Field-Effect Biosensors Modified with Tobacco Mosaic Virus Nanotubes as Enzyme Nanocarrier †
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of EIS Sensors
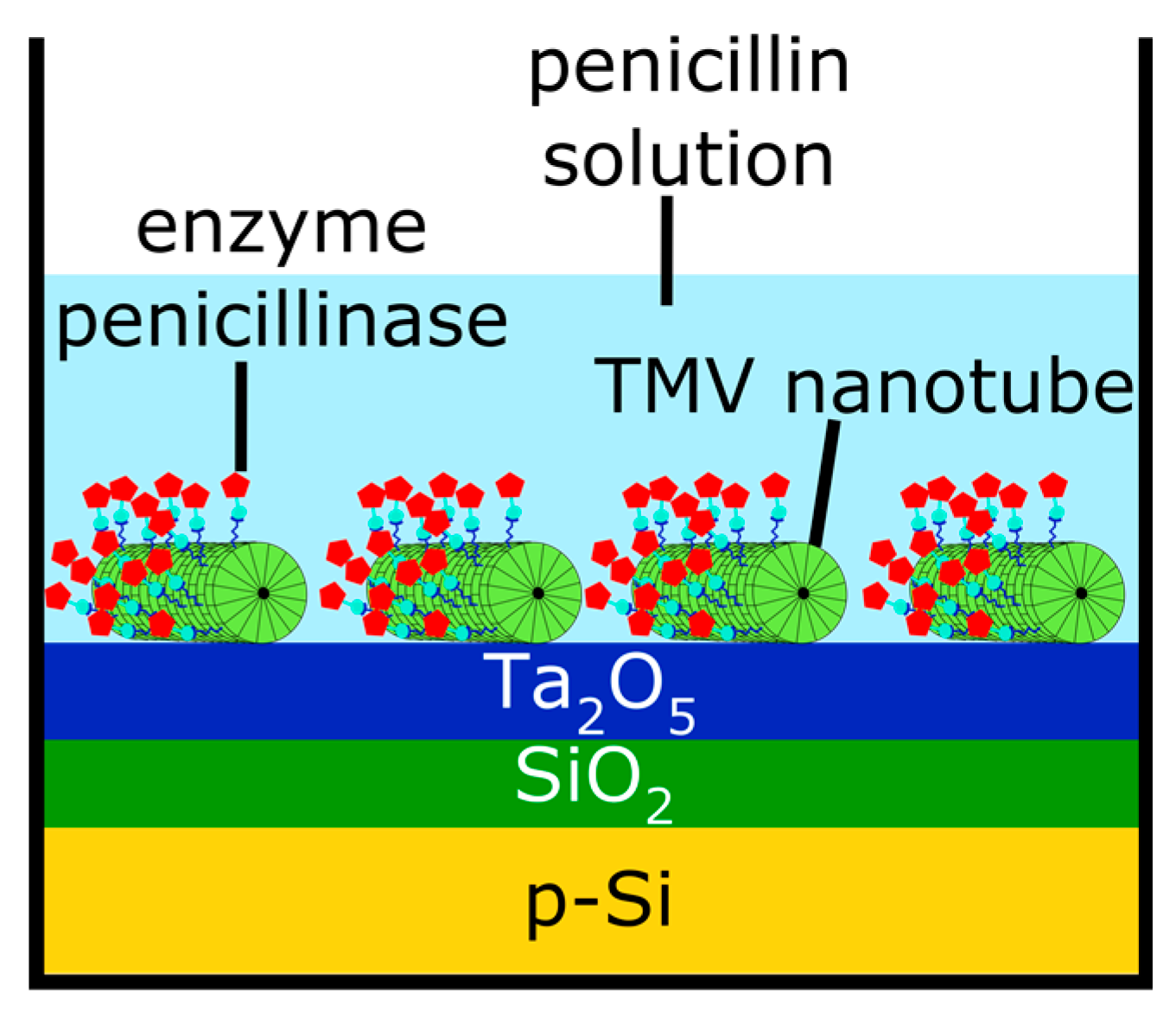
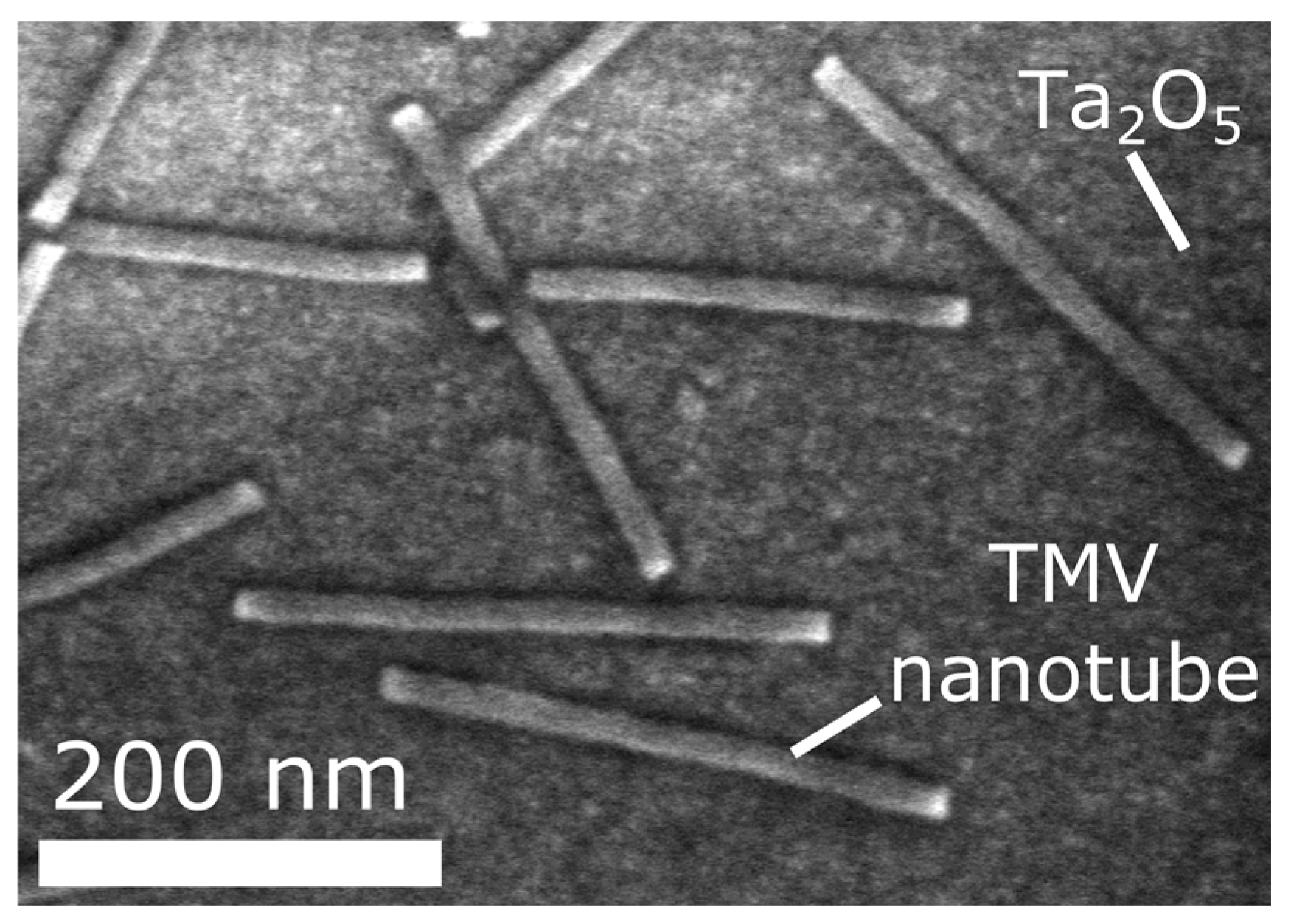
2.2. Loading of TMV Nanotubes and Enzyme Immobilization
2.3. Measurement Setup
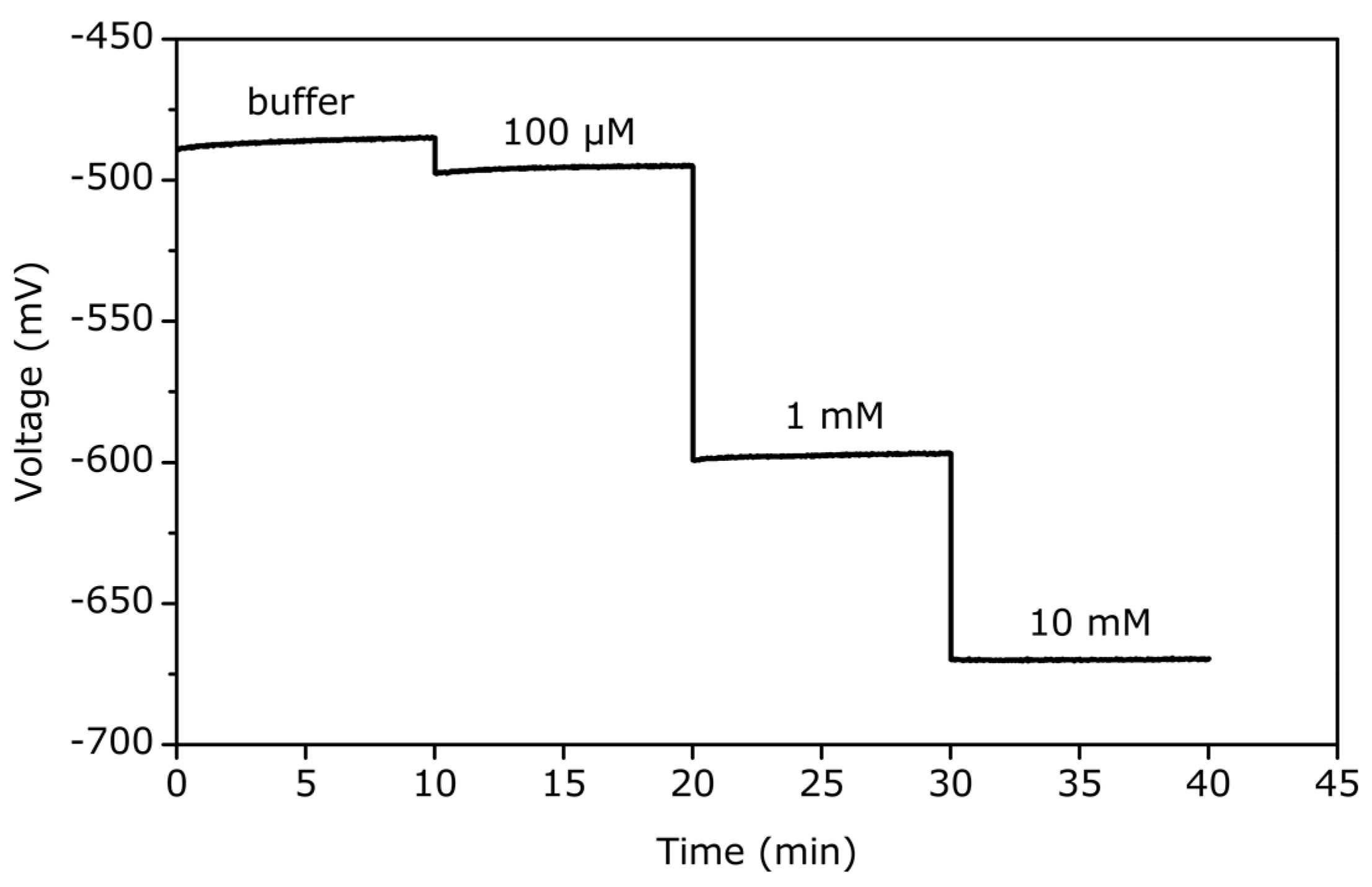
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Culver, J.N.; Brown, A.D.; Zang, F.; Gnerlich, M.; Gerasopoulos, K.; Ghodssi, R. Plant virus directed fabrication of nanoscale materials and devices. Virology 2015, 479, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Z.; Pomerantseva, E.; Gnerlich, M.; Brown, A.; Gerasopoulos, K.; McCarthy, M.; Culver, J.; Ghodssi, R. Tobacco mosaic virus: A biological building block for micro/nano/bio systems. J. Vac. Sci. Technol. 2013, 31, 050815. [Google Scholar] [CrossRef]
- Koch, C.; Wabbel, K.; Eber, F.J.; Krolla-Sidenstein, P.; Azucena, C.; Gliemann, H.; Eiben, S.; Geiger, F.; Wege, C. Modified TMV particles as beneficial scaffolds to present sensor enzymes. Front. Plant Sci. 2015, 6, 1137. