1. Introduction
Measuring NO2 is specially challenging for several reasons because nitrogen species in pollutant and toxic emissions are specified as NOx due to the diversity of possible nitrogen oxidation states. Moreover, because of its oxidizing character, its signal in sensors based on redox mechanisms is subject to strong cross interferences with other oxidizing species, like oxygen or ozone. Finally, because the range of concentrations at which it should be detected to trigger a safety alarm are very low (under 1 ppm).
Colorimetric methods offer a convenient approach for translating a qualitative chemical information from a color-changing reaction into a quantitative measurement of a molecular species [
1,
2]. An integrated, mobile colorimetric sensor can be particularly helpful for occasional chemical sensing measurements, such as informal air quality checks or risk assessment after an incident, where complex analytical methods are not available. In these situations, the main requirement is high availability, easy usage, and high specificity towards one single chemical species, combined with low cost. The main limiting factor for the widespread usage of chemical analysis techniques is the need of trained personnel to operate them (either because they involve some complicated manipulation, e.g., liquid chemical reagents, or because the readout is carried out by a complex instrument, e.g., spectrometer). In this contribution, we show how a well stablished colorimetric method can be adapted for easy operation and readout, making them suitable for the unqualified end user.
Instead of a complex spectrometric analysis, we introduce a simple and straight forward but quantitative RGB analysis: an efficient protocol to determine the true, quantitative color composition of an RGB signal, independent from omnipresent color deviations in an image caused by the hardware used or by the background illumination. As a result, even smartphone cameras can be used for making true color measurements, when our correction mechanisms are employed.
Second, as an application example, we will present a modified Saltzman reagent method for the selective detection of NO2 in air that can be applied using solid wet substrates, avoiding the manipulation of chemical solutions and simplifying the procedure. Our results show that the method can be adapted to detect NO2 concentrations ranging from 50 ppb to 300 ppm, with measure-to-result times in the range of minutes. We demonstrate that the color measurement can be carried out with the optical signals of RGB sensors, without losing quantitative performance.
2. Experimental Details
The Griess-Saltzman (GS) reagent was prepared by dissolving 5 grams of sulfanilic acid (reagent grade ACS crystals) in 940 mL of 2.5 M acetic acid. After dissolution, 5 mL of a 1% aqueous solution of N-(1-naphthy1)ethylenediamine dihydrochloride were added and the solution was diluted to volume in a 1-liter volumetric flask. To fabricate the sensor elements (from now on, “sensor pads”), we soaked 2.5 cm × 2.5 cm × 0.3 cm squares of sterilized cellulose absorbent pads with 0.2 mL of the indicator solution. Gas sensing experiments were conducted in a customized chamber of 15 mL in volume. Gas mixtures were introduced with thermal mass flow controllers (Bronkhorst High Tech 200 mL) by mixing synthetic air (SA) with NO2 in SA from certified gas cylinders. The spectral measurements were acquired with a UV-visible spectrometer (Specord 205, Analytik Jena) in an integrated sphere configuration using a customized sample holder to accommodate our wet pads. The color measurements were carried out in a home-made apparatus based on a TCS3200 RGB color sensor in combination with an illumination source made of 4 white LEDs. The sensor transforms the light intensity in each one of the red, green and blue bands into 3 frequency modulated square pulse signals.
