Interaction of Colloidal Silver Nanoparticles with Ni2+: Sensing Application †
Abstract
:1. Introduction
2. Materials and Methods
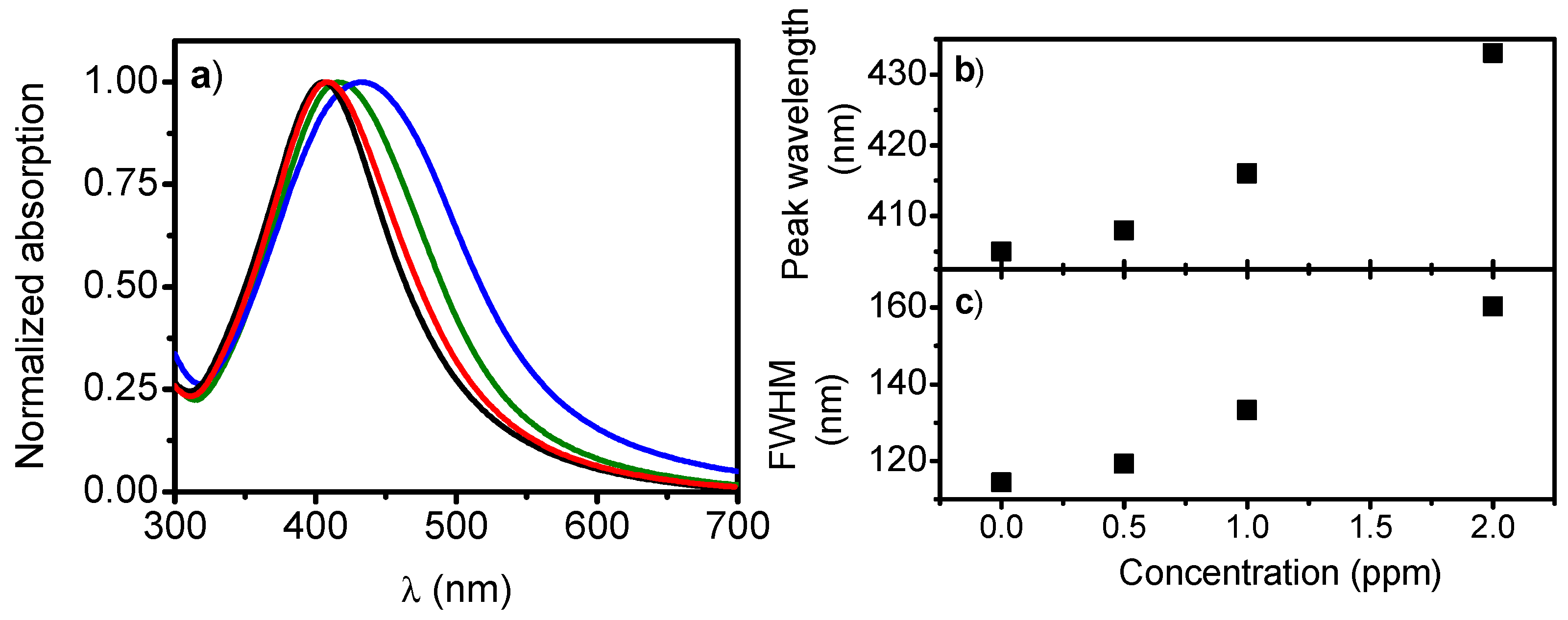
3. Results
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Edmundson, M.C.; Capeness, M. Exploring the potential of metallic nanoparticles within synthetic biology. New Biotechnol. 2014, 31, 572–578. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Bessar, H.; Venditti, I.; Fratoddi, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, V.; et al. Functionalized gold nanoparticles for topical delivery of Methotrexate for the possible treatment of psoriasis. Colloids Surf. B Biointerfaces 2016, 141, 141–147. [Google Scholar] [CrossRef]
- Zaniewski, A.M.; Schriver, M.; Lee, J.G.; Crommie, M.F.; Zettl, A. Electronic and optical properties of metal-nanoparticle filled grapheme sandwiches. Appl. Phys. Lett. 2013, 102, 023108. [Google Scholar] [CrossRef]
- Venditti, I. Gold nanoparticles in photonic crystals applications: A review. Materials 2017, 10, 97. [Google Scholar] [CrossRef]
- Tanabe, K. Optical radiation efficiencies of metal nanoparticles for optoelectronic applications. Mater. Lett. 2007, 61, 4573–4575. [Google Scholar] [CrossRef]
- Venditti, I.; Barbero, N.; Russo, M.V.; Di Carlo, A.; Decker, F.; Fratoddi, I.; Barolo, C.; Dini, D. Electrodeposited ZnO with squaraine sentisizers as photoactive anode of DSCs. Mater. Res. Express 2014, 1, 015040. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; O’Neil, D.; El-Sayed, M.A. Hollow and solid metallic nanoparticles in sensing and in nanocatalysis. Chem. Mater. 2014, 26, 44–58. [Google Scholar] [CrossRef]
- Prosposito, P.; D’Amico, L.; Casalboni, M.; Motta, N. Periodic arrangement of mono-dispersed gold nanoparticles for high performance polymeric solar cells. IEEE Conf. Publ. IEEE-NANO 2015, 378–380. [Google Scholar] [CrossRef]
- Fratoddi, I.; Zampetti, E.; Venditti, I.; Battocchio, C.; Russo, M.V.; Macagnano, A.; Bearzotti, A. Platinum nanoparticles on electrospun titania nanofibers as hydrogen sensing material working at room temperature. Nanoscale 2014, 6, 9177–9184. [Google Scholar] [CrossRef] [PubMed]
- Moores, A.; Goettmann, F. The plasmon band in noble metal nanoparticles: An introduction to theory and applications. New J. Chem. 2006, 30, 1121–1132. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-J.; Liu, C.-Y.; Sun, L.-W. Catalytic properties of silver nanoparticles supported on silica spheres. J. Phys. Chem. B 2005, 109, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, F.; Carlini, L.; Ugolini, A.; Visaggio, D.; Luisetto, I.; Visca, P.; Fratoddi, I.; Venditti, I.; Simonelli, L.; Marini, C.; et al. Synthesis and structural characterization of silver nanoparticles stabilized with 3-mercapto-1-propansulfonate and 1-thioglucose mixed thiols for antibacterial applications. Materials 2016, 9, 1028. [Google Scholar] [CrossRef]
