Study on Cell-Capturing Microfluidic Device in High Flow Rates through Controlling Shape of Microstructures and Their Alignments †
Abstract
:1. Introduction
2. Materials and Methods
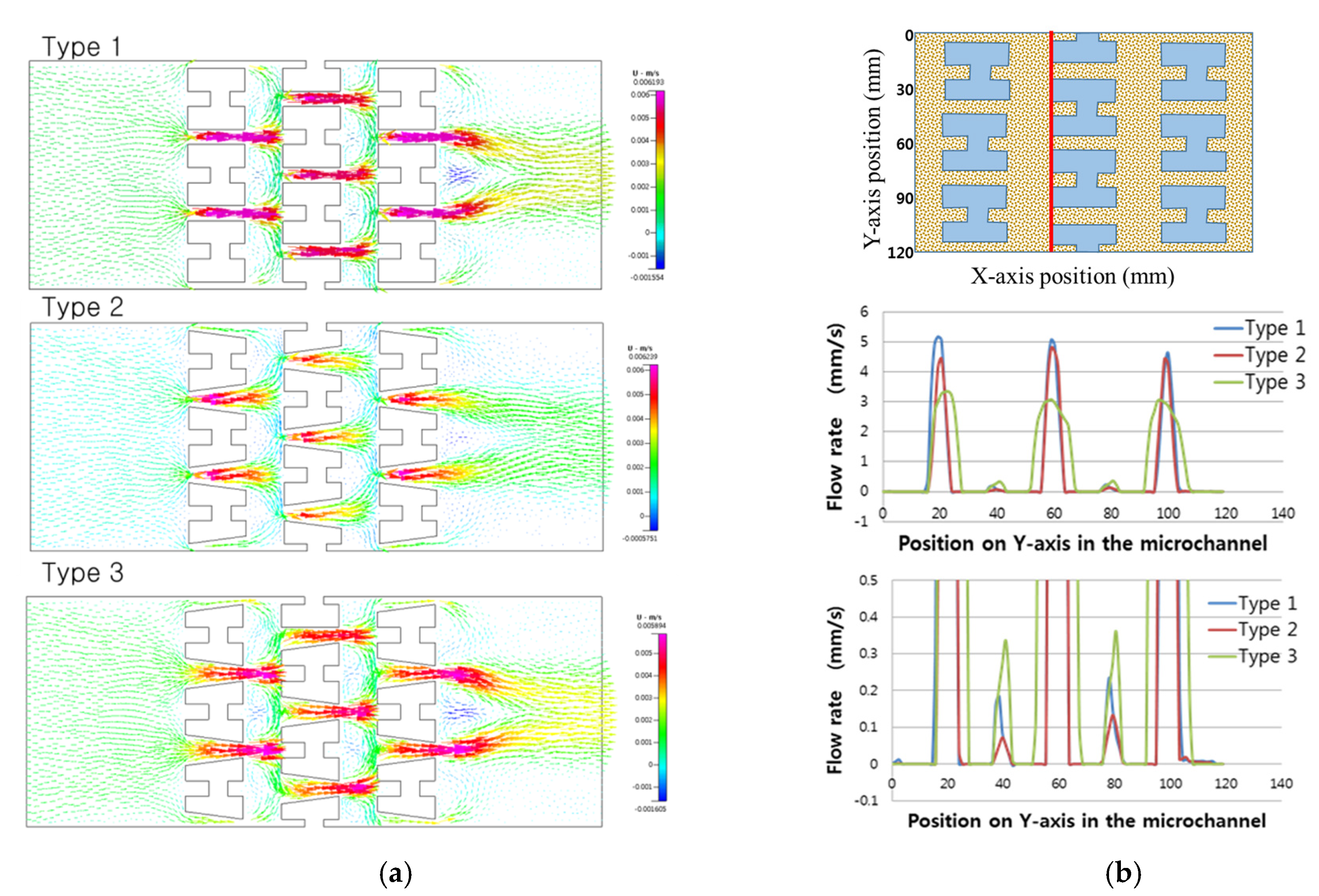
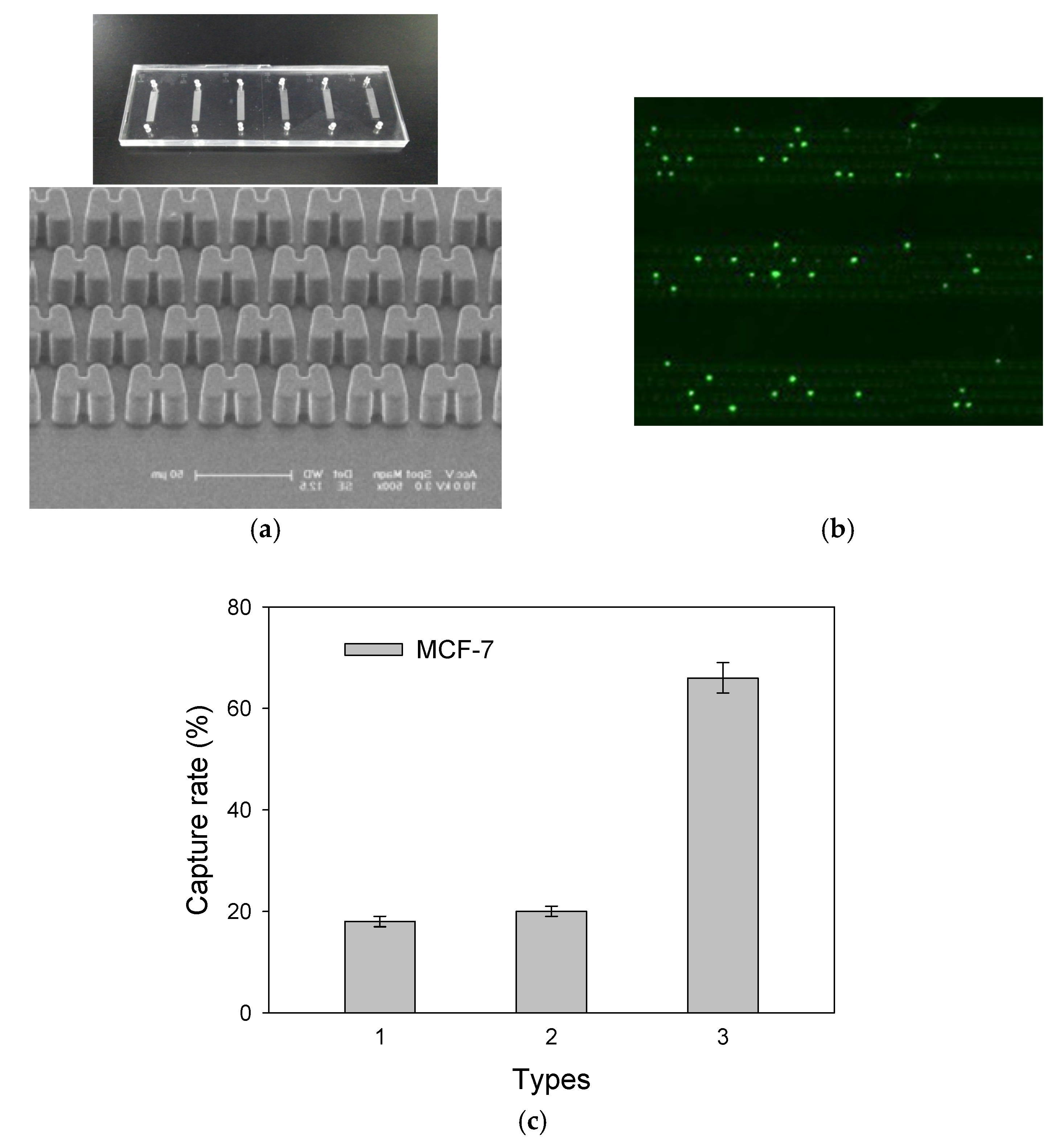
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sackmann, E.K.; Fulton, A.I.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.W.; Reyes, C.D.; Lopez, G.P. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Carlo, D.D.; Wu, L.Y.; Lee, L.P. Dynamic single cell culture array. Lab Chip 2006, 206, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Yoon, G.-W.; Park, J.W.; Ihm, C.; Lee, D.-S.; Yoon, J.-B. Fabrication of a membrane filter with controlled pore shape and its application to cell separation and strong single cell trapping. J. Micromech. Microeng. 2015, 11, 105007. [Google Scholar] [CrossRef]
- Dura, B.; Dougan, S.K.; Barisa, M.; Hoehl, M.M.; Lo, C.T.; Ploegh, H.L.; Voldman, J. Profiling lymphocyte interactions at the single-cell level by microfluidic cell pairing. Nat. Commun. 2015, 6, 5940. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, W.; Xin, H.; Deng, G. Single, Cell Isolation and Analysis. Front. Cell Dev. Biol. 2016, 4, 116. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, N.L.; Cho, S.M.; Jung, M.Y.; Ihm, C.; Lee, D.