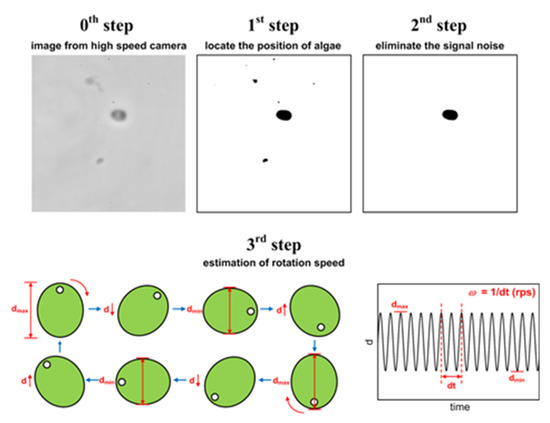
Figure 2.
Image processing for quantifying the rotational speed of the cell.
3. Results and Discussions
The lipid content of the microalgae affects the complex permittivity of the cell (εc), as it is defined by the dielectric properties of its membrane, cytoplasm, and volume factor of lipid. Such dependence can be described by analytical modeling [3]. Figure 3 shows the effect of the lipid content on the imaginary part of the Clausius-Mossotti factor predicted by the multi-shell model. We find that the reverse frequency at which the imaginary part of the Clausius-Mossotti factor switches sign, decreases when the lipid content of the microalgae increases. Since the rotational speed of the cell is proportional to the imaginary part of the Clausius-Mossotti factor as Equation (1) suggests, we are able to determine this reverse frequency from the measuring spectrum of electrorotation, and a fatter microalgae leads to a lower reverse frequency.
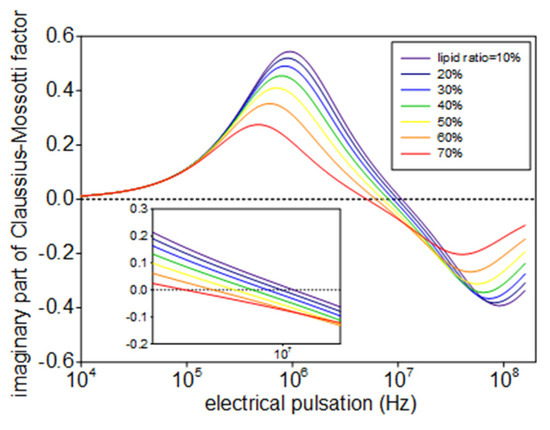
Figure 3.
Effect of the lipid content on the imaginary part of the Clausius-Mossotti factor.
The metabolism of carbohydrates inside microalgae can be shifted by the deprivation of nitrogen and the flow of carbon is directed into lipids. As S. abundans is subjected to nitrogen starvation, its lipid abundance increases gradually with the duration of nitrogen starvation. The accumulation of lipids is commonly reported as a 1- to 2-week process, following by a period of several days with stable lipid abundance. As a result, a decrease in the reverse frequency is expected when comparing the electrorotation spectrum of cells at the beginning of the nitrogen starvation (day 0 in Figure 4) and those after 14 days of starvation. The reverse frequencies are nearly identical for microalgae cells enduring 14 days and 18 days of starvation. This is because these microalgae cells contain similar lipid abundances. Nevertheless, the magnitudes of the rotational speed are considerably different. This may be ascribed to the dissimilar cell shapes and sizes, and these will be further investigated and accommodated into the theoretical model in the future.
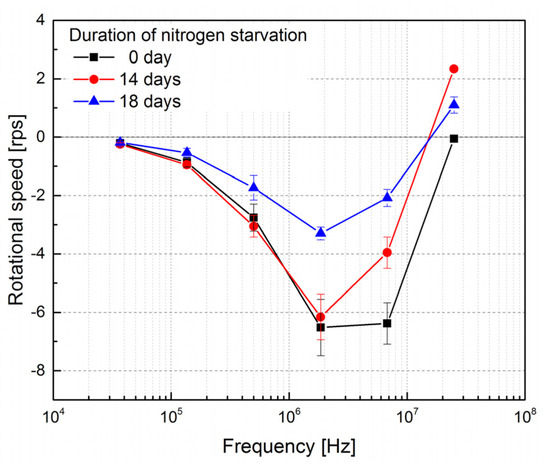
Figure 4.
The experimental electrorotation spectra of microalgae cells enduring different periods of nitrogen starvation.
4. Conclusions
In this study, we demonstrate the potential of using the electrorotation of single microalgae cells to gauge its lipid abundance with a microfluidic device. The rotational speeds of single microalgae cells exposing to an electrorotational field with different frequencies are quantified so that the reverse frequency, at which the cell rotation switches direction, can be determined. As the lipid content increases, the reverse frequency decreases accordingly. This trend matches the theoretical prediction based on the multi-shell model. Our results suggests that electrorotation might be a powerful tool to characterize the dielectric properties of microalgae cells.
Acknowledgments
This work is supported by the Ministry of Science and Technology of Taiwan under the Dragon Gate Project (MOST 105-2911-I-007-525), and LASIPS Laboratory of Excellence.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gawad, S.; Schild, L.; Renaud, P.H. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 2001, 1, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hölzel, R.; Pethig, R.; Wang, X.B. Differences in the AC electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Phys. Med. Biol. 1992, 37, 1499–1517. [Google Scholar] [CrossRef] [PubMed]
- Français, O.; Le Pioufle, B. Single Cell Electrical Characterization Techniques. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Berlin, Germany, 2016; pp. 1–18. [Google Scholar]
- Trainito, C.I.; Français, O.; Le Pioufle, B. Monitoring the permeabilization of a single cell in a microfluidic device, through the estimation of its dielectric properties based on combined dielectrophoresis and electrorotation in situ experiments. Electrophoresis 2015, 36, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Oxford, UK, 1881. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).