Oxyhydrogen and Hydrogen Detection by Gasochromic Coloration of Highly Porous Tungsten Oxide with Fractal-Like Pd Nanoparticles †
Abstract
:1. Introduction
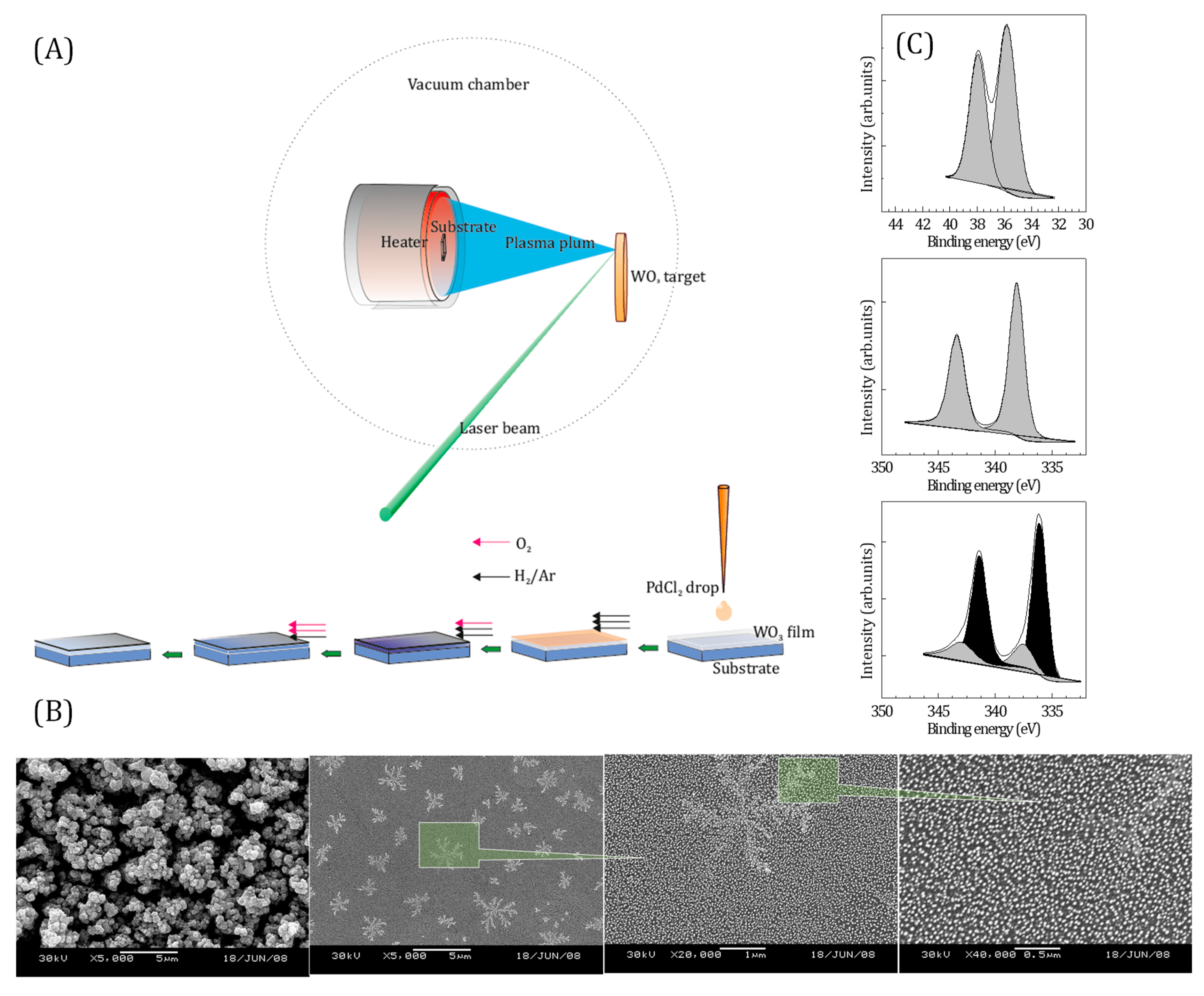
2. Materials and Methods
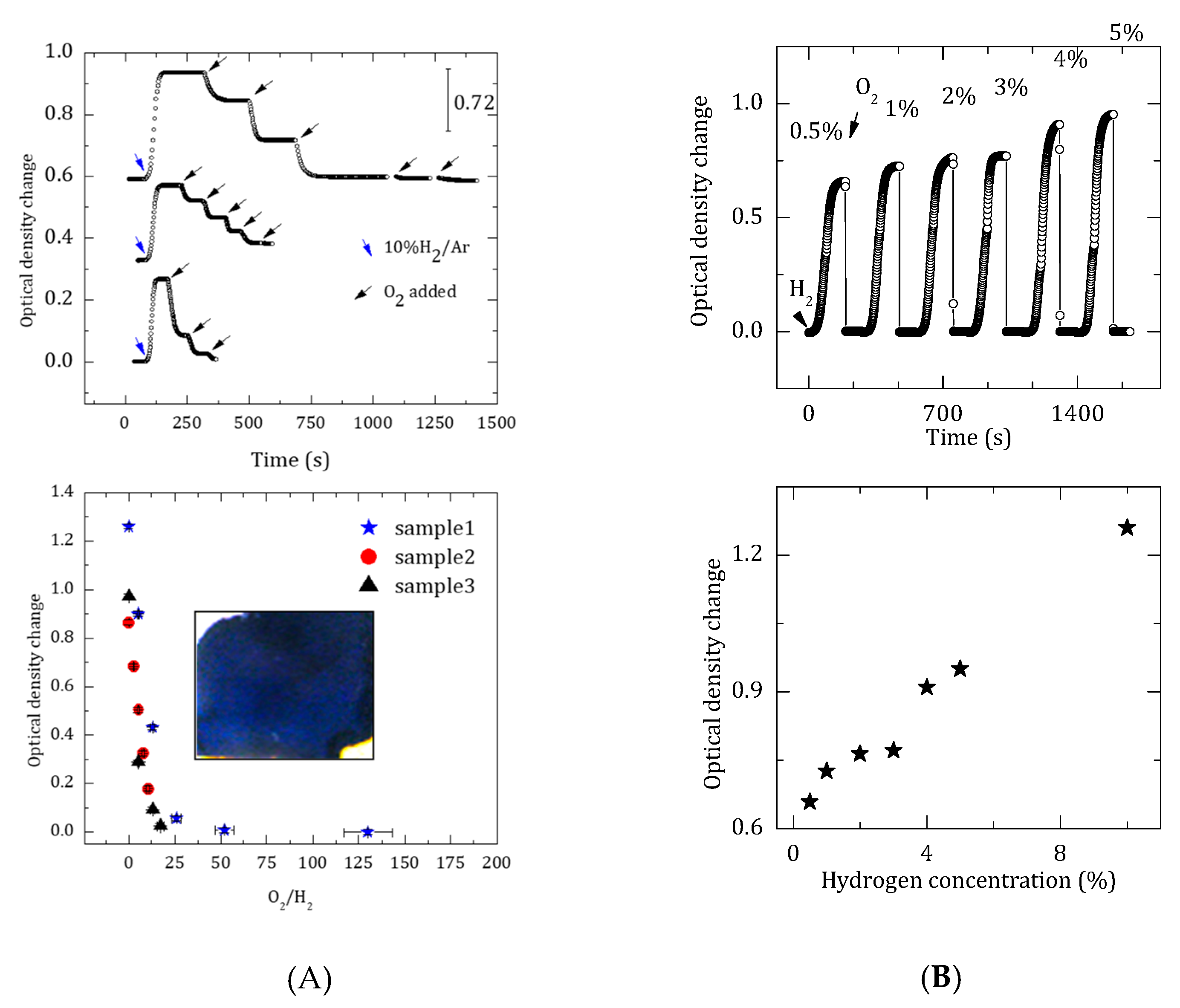
3. Results and Discussion
4. Conclusions
Conflicts of Interest
References
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Solar Energy Mater. Solar Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Georg, A.; Graf, W.; Neumann, R.; Wittwer, V. Mechanism of the gasochromic coloration of porous WO3 films. Solid State Ion. 2000, 127, 319–328. [Google Scholar] [CrossRef]
- Wittwer, V.; Datz, M.; Ell, J.; Georg, A.; Graf, W.; Walze, G. Gasochromic windows. Solar Energy Mater. Solar Cells 2004, 84, 305–314. [Google Scholar] [CrossRef]
- Orel, B.; Krašovec, U.O.; Grošelj, N.; Kosec, M.; Dražič, G.; Reisfeld, R. Gasochromic behavior of sol-gel derived Pd doped peroxopolytungstic acid (W-PTA) nano-composite films. J. Sol-Gel Sci. Technol. 1999, 14, 291–308. [Google Scholar] [CrossRef]
- Chan, C.C.; Hsu, W.C.; Chang, C.C.; Hsu, C.S. Preparation and characterization of gasochromic Pt/WO3 hydrogen sensor by using the Taguchi design method. Sens. Actuators B Chem. 2010, 145, 691–697. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Lee, Y.A.; Seo, H. Eye-readable gasochromic and optical hydrogen gas sensor based on CuS-Pd. RSC Adv. 2015, 5, 9028–9034. [Google Scholar] [CrossRef]