3. Results and Discussion
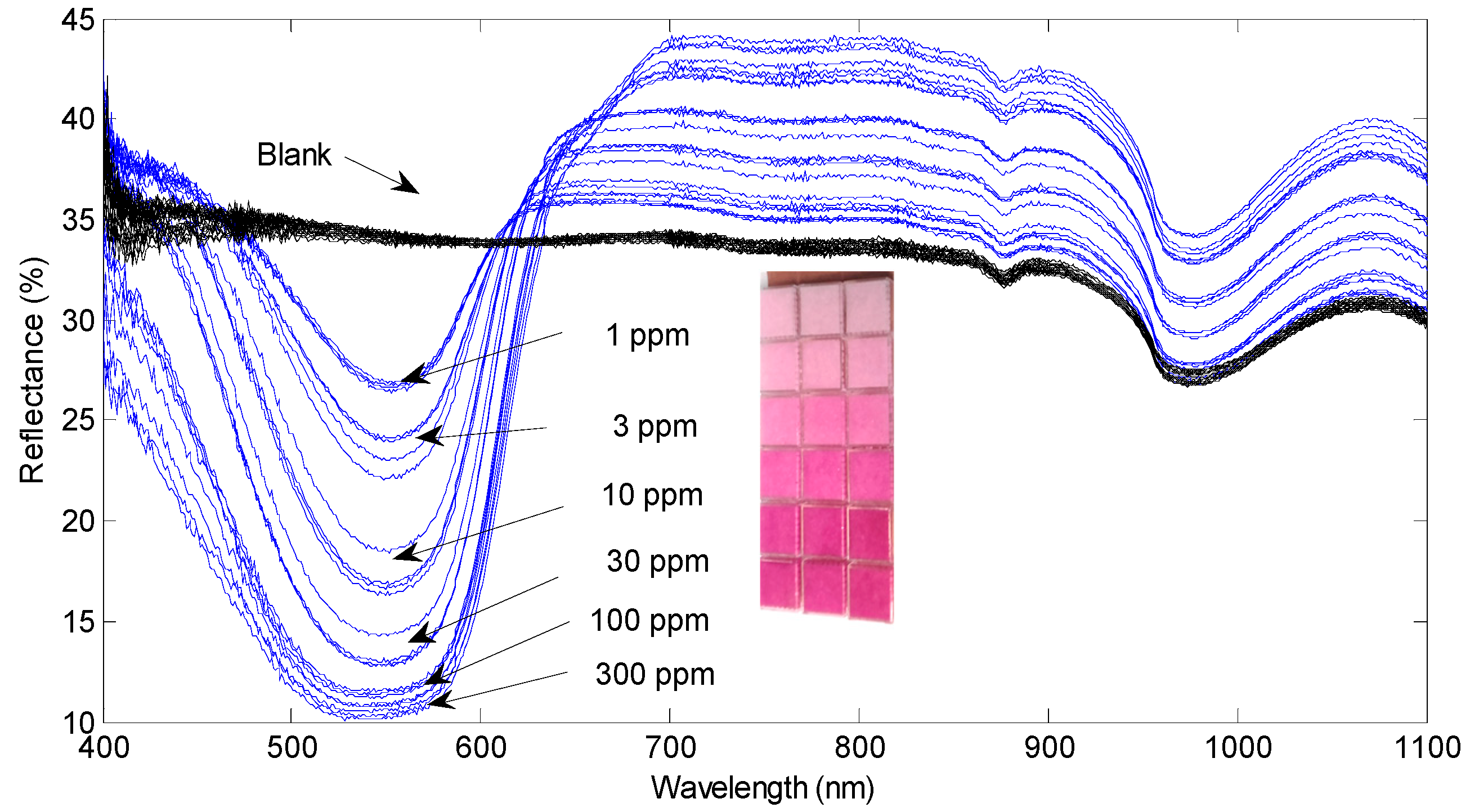
Figure 1a shows the UV-vis reflectance spectra of the GS-soaked pads exposed to different concentrations of NO
2 for 5 min. The spectra are dominated by an asymmetric absorption band centered around 540 nm [
3], which is the responsible of the characteristic deep-purple coloration developed by the GS reagent in presence of NO
2. It was determined that the reflectance at 540 nm presented, approximately, an exponential dependence on the NO
2 concentration.
RGB signals from a conventional color sensor can be used to monitor de color changes in our NO
2 sensing pads. For the sake of simplicity, we chose the mean value of the R, G and B detector signals as our color magnitude.
