1. Introduction
There are several well established methods to detect and quantitate contaminants (e.g., pesticides) in drinking and source water by bioanalytical means. Most of them require a laboratory environment with a dedicated and usually bulky instrumentation as well as specifically trained personnel. The target of this work is the development of a bioassay platform with the following characteristics: (i) Reliable detection of several water contaminants, with sensitivities within the regulatory scope, and a time to result of less than 1 h. (ii) User-friendly, featuring a high degree of automation and operation autonomy combined with robustness of operation. The detection platform has to be operated by personnel not specially trained in executing bioassays. (iii) Cost efficiency concerning instrumentation as well as consumables.
It represents quite a challenge to fulfill all these requirements in one and the same bioanlytical platform as immuno-reactions, which are mostly the basis for biosensing devices, are inherently decisive and prone to variation. The sources of variation primarily reside in the inherent molecular instability of the biologicals (e.g., antibodies), appropriateness of sensing surfaces (immobilized capture molecules) and finally in the sensing principle and the corresponding instrumentation. In view of the bioassay features listed above, approaches are required where such variations can be autonomously compensated for. Different biosensing principles can be envisaged for detecting pesticides in water. Within this work, label-free assays were utilized for pesticide detection and quantitation. The biosensing surface plays a crucial role and the surface functionalization technology of CSEM can offer a variety of advantageous and competitive aspects. A special method designed is to couple target molecules to a dextran polymer (AtraDex) and covalently bind these polymers to OptoDex™ surfaces. The advantages are: (i) robust immobilization via multiple photo-bonding sites; (ii) well designable surface properties; and (iii) suppression of non-specific binding due to the dextran basis of both, capture and cross-linker (OptoDex™) molecules. In addition, AtraDex surfaces are very stable in ambient environment and re-generable. The synthesis of small molecules to dextran polymers seems therefore the method of choice also for other pesticides (or drugs) and water contaminants.
2. Materials and Methods
2.1. Reagents
All chemicals, unless specified, were obtained from Sigma-Aldrich (Switzerland). Mouse IgG and goat-anti mouse IgG antibodies were purchased from Thermo-Fisher (Switzerland). Gold nanoparticles of 40 nm diameter was purchased from Metalor (Switzerland), the conjugate of gold nanoparticle and goat anti-mouse IgG antibody was prepared in house. Atrazine was obtained from Labor Dr. Ehrenstorfer, Augsburg. Aqueous solutions of atrazine were prepared and final concentrations were quantitated optically (ε
263 = 35’000 M
−1·cm
−1 [
1]). Monoclonal anti-atrazine antibodies were purchased either from Enzo Life Sciences, Switzerland (monoclonal anti-atrazine antibody [1F9]), or from Abcam
TM, Cambridge UK (monoclonal anti-atrazine antibody [8.F.20]). Concentrated stock solutions of antibodies were kept at 4 °C (Enzo) or at −20 °C (Abcam). Prior to use, the antibodies were diluted with phosphate buffered saline pH 7.4 (PBS). Atrazine multivalent antigen-carrier dextran conjugate (AtraDex) was synthesized in house. The photo-crosslinkable polymer (OptoDex
®) used to functionalize the chips and related technologies were developed by CSEM (Switzerland).
