Fabrication of Sharp Tip-Separable Microneedle Device for Trans-Dermal Drug Delivery Systems †
Abstract
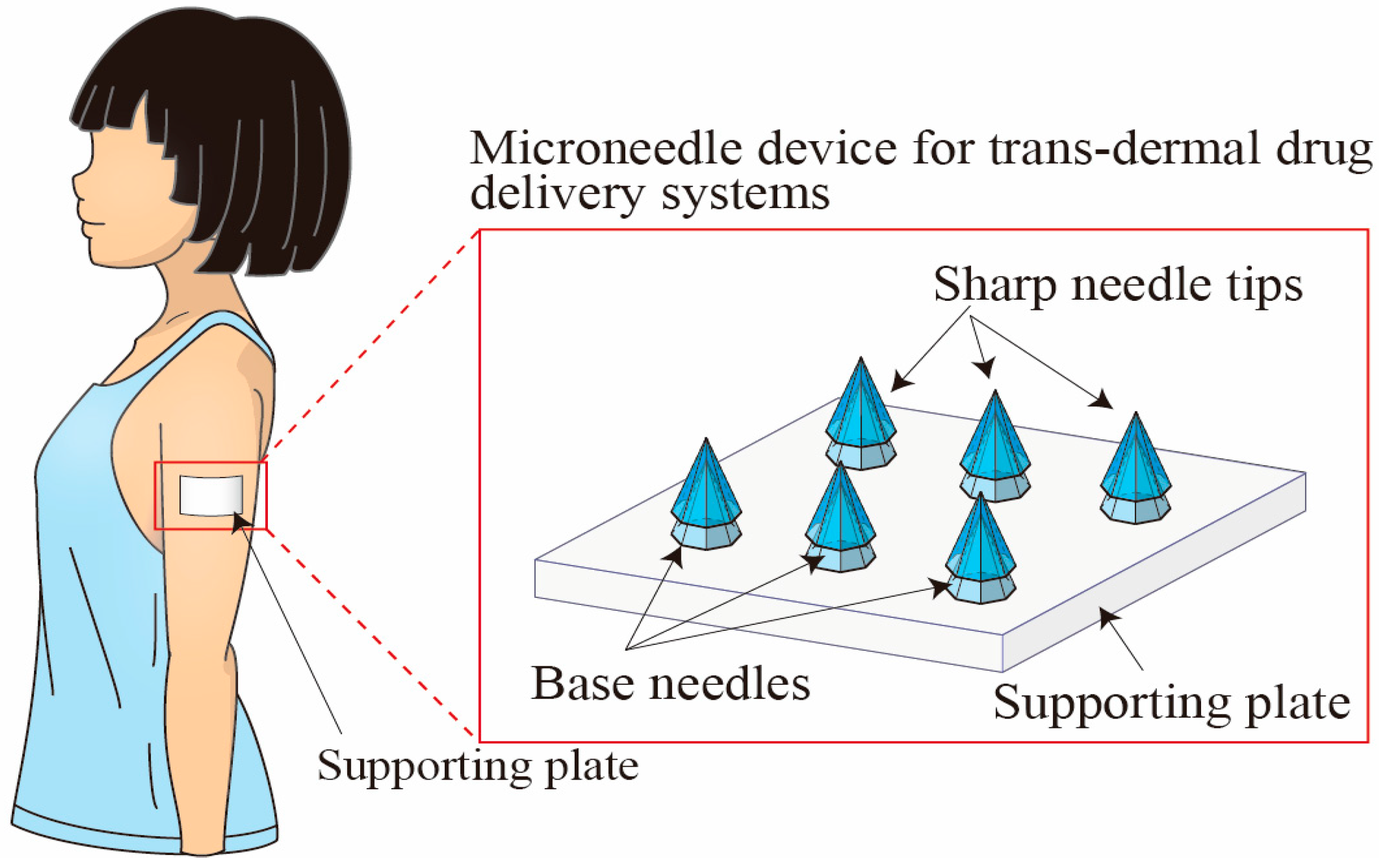
:1. Introduction
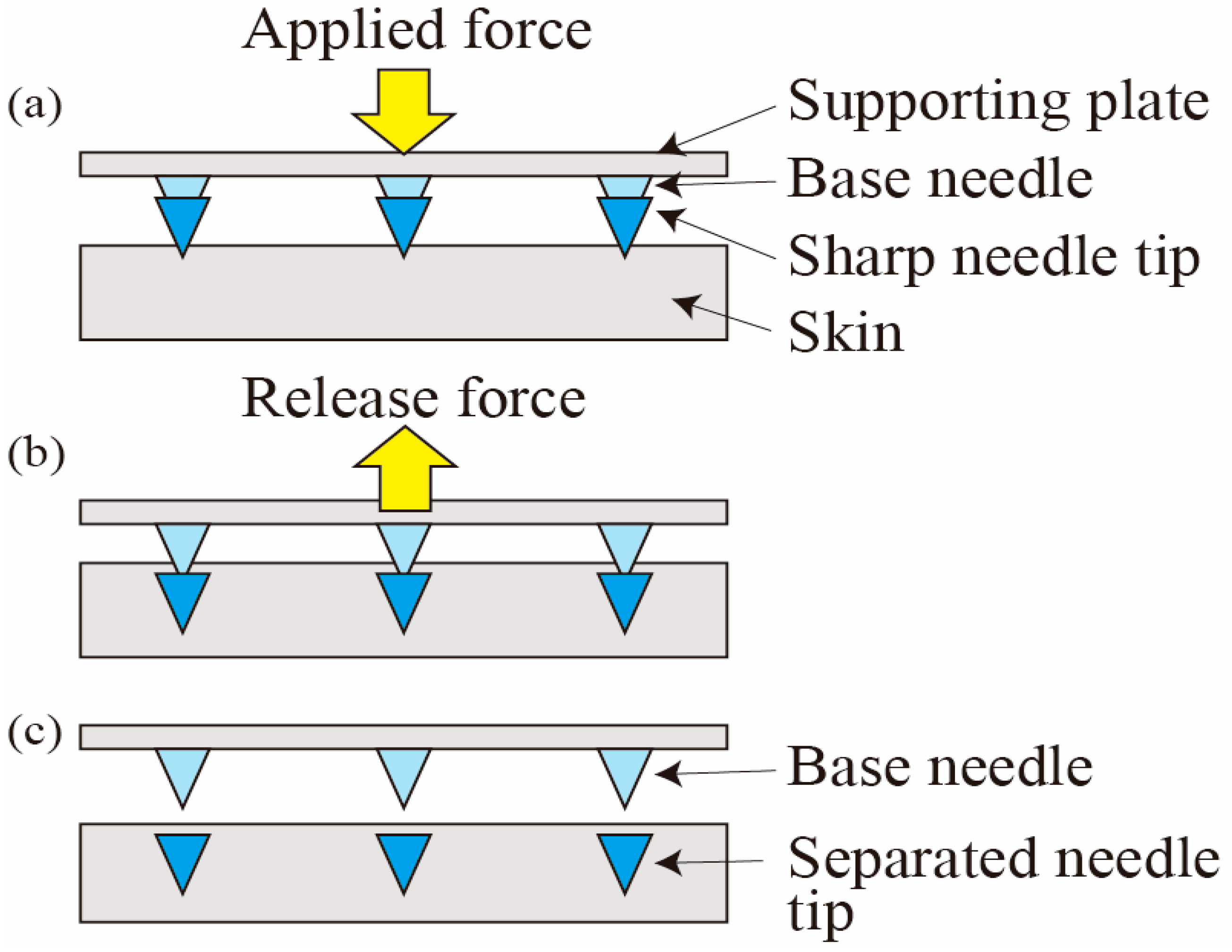
2. Alignment Mechanism
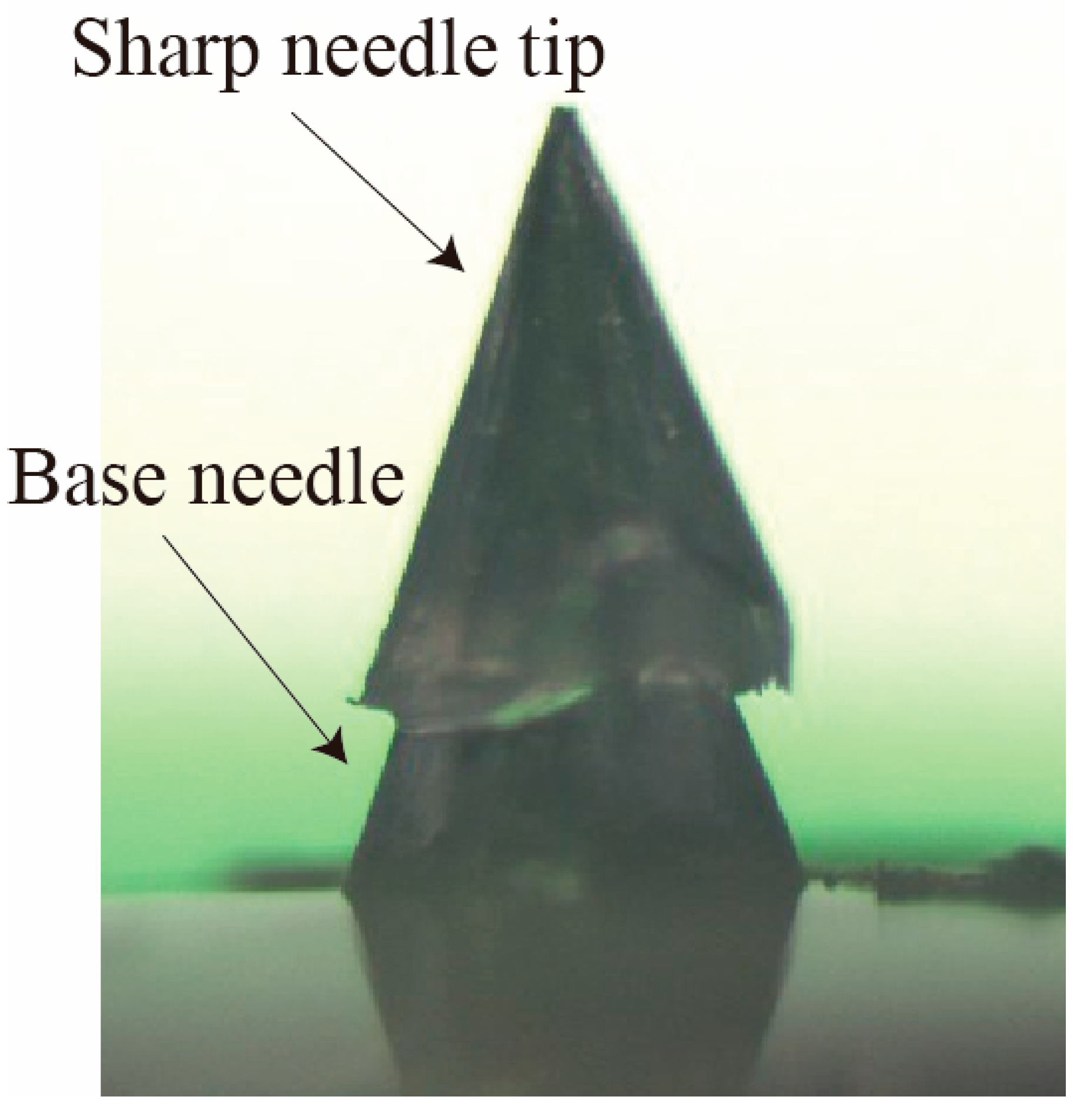
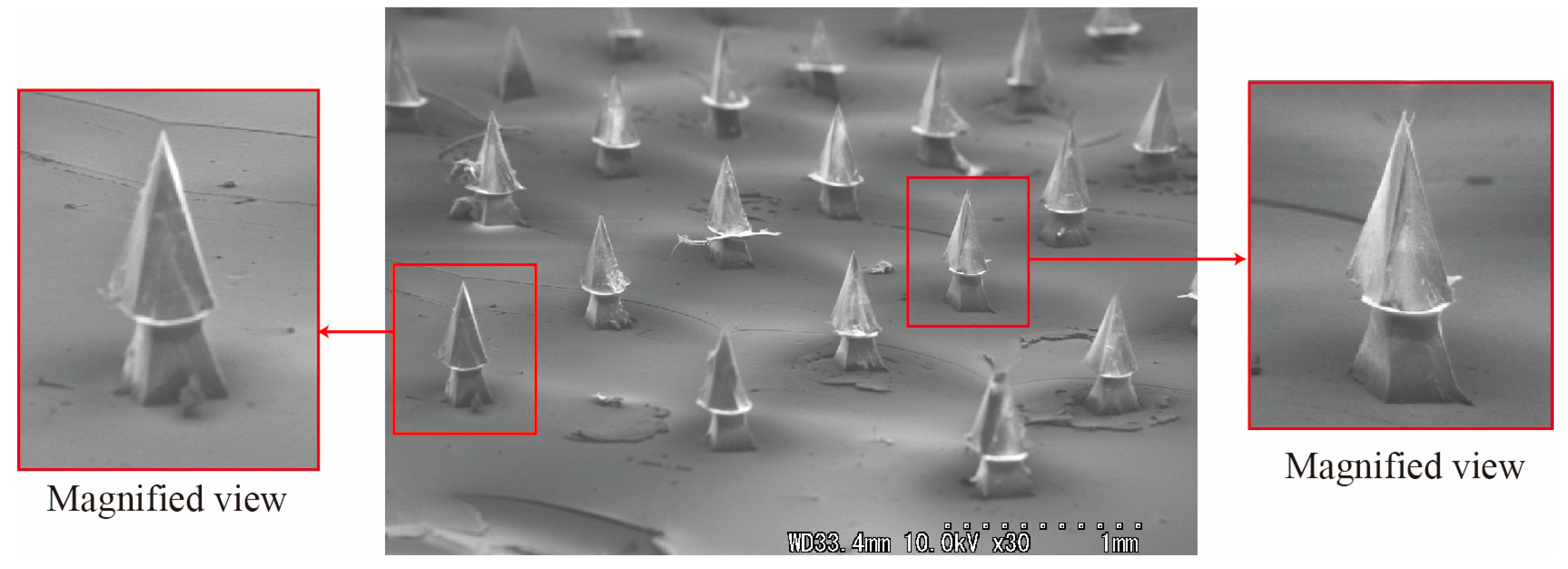
3. Experiments
4. Conclusions
- (1)
- An alignment mechanism for producing a sharp tip-separable microneedle device for trans-dermal drug delivery systems was developed.
- (2)
- A sharp tip-separable microneedle device was successfully produced by using the developed alignment mechanism. The fabricated needle was about 400 μm high. The overlapping distance between