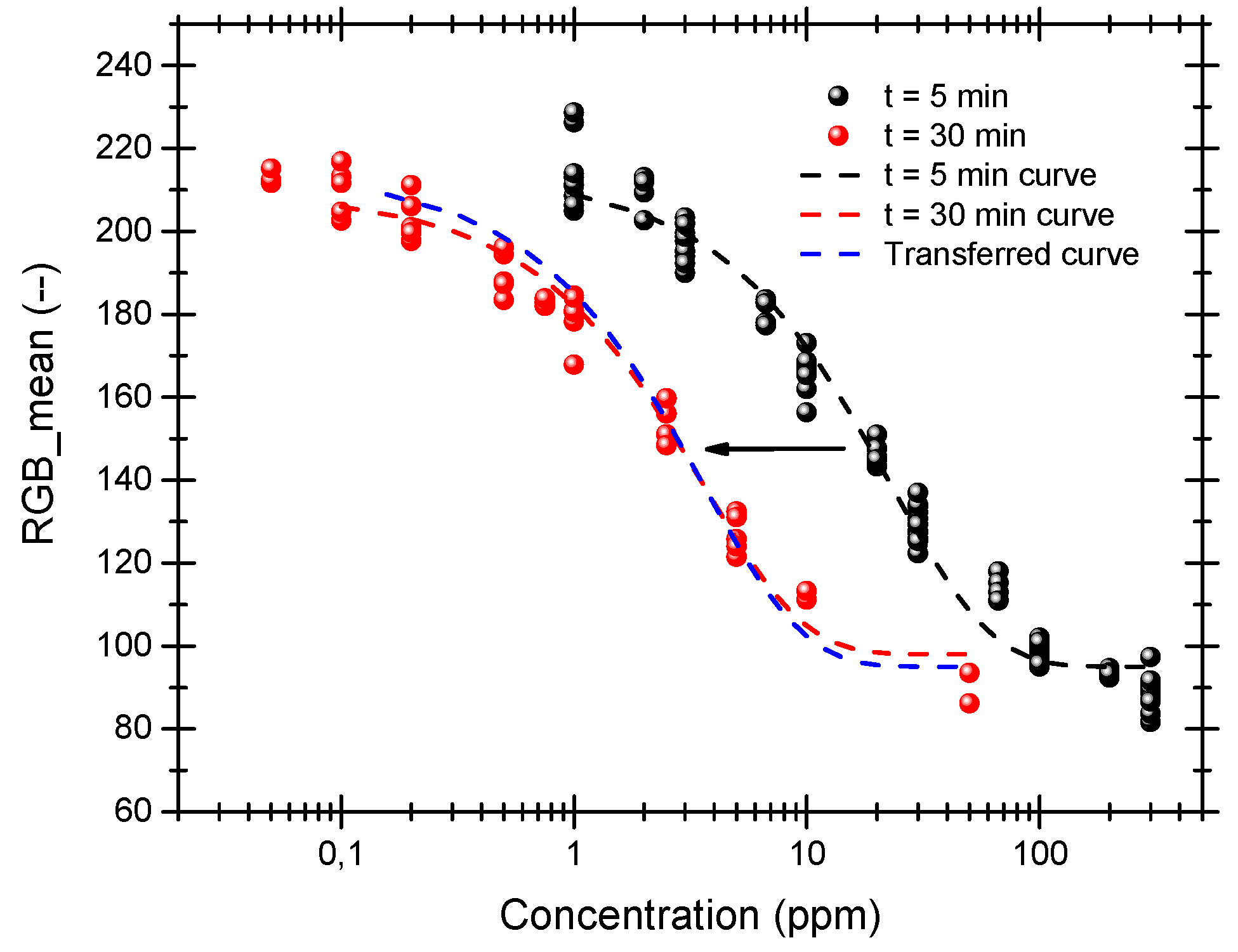
Figure 2 shows the color developed by our pads in two different situations. In a first set of experiments, the samples were exposed for 5 min to NO
2 concentrations ranging from 1 to 300 ppm. Then, a second set of experiments was carried out, exposing now the pads for 30 min to NO
2 concentrations from 50 ppb to 50 ppm. Several sensing pads have been use for each of the NO
2 concentrations, showing that the process is reproducible and repetitive.
An exponential trend was observed in both cases, proving that the information provided by a simple RGB detector is sufficient to monitor the color development in a comparable way to the UV-vis reflectance measurements.
We observed that the detection range of [NO2] can be arbitrarily extended simply by controlling the exposure time (i.e., for low detection range, larger times and vice versa). Therefore, the exposure time can be used as a parameter to tune the dynamic color range for a specific span of NO2 concentrations. In our experiments we could show that this span extended over four orders of magnitude, but there is no reason to think that this could not be extended further, provided that the waiting times were acceptable.
Another important conclusion that can be extracted is that NO
2 and exposure time play an equivalent and exchangeable role. One of the important implications of this feature is that only one calibration curve is needed to fully characterize the sensor pad for a very wide NO
2 detection range. Any calibration curve at a given exposure time can be time-shifted to detect another NO
2 concentration range. An example of this is show in
Figure 2 (blue line) where calibration curve at
t = 5 min have been time-shifted to
t = 30 min. The matching between the time-shifted curve and the actual curve at
t = 30 min is clear and prove the robustness of our system.
4. Conclusions
We have presented a convenient method to implement a Griess-Saltzman-type reaction for the colorimetric detection of NO2. Our soaked sensor pads are one use devices, easy to fabricate, very cheap and can be stored for a long time, preserving their sensing properties, characterized by a high specificity and response times fast enough for ambient monitoring applications.
We have shown that it can be used to tailor the response of the pads to the desired range of NO2 concentrations, and demonstrated that the same pads can be used to detect NO2 in concentrations ranging from 50 ppb to 300 ppm.