- Guo, H.; Xing, B.; Hamlet, L.C.; Chica, A.; He, L. Surface-enhanced Raman scattering detection of silver nanoparticles in environmental and biological samples. Sci. Total Environ. 2016, 554, 246–252. [Google Scholar] [CrossRef]
- Kumar, V.V.; Savarimuthu, P.A. Silver nanoparticles based selective colorimetric sensor for Cd2+, Hg2+ and Pb2+ ions: Tuning sensitivity and selectivity using co-stabilizing agents. Sens. Actuators B 2014, 191, 31–36. [Google Scholar] [CrossRef]
- Dubas, S.T.; Pimpan, V. Green synthesis of silver nanoparticles for ammonia sensing. Talanta 2008, 76, 29–33. [Google Scholar] [CrossRef]
- Ratnarathorn, N.; Chailapakul, O.; Henry, C.S.; Dungchai, W. Simple silver nanoparticle colorimetric sensing for copper by paper-based devices. Talanta 2012, 99, 552–557. [Google Scholar] [CrossRef]
- Wang, C.C.; Luconi, M.O.; Masi, A.N.; Fernández, L.P. Derivatized silver nanoparticles as sensor for ultra-tracenitrate determination based on light scattering phenomenon. Talanta 2009, 77, 1238–1243. [Google Scholar] [CrossRef]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Venditti, I.; Russo, M.V.; Falconieri, M. Growth control and long range self-assembly of polymethylmethacrylate nanospheres. J. Appl. Pol. Sci. 2006, 102, 4493–4499. [Google Scholar] [CrossRef]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interface Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochi, F.; Venditti, I.; Fratoddi, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; Matteis, F.D.; Casciardi, S.; Prosposito, P. Interaction of Colloidal Silver Nanoparticles with Ni2+: Sensing Application. Proceedings 2017, 1, 427. https://doi.org/10.3390/proceedings1040427
Mochi F, Venditti I, Fratoddi I, Battocchio C, Carlini L, Iucci G, Casalboni M, Matteis FD, Casciardi S, Prosposito P. Interaction of Colloidal Silver Nanoparticles with Ni2+: Sensing Application. Proceedings. 2017; 1(4):427. https://doi.org/10.3390/proceedings1040427
Chicago/Turabian StyleMochi, Federico, Iole Venditti, Ilaria Fratoddi, Chiara Battocchio, Laura Carlini, Giovanna Iucci, Mauro Casalboni, Fabio De Matteis, Stefano Casciardi, and Paolo Prosposito. 2017. "Interaction of Colloidal Silver Nanoparticles with Ni2+: Sensing Application" Proceedings 1, no. 4: 427. https://doi.org/10.3390/proceedings1040427
APA StyleMochi, F., Venditti, I., Fratoddi, I., Battocchio, C., Carlini, L., Iucci, G., Casalboni, M., Matteis, F. D., Casciardi, S., & Prosposito, P. (2017). Interaction of Colloidal Silver Nanoparticles with Ni2+: Sensing Application. Proceedings, 1(4), 427. https://doi.org/10.3390/proceedings1040427