the needle and base parts was controlled with an accuracy of less than 21 μm. Furthermore, the alignment error in x-y directions was controlled with an accuracy of less than 19 μm.
- (3)
- An arrayed tip-separable microneedle device was produced by applying the developed mechanism.
Conflicts of Interest
References
- Shikida, M.; Kitamura, S.; Miyake, C.; Bessho, K. Micromachined pyramidal shaped biodegradable microneedle and its skin penetration capability. Microsyst. Technol. 2014, 20, 2239–2245. [Google Scholar] [CrossRef]
- Imaeda, K.; Bessho, K.; Shikida, M. Sharp tip-separable microneedle device for trans-dermal drug delivery systems. In Proceedings of the 2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 1715–1718. [Google Scholar]
- Nabekura, Y.; Imaeda, K.; Hasegawa, Y.; Shikida, M. Insertion of tip-separable microneedle device for trans-dermal drug delivery systems. In Proceedings of the Technical Digit Asia-Paciific Conference Transducers and Micro-Nano Technology, Kanazawa, Japan, 26–29 June 2016; pp. 255–256. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabekura, Y.; Fukuyu, H.; Hasegawa, Y.; Shikida, M. Fabrication of Sharp Tip-Separable Microneedle Device for Trans-Dermal Drug Delivery Systems. Proceedings 2017, 1, 304. https://doi.org/10.3390/proceedings1040304
Nabekura Y, Fukuyu H, Hasegawa Y, Shikida M. Fabrication of Sharp Tip-Separable Microneedle Device for Trans-Dermal Drug Delivery Systems. Proceedings. 2017; 1(4):304. https://doi.org/10.3390/proceedings1040304
Chicago/Turabian StyleNabekura, Yuki, Hitoshi Fukuyu, Yoshihiro Hasegawa, and Mitsuhiro Shikida. 2017. "Fabrication of Sharp Tip-Separable Microneedle Device for Trans-Dermal Drug Delivery Systems" Proceedings 1, no. 4: 304. https://doi.org/10.3390/proceedings1040304
APA StyleNabekura, Y., Fukuyu, H., Hasegawa, Y., & Shikida, M. (2017). Fabrication of Sharp Tip-Separable Microneedle Device for Trans-Dermal Drug Delivery Systems. Proceedings, 1(4), 304. https://doi.org/10.3390/proceedings1040304