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Liu, A.; Cao, B. Virus-based chemical and biological sensing. Angew. Chem. Int. Ed. 2009, 48, 6790–6810. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, M.; Koch, C.; Eiben, S.; Geiger, F.; Eber, F.; Gliemann, H.; Poghossian, A.; Wege, C.; Schöning, M.J. Tobacco mosaic virus as enzyme nanocarrier for electrochemical biosensors. Sens. Actuators B 2016, 238, 716–722. [Google Scholar] [CrossRef]
- Poghossian, A.; Thust, M.; Schöning, M.J.; Müller-Veggian, M.; Kordos, P.; Lüth, H. Cross-sensitivity of a capacitive penicillin sensor combined with a diffusion barrier. Sens. Actuators B 2000, 68, 260–265. [Google Scholar] [CrossRef]
- Rolka, D.; Poghossian, A.; Schöning, M.J. Integration of a capacitive EIS sensor into a FIA system for pH and penicillin determination. Sensors 2004, 4, 84–94. [Google Scholar] [CrossRef]
- Siqueira, J.; Abouzar, M.; Bäcker, M.; Zucolotto, V.; Poghossian, A.; Oliveira, O.; Schöning, M.J. Carbon nanotubes in nanostructured films: Potential application as amperometric and potentiometric field-effect (bio-)chemical sensors. Phys. Status Solidi A 2009, 206, 462–467. [Google Scholar] [CrossRef]