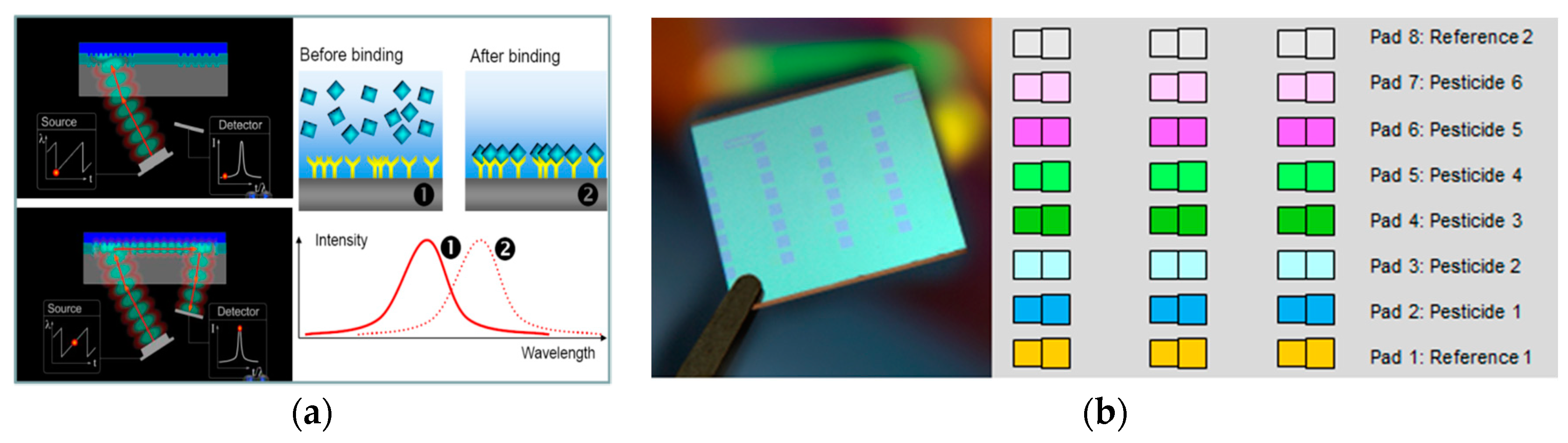
2.2. Waveguide Grating Biosensor
Immunoassays for the detection of atrazine were performed with the so-called wavelength-interrogated optical sensing platform (WIOS) developed at CSEM. Details of the instrumentation have been given elsewhere [
2]. Briefly, the detection of WIOS is based on changes of effective refractive index of a waveguide grating upon adsorption of biomolecules. The waveguide chips for WIOS consist of a thin Ta
2O
5 film deposited on a nano-structured glass substrate (
Figure 1). The sensitivity of the instrument was shown to be below 10
−6 refractive index units for bulk refractometry and the limit of detection for the adsorption of small molecules corresponded to a surface coverage of 0.3 pg/mm
2. This feature makes it possible to analyze several pesticides with the different pads and some as reference for the correction of analytic matrix variations.
2.3. Chip Functionalization
A dextran-based atrazine multivalent antigen-carrier conjugate (AtraDex) was synthesized from amino dextran and carboxy-atrazine (6-(Aza-6-cloro-4-isopropylamino-pyrimidin-2yl)-amino) hexanoic acid): custom synthesized according to Fatibello et al. [
3], applying EDC/NHS coupling chemistries. Multivalent atrazine carrier AtraDex was immobilized on the waveguide input gratings using the OptoDex
® technology developed by CSEM. OptoDex
® is a dextran-based photo-crosslinkable polymer that covalently binds biomolecules to surfaces upon UV exposure. The reagents are spotted onto any of the eight measurement pads using a NanoPlotterTM dispensing system (GeSim, Germany). In addition to the excellent stability of the bound molecules the polymer layer provides passivation, preventing the non-specific adsorption of biomolecules. In each WIOS chip, four measurement pads were functionalized with an optimized amount of AtraDex and four others were used as reference channels.