- Ranjbar, M.; Zad, A.I.; Mahdavi, S.M. Gasochromic tungsten oxide thin films for optical hydrogen sensors. J. Phys. D Appl. Phys. 2008, 41, 055405. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Jaiswal, J.; Chandra, R. A fast response/recovery of hydrophobic Pd/V2O5 thin films for hydrogen gas sensing. Sens. Actuators B Chem. 2016, 236, 16–26. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjbar, M.; Sberveglieri, G. Oxyhydrogen and Hydrogen Detection by Gasochromic Coloration of Highly Porous Tungsten Oxide with Fractal-Like Pd Nanoparticles. Proceedings 2017, 1, 487. https://doi.org/10.3390/proceedings1040487
Ranjbar M, Sberveglieri G. Oxyhydrogen and Hydrogen Detection by Gasochromic Coloration of Highly Porous Tungsten Oxide with Fractal-Like Pd Nanoparticles. Proceedings. 2017; 1(4):487. https://doi.org/10.3390/proceedings1040487
Chicago/Turabian StyleRanjbar, Mehdi, and Giorgio Sberveglieri. 2017. "Oxyhydrogen and Hydrogen Detection by Gasochromic Coloration of Highly Porous Tungsten Oxide with Fractal-Like Pd Nanoparticles" Proceedings 1, no. 4: 487. https://doi.org/10.3390/proceedings1040487
APA StyleRanjbar, M., & Sberveglieri, G. (2017). Oxyhydrogen and Hydrogen Detection by Gasochromic Coloration of Highly Porous Tungsten Oxide with Fractal-Like Pd Nanoparticles. Proceedings, 1(4), 487. https://doi.org/10.3390/proceedings1040487