-S. Microdevice for separation of circulating tumor cells using embedded magnetophoresis with v-shaped Ni-Co nanowires and immuno-nanomagnetic beads. J. Electron. Telecommun. Res. Inst. ETRI 2015, 37, 233–240. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-S.; Park, J.W.; Jung, M.Y.; Ihm, C. Study on Cell-Capturing Microfluidic Device in High Flow Rates through Controlling Shape of Microstructures and Their Alignments. Proceedings 2017, 1, 516. https://doi.org/10.3390/proceedings1040516
Lee D-S, Park JW, Jung MY, Ihm C. Study on Cell-Capturing Microfluidic Device in High Flow Rates through Controlling Shape of Microstructures and Their Alignments. Proceedings. 2017; 1(4):516. https://doi.org/10.3390/proceedings1040516
Chicago/Turabian StyleLee, Dae-Sik, Jeong Won Park, Moon Youn Jung, and Chunwha Ihm. 2017. "Study on Cell-Capturing Microfluidic Device in High Flow Rates through Controlling Shape of Microstructures and Their Alignments" Proceedings 1, no. 4: 516. https://doi.org/10.3390/proceedings1040516
APA StyleLee, D.-S., Park, J. W., Jung, M. Y., & Ihm, C. (2017). Study on Cell-Capturing Microfluidic Device in High Flow Rates through Controlling Shape of Microstructures and Their Alignments. Proceedings, 1(4), 516. https://doi.org/10.3390/proceedings1040516



