Detection of Aβ(1-40) Protein in Human Serum as a Causative Agent of Alzheimer’s Disease by Strain Gauge Cantilever Biosensor Immobilizing Liposome Incorporating Cholesterol †
Abstract
1. Introduction
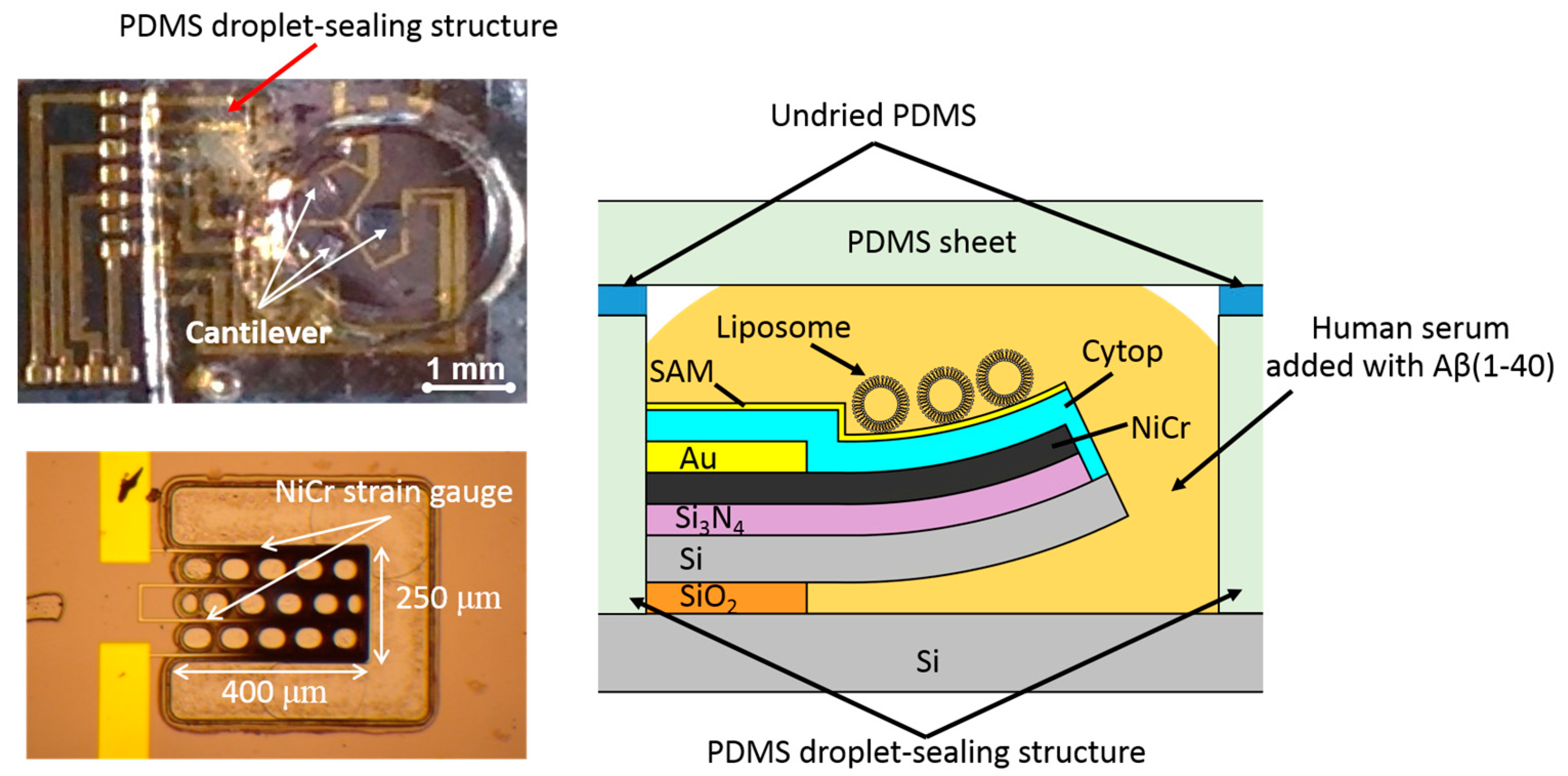
2. Experimental Methods
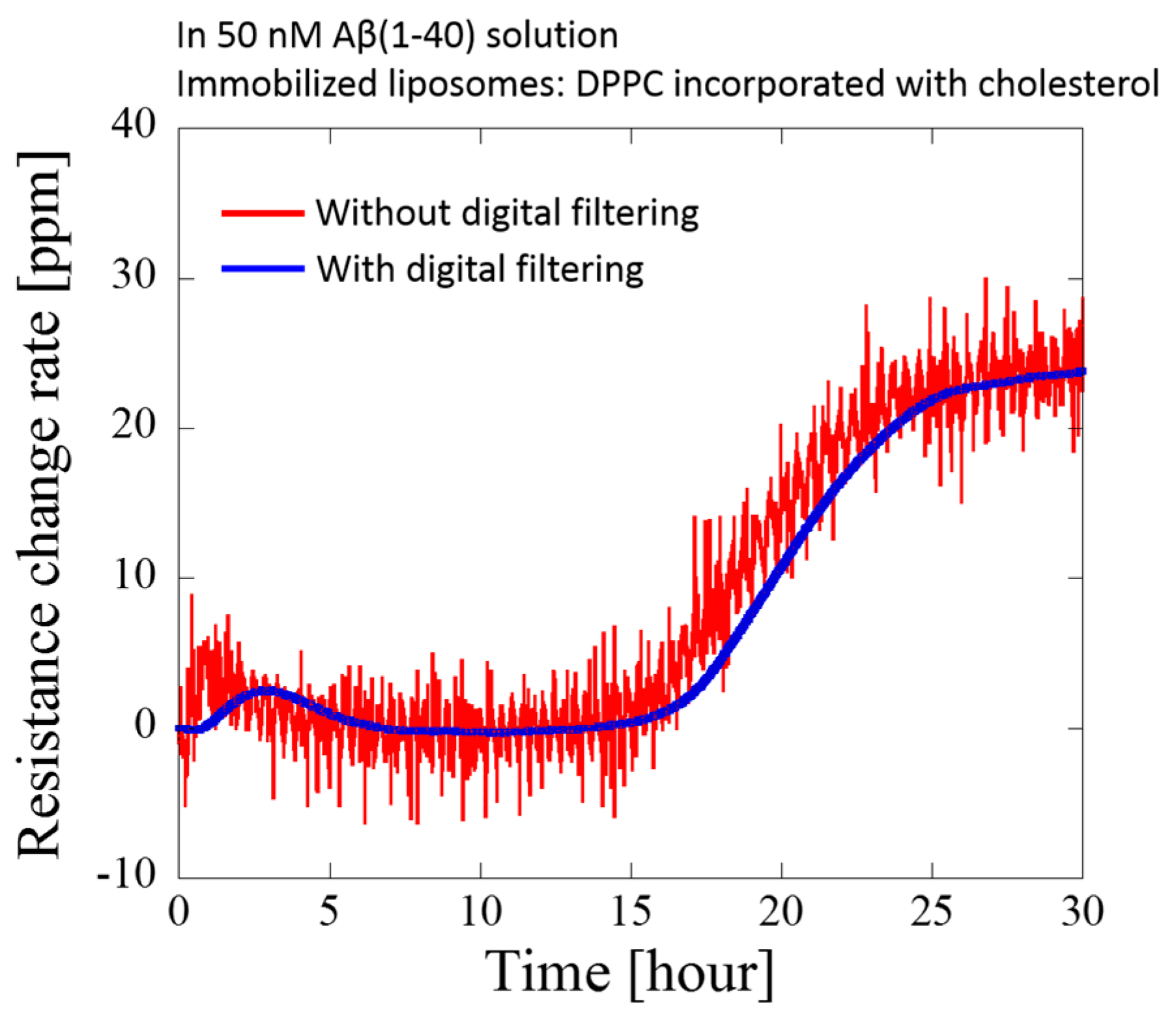
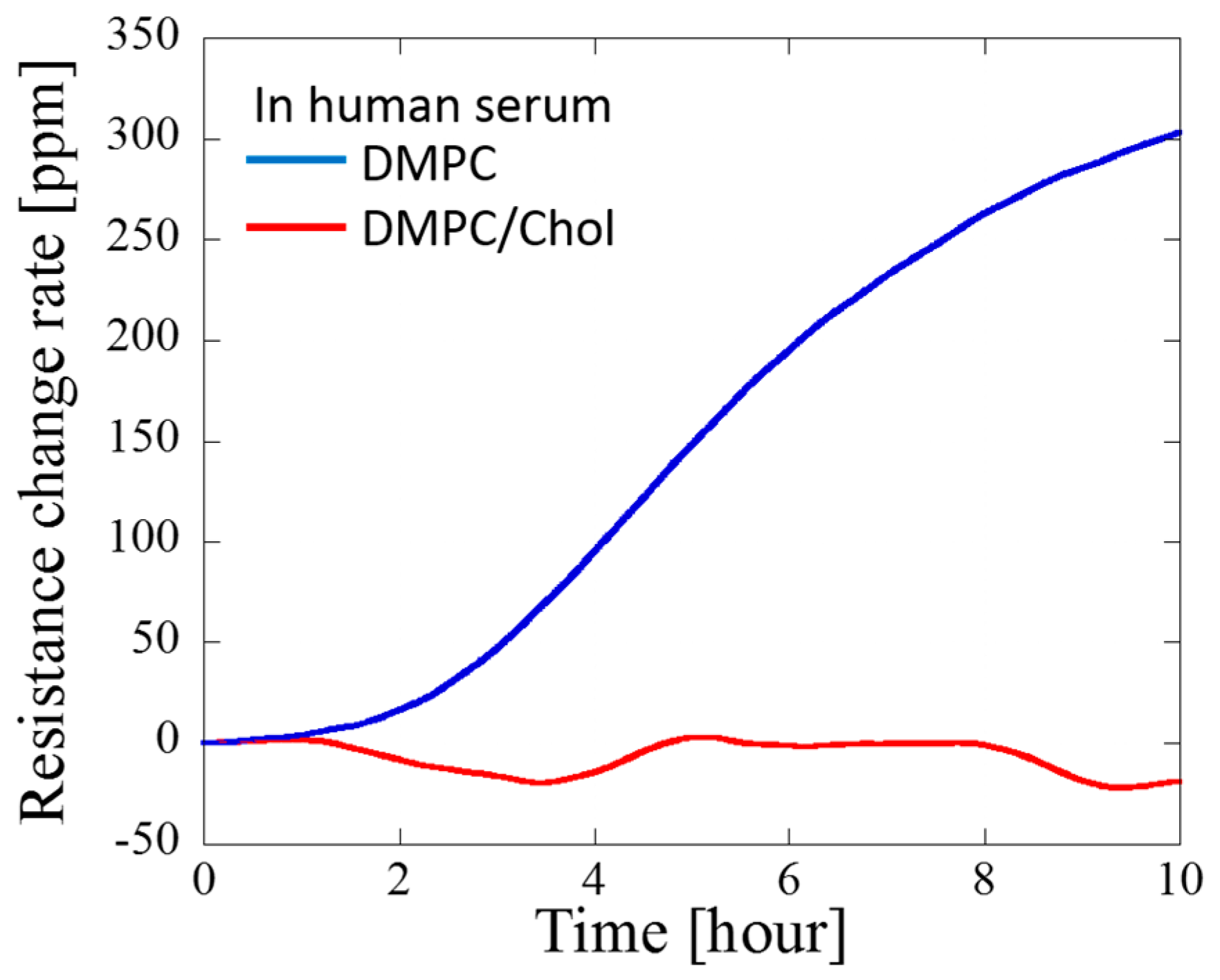
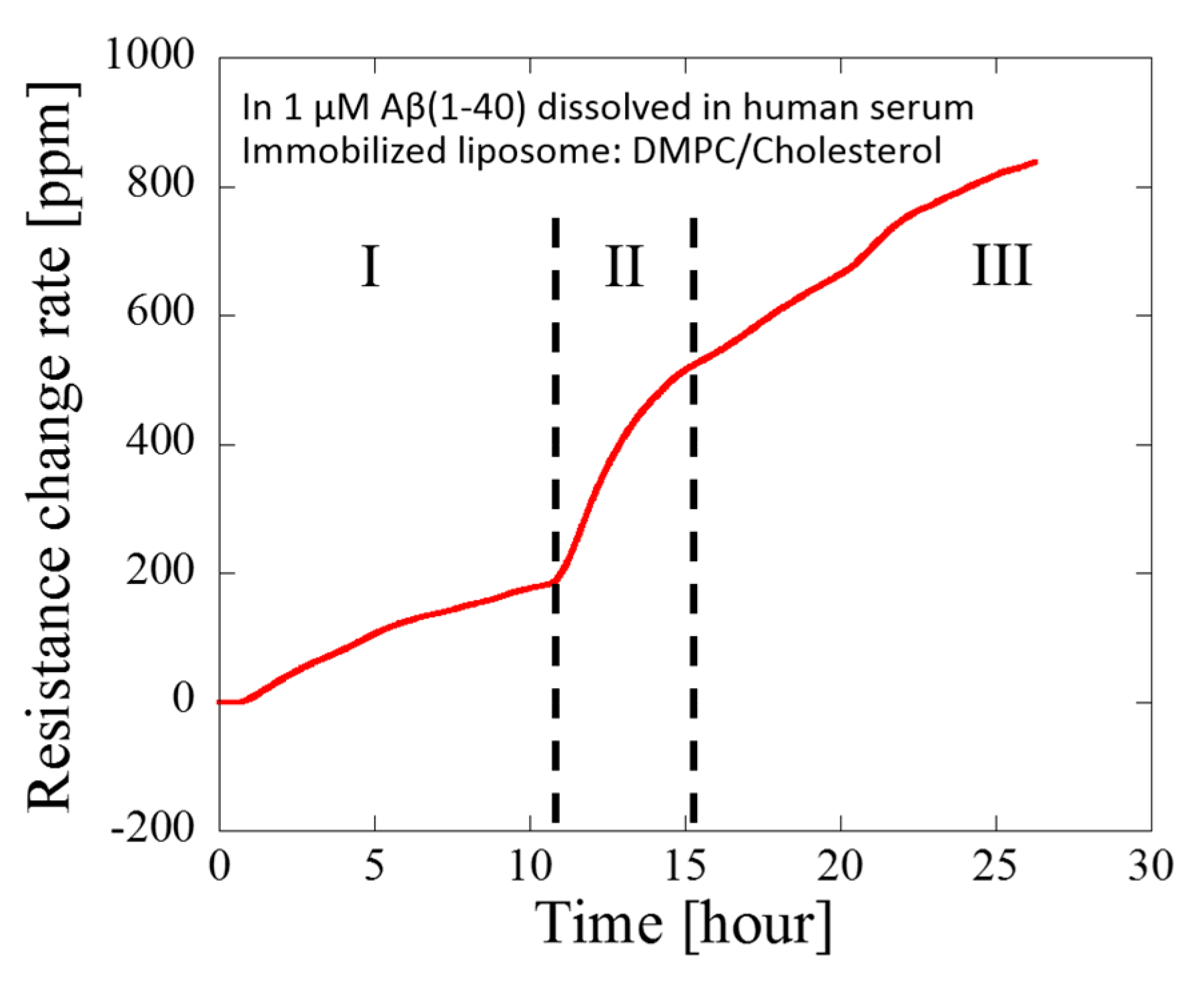
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2003, 297, 353–356. [Google Scholar] [CrossRef]
- Zhang, Z.; Murakami, Y.; Taniguchi, T.; Sohgawa, M.; Yamashita, K.; Noda, M. A cantilever-based biosensor for real-time monitoring of interactions between amyloid-β(1-40) and membranes comprised of phosphatidylcholine lipids with different hydrophobic acyl chains. Electroanalysis 2017, 29, 722–729. [Google Scholar] [CrossRef]