3. Results
3.1. Competitive Immunoassay for Detection of Atrazine
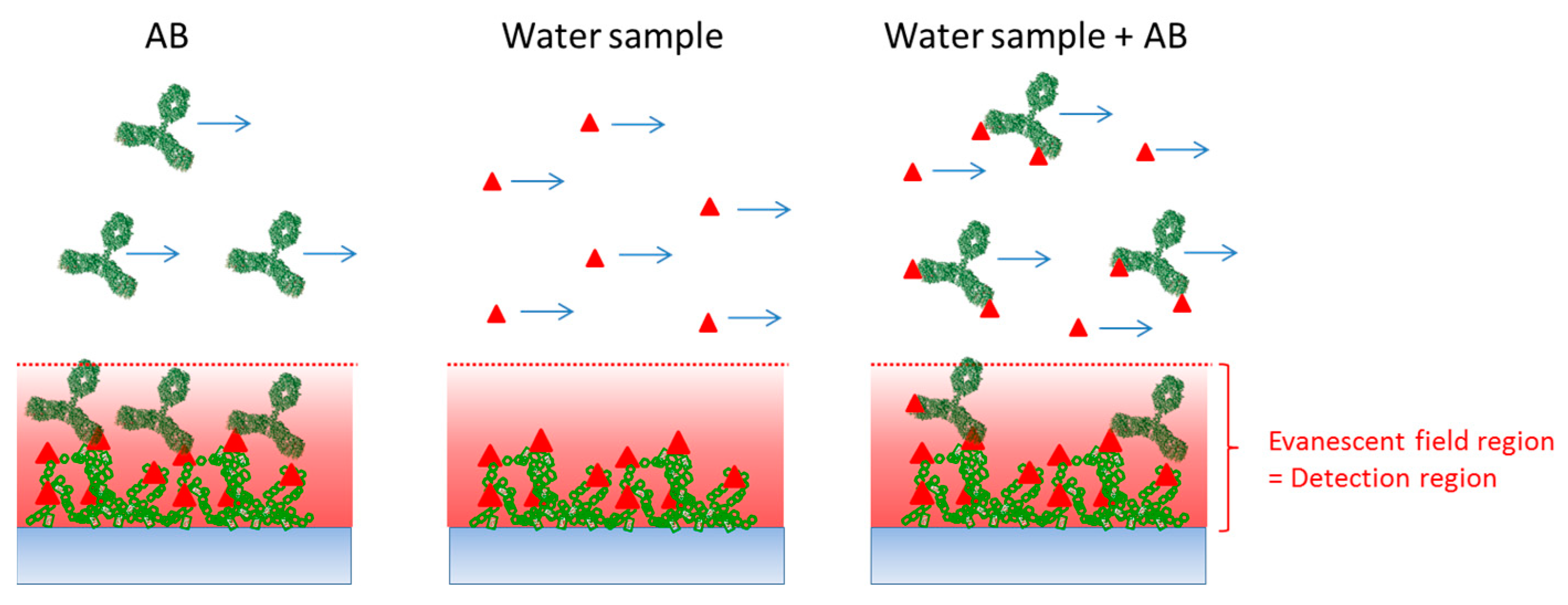
The assay format is a competitive one, where analogues of the molecules to be detected (in the present case analogues of atrazine) are immobilized on the surface (
Figure 2). The competitive assay offers the advantage that the immobilized target molecules are usually more robust than antibodies. This is an essential prerequisite for surface regeneration and long shelf-life of the biosensor.
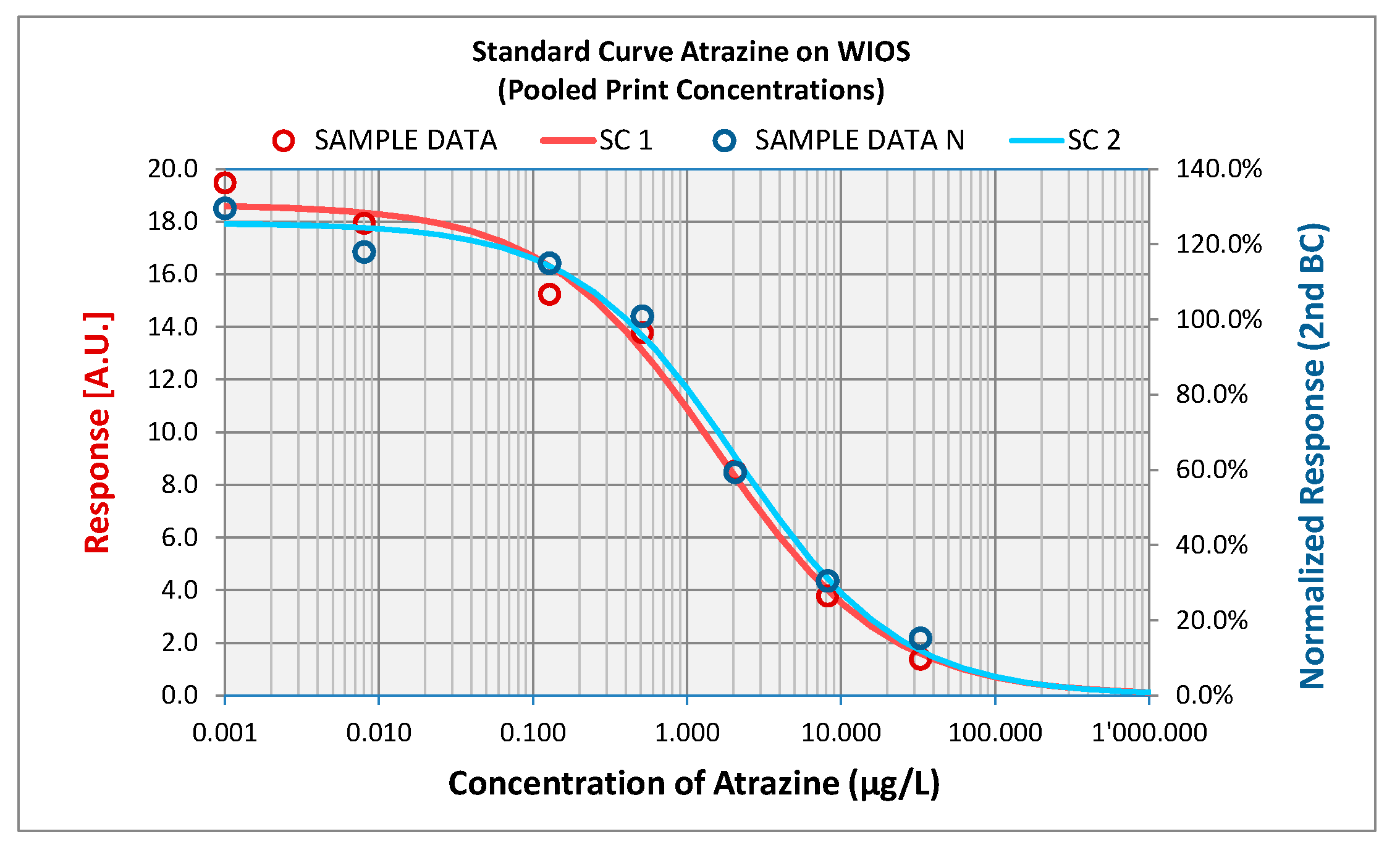
An advantage of the system over conventional detection systems is to be able to measure eight channels simultaneously. This feature makes it possible to analyze several contaminants in the same water sample. An example of atrazine calibration curves obtained by signal amplification using gold-labeled secondary antibody can be seen in
Figure 3. Standard curves were fitted to dose-response data by using a 4-parameter model. As the assays conducted with the WIOS label-free was always of the competitive type, standard curves have their maximum at zero concentration and their minimum at infinite concentrations. The detection limit is reached at 0.05 µg/L of free atrazine.
3.2. Regenerability and Stability of Sensor Surface
Regenerability of analytical chips for biodetection in general, and for contaminants in water in particular, is a challenge with considerable commercial consequences. Optical chips for label-free detection attain reasonable cost efficiency when both multiplexing and regenerability can be considered. It is therefore essential to provide appropriately functionalized surfaces which are covalently bonded, immunologically competent and regenerable. OptoDexTM based surface