A Novel, Low-Cost, Portable PID Sensor for Detection of VOC †
Abstract
:1. Introduction
2. Experimental
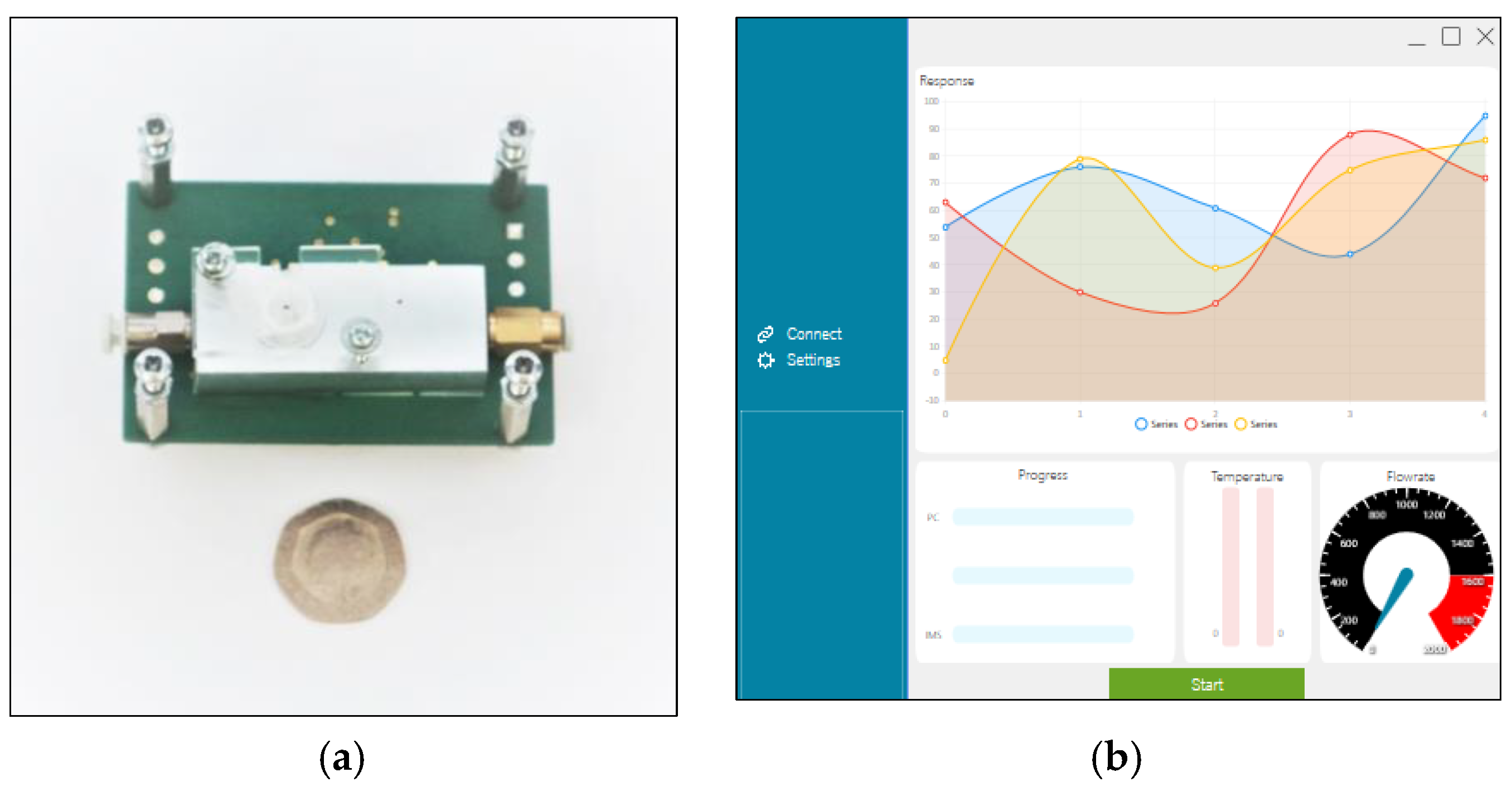
2.1. Design Overview
2.2. Ionisation
2.3. Detection and Electronics
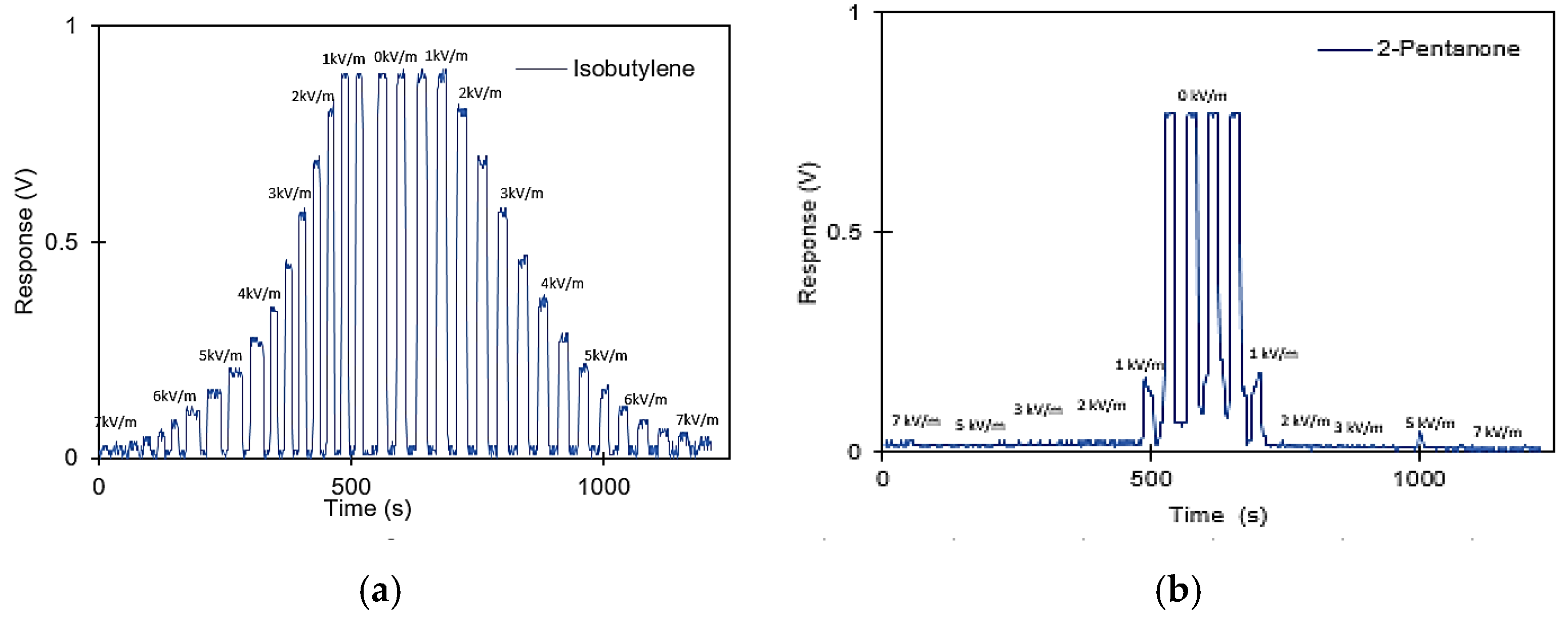
3. Results and Discussion
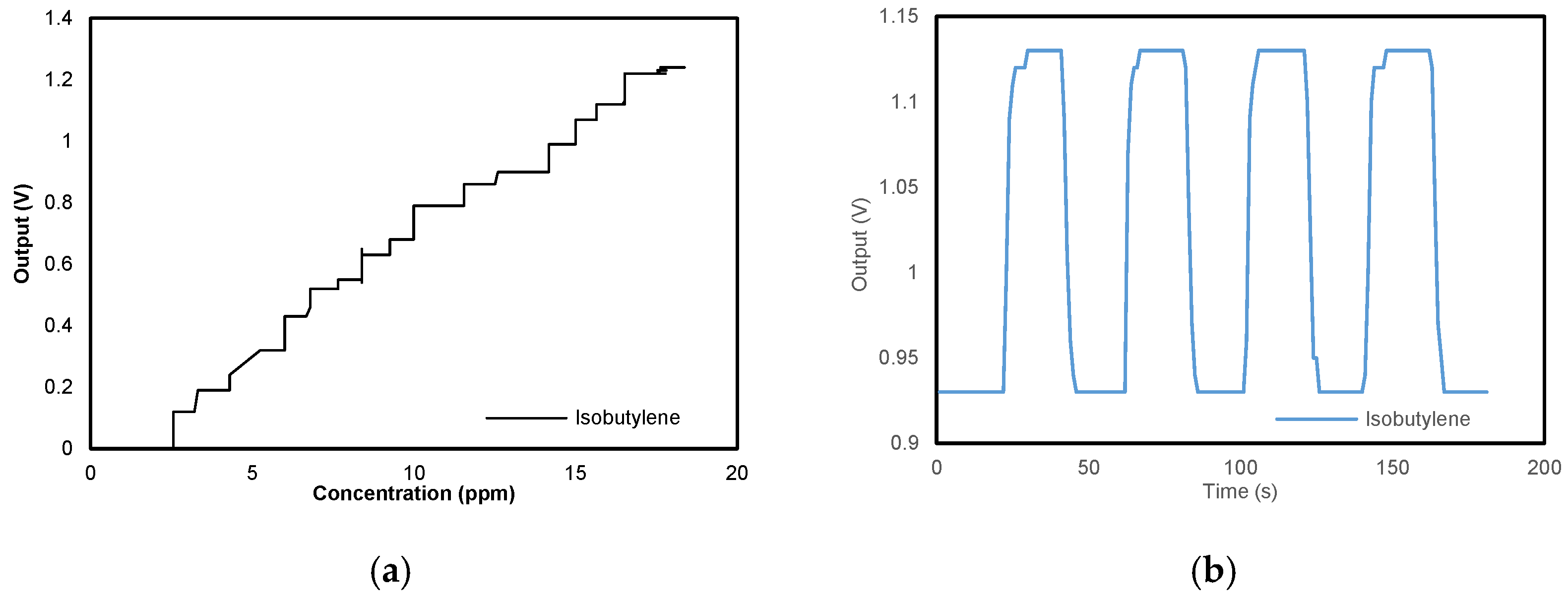
3.1. Concentration Information
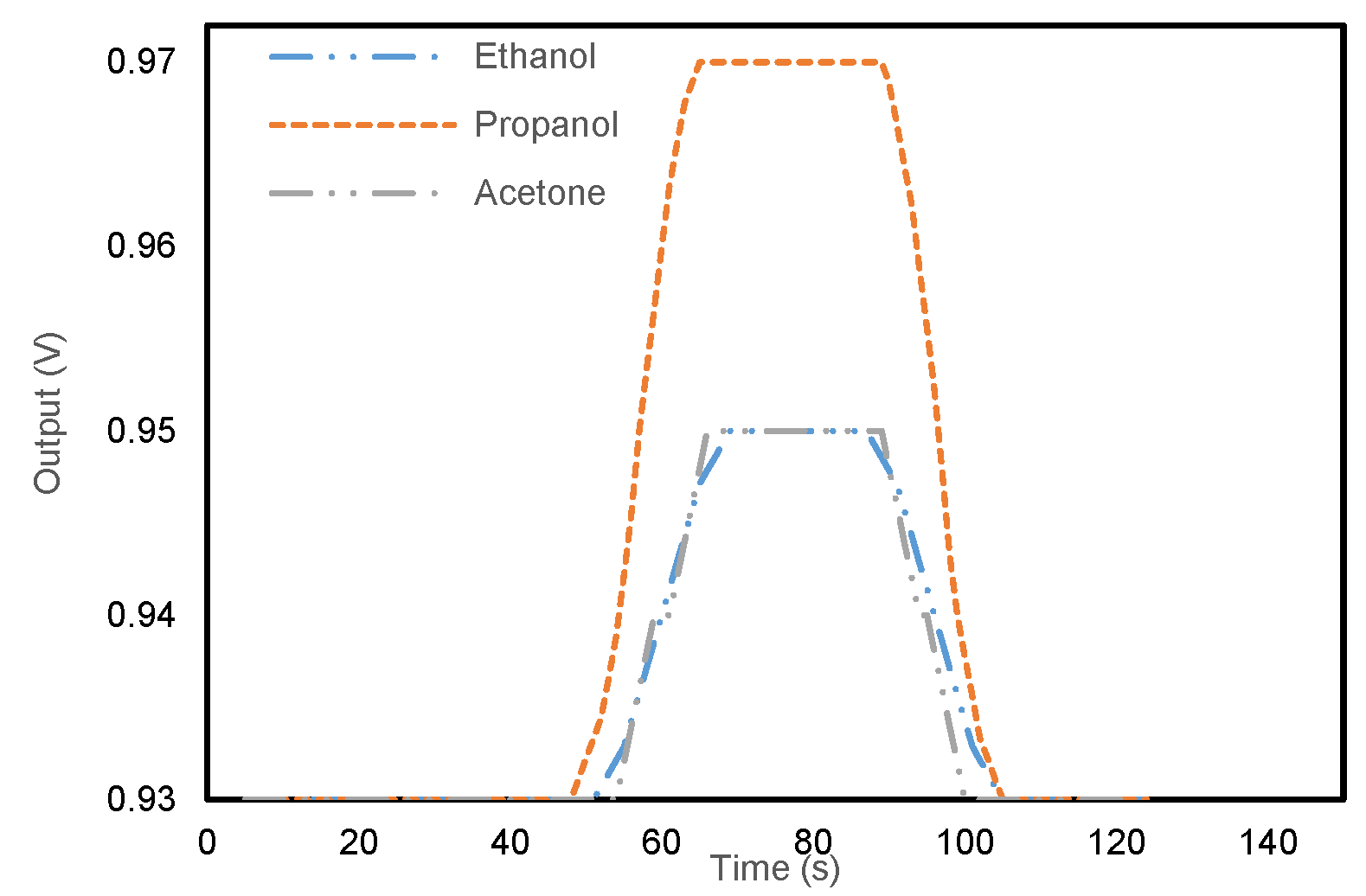