- Siqueira, J.; Maki, R.M.; Paulovich, F.V.; Werner, C.F.; Poghossian, A.; de Oliveira, C.F.; Zucolotto, V.; Oliveira, O.N., Jr.; Schöning, M.J. Use of information visualization methods eliminating cross talk in multiple sensing units investigated for a light-addressable potentiometric sensor. Anal. Chem. 2010, 82, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Beging, S.; Leinhos, M.; Jablonski, M.; Poghossian, A.; Schöning, M.J. Studying the spatially resolved immobilisation of enzymes on a capacitive field-effect structure by means of nano-spotting. Phys. Status Solidi A 2015, 212, 1353–1358. [Google Scholar] [CrossRef]
- Poghossian, A.; Weil, M.; Cherstvy, A.G.; Schöning, M.J. Electrical monitoring of polyelectrolyte multilayer by means of capacitive field-effect devices. Anal. Bioanal. Chem. 2013, 405, 6425–6436. [Google Scholar] [CrossRef] [PubMed]
- Dzyadevych, S.V.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezic-Renault, N. Enzyme biosensors based on ion-selective field-effect transistors. Anal. Chim. Acta 2006, 568, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Schöning, M.J. Label-free sensing of biomolecules with field-effect devices for clinical applications. Electroanal 2014, 26, 1197–1213. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonski, M.; Koch, C.; Bronder, T.S.; Poghossian, A.; Wege, C.; Schöning, M.J. Field-Effect Biosensors Modified with Tobacco Mosaic Virus Nanotubes as Enzyme Nanocarrier. Proceedings 2017, 1, 505. https://doi.org/10.3390/proceedings1040505
Jablonski M, Koch C, Bronder TS, Poghossian A, Wege C, Schöning MJ. Field-Effect Biosensors Modified with Tobacco Mosaic Virus Nanotubes as Enzyme Nanocarrier. Proceedings. 2017; 1(4):505. https://doi.org/10.3390/proceedings1040505
Chicago/Turabian StyleJablonski, Melanie, Claudia Koch, Thomas S. Bronder, Arshak Poghossian, Christina Wege, and Michael J. Schöning. 2017. "Field-Effect Biosensors Modified with Tobacco Mosaic Virus Nanotubes as Enzyme Nanocarrier" Proceedings 1, no. 4: 505. https://doi.org/10.3390/proceedings1040505
APA StyleJablonski, M., Koch, C., Bronder, T. S., Poghossian, A., Wege, C., & Schöning, M. J. (2017). Field-Effect Biosensors Modified with Tobacco Mosaic Virus Nanotubes as Enzyme Nanocarrier. Proceedings, 1(4), 505. https://doi.org/10.3390/proceedings1040505





