Operando Investigations of Differently Prepared In2O3-Gas Sensors †
Abstract
:1. Introduction
2. Materials and Methods
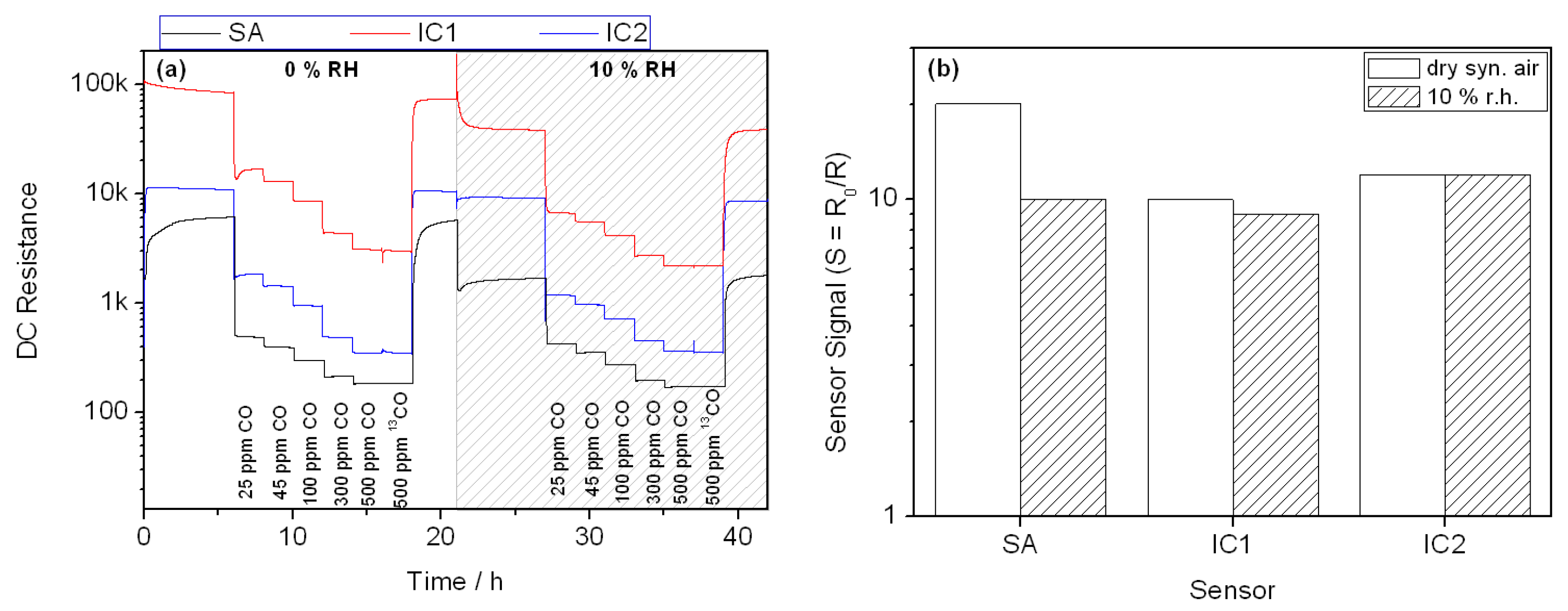
3. Results
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Yamaura, H.; Jinkawa, T.; Tamaki, J.; Moriya, K.; Miura, N.; Yamazoe, N. Indium oxide-based gas sensor for selective detection of CO. Sens. Actuators B 1996, 36, 325–332. [Google Scholar] [CrossRef]
- Gurlo, A.; Bârsan, N.; Ivanovskaya, M.; Weimar, U.; Göpel, W. In2O3 and MoO3–In2O3 thin film semiconductor sensors: Interaction with NO2 and O3. Sens. Actuators B Chem. 1998, 47, 92–99. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Gurlo, A.; Bogdanov, P. Mechanism of O3 and NO2 detection and selectivity of In2O3 sensors. Sens. Actuators B Chem. 2001, 77, 264–267. [Google Scholar] [CrossRef]
- Sänze, S.; Gurlo, A.; Hess, C. Monitoring gas sensors at work: Operando Raman-FTIR study of ethanol detection by indium oxide. Angew. Chem. Int. Ed. 2013, 52, 3607–3610. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, J. Synthesis of In2O3 nanoparticles via a green and solvent-free method. Green Process. Synth. 2016, 5, 389–394. [Google Scholar] [CrossRef]
- Grossmann, K.; Pavelko, R.G.; Barsan, N.; Weimar, U. Interplay of H2, water vapor and oxygenat the surface of SnO2 based gas sensors—An operando investigation utilizing deuterated gases. Sens. Actuators B Chem. 2012, 166–167, 787–793. [Google Scholar] [CrossRef]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the active oxygen species in SnO2 based gas sensing materials: an operando IR spectroscopy study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Gurlo, A.; Barsan, N.; Weimar, U. Gas Sensors Based on Semiconducting Metal Oxides. In Metal Oxides: Chemistry and Applications, 1st ed.; Fierro, J.L.G., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 704–706. [Google Scholar]
- Wicker, S. Influence of Humidity on the Gas Sensing Characteristics of SnO2—DRIFTS Investigation of Different Base Materials and Dopants. Ph.D. Thesis, Eberhard Karls Universität, Tübingen, Germany, 2016. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Can, I.; Weimar, U.; Barsan, N. Operando Investigations of Differently Prepared In2O3-Gas Sensors. Proceedings 2017, 1, 432. https://doi.org/10.3390/proceedings1040432
Can I, Weimar U, Barsan N. Operando Investigations of Differently Prepared In2O3-Gas Sensors. Proceedings. 2017; 1(4):432. https://doi.org/10.3390/proceedings1040432
Chicago/Turabian StyleCan, Inci, Udo Weimar, and Nicolae Barsan. 2017. "Operando Investigations of Differently Prepared In2O3-Gas Sensors" Proceedings 1, no. 4: 432. https://doi.org/10.3390/proceedings1040432
APA StyleCan, I., Weimar, U., & Barsan, N. (2017). Operando Investigations of Differently Prepared In2O3-Gas Sensors. Proceedings, 1(4), 432. https://doi.org/10.3390/proceedings1040432