engineering is additionally beneficial due to its property of suppressing non-specific binding. The later point is of major importance regarding contaminant analysis in water. To date, the investigations indicate that surfaces with photo-bonded AtraDex can be regenerated more than 80 times, stored in buffer at least for 2 months and in dry state for at least one year without losing their properties. The quality of generated data has been significantly improved with inclusion of the built-in calibration.
3.3. Matrix Effects on the Atrazine Assay
Further piloting experiments concerned investigations of matrix and interfering effects. Interferents are assay-disturbing components which may arise in real samples. Performed investigations are dual aimed. For bioanalytics it is essential to know which matrix may possibly interfere with the assay and thus falsify the result. Moreover, is it possible to identify interfering effects and correct the primary result accordingly? Matrix effects have been studied so far with tap water and lake water (Lake of Neuchatel, Switzerland). The matrix component in these assays accounts for 94% by volume. The results are summarized in
Table 1. As compared to deionized water, neither tap water nor lake water interfere with the atrazine detection assay.
4. Conclusions
In summary, we can offer a system for online monitoring of water contaminants with the following properties: (a) Competitive immunoassay with antigen-carrier (AtraDex) on surface; (b) Label-free sensing for real-time measurement and reaction kinetics; (c) Multiple sensing surfaces with different characteristics; (d) In-assay calibration/normalization; (e) Regenerable biochip more than 80 times; (f) Detection limit of atrazine achieved at 0.05 µg/L; (g) Computational bioanalytics; (h) Compensation of interfering effects.