- Coppus, A.M.W.; Schuur, M.; Vergeer, J.; Janssens, A.C.J.W.; Oostra, B.A.; Verbeek, M.M.; van Duijn, C.M. Plasma β amyloid and the risk of Alzheimer’s disease in down syndrome. Neurobiol. Aging 2012, 33, 1988–1994. [Google Scholar] [CrossRef]
- Komura, S.; Andelman, D. Physical aspects of heterogeneities in multi-component lipid membranes. Adv. Coll. Int. Sci. 2014, 208, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, C.; Klimov, D.K. Calcium enhances binding Aβ monomer to DMPC lipid bilayer. Biophys. J. 2015, 108, 1807–1818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuzaki, K. Physicochemical interactions of amyloid β-peptide with lipid bilayers. Biochim. Biophys. Acta 2007, 1768, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, T.; Murakami, Y.; Sohgawa, M.; Yamashita, K.; Noda, M. Detection of Aβ(1-40) Protein in Human Serum as a Causative Agent of Alzheimer’s Disease by Strain Gauge Cantilever Biosensor Immobilizing Liposome Incorporating Cholesterol. Proceedings 2017, 1, 503. https://doi.org/10.3390/proceedings1040503
Taniguchi T, Murakami Y, Sohgawa M, Yamashita K, Noda M. Detection of Aβ(1-40) Protein in Human Serum as a Causative Agent of Alzheimer’s Disease by Strain Gauge Cantilever Biosensor Immobilizing Liposome Incorporating Cholesterol. Proceedings. 2017; 1(4):503. https://doi.org/10.3390/proceedings1040503
Chicago/Turabian StyleTaniguchi, Tomoya, Yuki Murakami, Masayuki Sohgawa, Kaoru Yamashita, and Minoru Noda. 2017. "Detection of Aβ(1-40) Protein in Human Serum as a Causative Agent of Alzheimer’s Disease by Strain Gauge Cantilever Biosensor Immobilizing Liposome Incorporating Cholesterol" Proceedings 1, no. 4: 503. https://doi.org/10.3390/proceedings1040503
APA StyleTaniguchi, T., Murakami, Y., Sohgawa, M., Yamashita, K., & Noda, M. (2017). Detection of Aβ(1-40) Protein in Human Serum as a Causative Agent of Alzheimer’s Disease by Strain Gauge Cantilever Biosensor Immobilizing Liposome Incorporating Cholesterol. Proceedings, 1(4), 503. https://doi.org/10.3390/proceedings1040503