3.2. Composition
4. Conclusions
Conflicts of Interest
References
- Szulczyński, B.; Gębicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Li, H.; Zhang, Y.-J.; Bi, F.; Meng, L.-S.; Zhang, X.-M.; Mao, J.-Y.; Cheng, N.-L.; Fang, B.; Yang, Y.; et al. Fast determination of monocyclic aromatic hydrocarbons in ambient air using a portable gas chromatography–photoionization detector. Chromatographia 2017. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbroko, S.O.; Covington, J. A Novel, Low-Cost, Portable PID Sensor for Detection of VOC. Proceedings 2017, 1, 482. https://doi.org/10.3390/proceedings1040482
Agbroko SO, Covington J. A Novel, Low-Cost, Portable PID Sensor for Detection of VOC. Proceedings. 2017; 1(4):482. https://doi.org/10.3390/proceedings1040482
Chicago/Turabian StyleAgbroko, Samuel O., and James Covington. 2017. "A Novel, Low-Cost, Portable PID Sensor for Detection of VOC" Proceedings 1, no. 4: 482. https://doi.org/10.3390/proceedings1040482
APA StyleAgbroko, S. O., & Covington, J. (2017). A Novel, Low-Cost, Portable PID Sensor for Detection of VOC. Proceedings, 1(4), 482. https://doi.org/10.3390/proceedings1040482





