1. Introduction
Ionic liquids (ILs) are interesting for consumer-electronic products due to their advantageous properties, such as low toxicity and negligible volatility at room temperature. However for the use in consumer products, they have to be immobilized on a transducer. This can be achieved by jellifying the ionic liquid by means of a gelator. Organic/inorganic polymers or metal oxide (MOX) nanoparticles can be used as gelators, resulting in a so-called ionogel [
1]. It was shown that a variety of gaseous analytes can be detected by ionogel-based sensors, e.g., H
2S, ethylene, NO
2 and CO
2 [
2]. A composite material of Poly Ionic Liquid (PIL) with La
2O
3 was shown by Willa et al. [
3] to be sensitive to CO
2. They ascribed the detection mechanism to a pre-concentration of CO
2 by PIL and subsequent detection of CO
2 by the La
2O
2CO
3 particles.
The composite material in the present work is based on a novel ionogel. However, instead of a PIL, a supported ionic liquid was used as main component of the composite material. Furthermore the capability for mass production was studied by depositing the material by ink-jet printing on a chemiresistive transducer. The sensor is operated at elevated room-temperature, which reduces the power consumption to a very low level.
2. Materials and Methods
2.1. Materials
The semiconducting metal oxides were obtained as optimized inks for the inkjet printing process. These inks consist of a dispersion of nanoparticles with stabilizing agent in aqueous solvent system. The polymer and the ionic liquid were obtained from a commercial supplier. All material were used as-received, without purification prior to application. Interdigitated electrodes are prepared via sputtering of Ti (20 nm) and Pt (200 nm) onto Si substrates with native oxide.
2.2. Film Fabrication
First, the metal oxide film was inkjet printed by using the material cartridge DMC-11610 with a Dimatix DMP-3000 inkjet printer. After drying, the sintering process was conducted at optimized temperatures for each metal oxide phase. The final film thickness is about 100 nm.
The ionogel was prepared by dissolving the polymer and the ionic liquid in a common solvent. Subsequently the Ionogel was inkjet printed on top of the as-prepared MOX film and annealed overnight at 80 °C. The thickness of the ionogel film is about 1000 nm.
2.3. Measurements
Measurements were performed with a Keitheley 2636B SMU unit. The devices were probed in a setup which is open to the environment in a clean room facility. In order to prevent interference from the atmosphere, gas was purged with 2 L/min onto the devices with a constant separation of 1cm between the gas outlet and the device.
3. Results
3.1. Sensitivity to Carbon Dioxide (CO2)
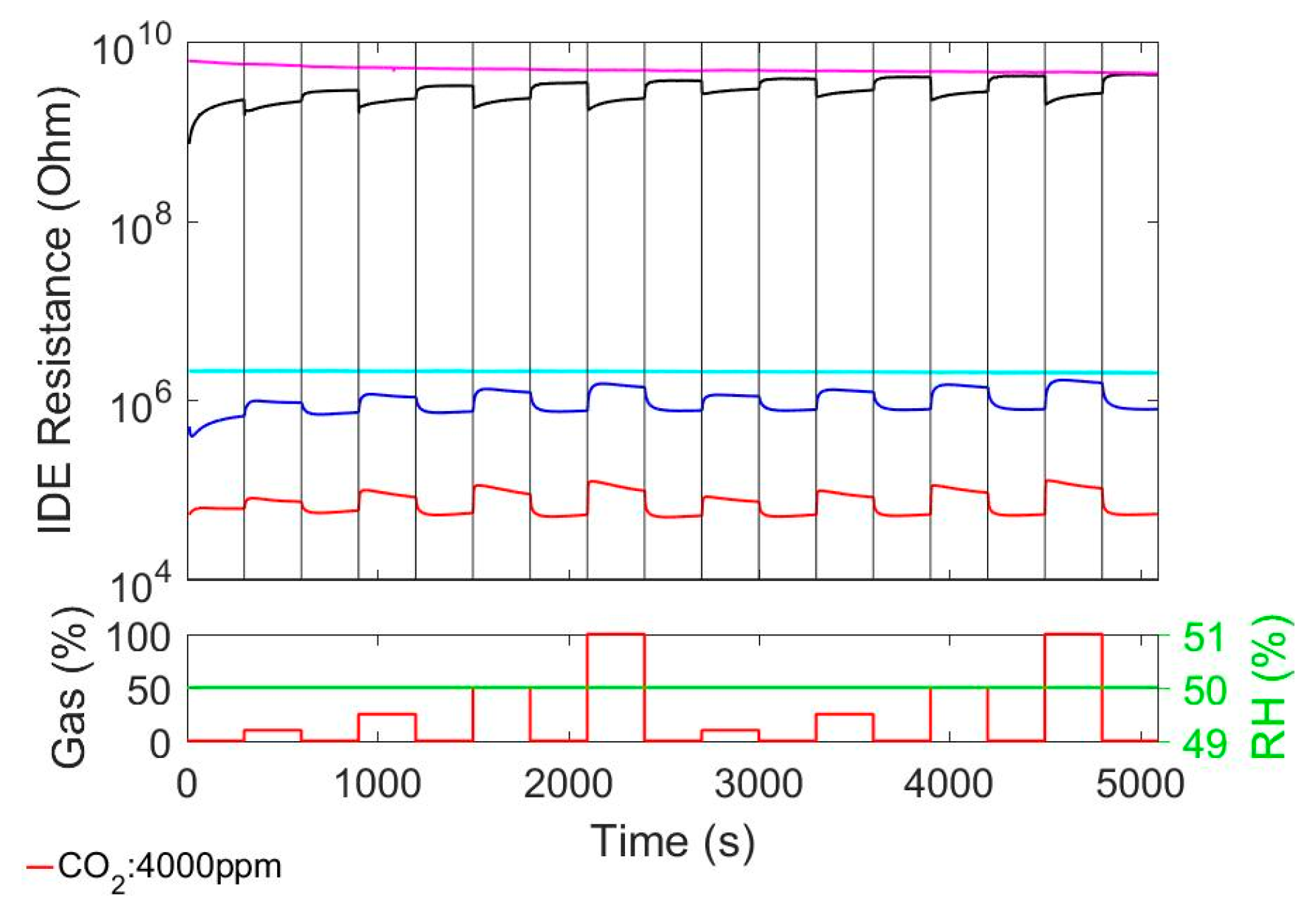
A comparison of CO
2 sensitivity between pure MOX films, Ionogel film and our composite MOX@Ionogel is presented in
Figure 1. The pure MOX films do not show any CO
2 response at all, even at 4000 ppm. The ionogel reacts with decreasing resistance on the presence of CO
2 in the surrounding. This decrease is proportional to the concentration of CO
2. The response and recovery times (t
90) are below 5 s, each. The response of MOX@Ionogel (with MOX = SnO
2 or In
2O
3) is in both cases slightly larger than for the pure Ionogel. However, it is more intriguing that the response to CO
2 leads to an increasing resistance, which is in contrast to the behavior of pure Ionogel. Furthermore it can be observed that upon coating the MOX with the Ionogel, the resistance of SnO
2@Ionogel decreases by about 3 orders of magnitude while the resistance of In
2O
3@Ionogel decreases by about 1.5 orders of magnitude.
3.2. Cross-Sensitivity to Relative Humidity (RH)
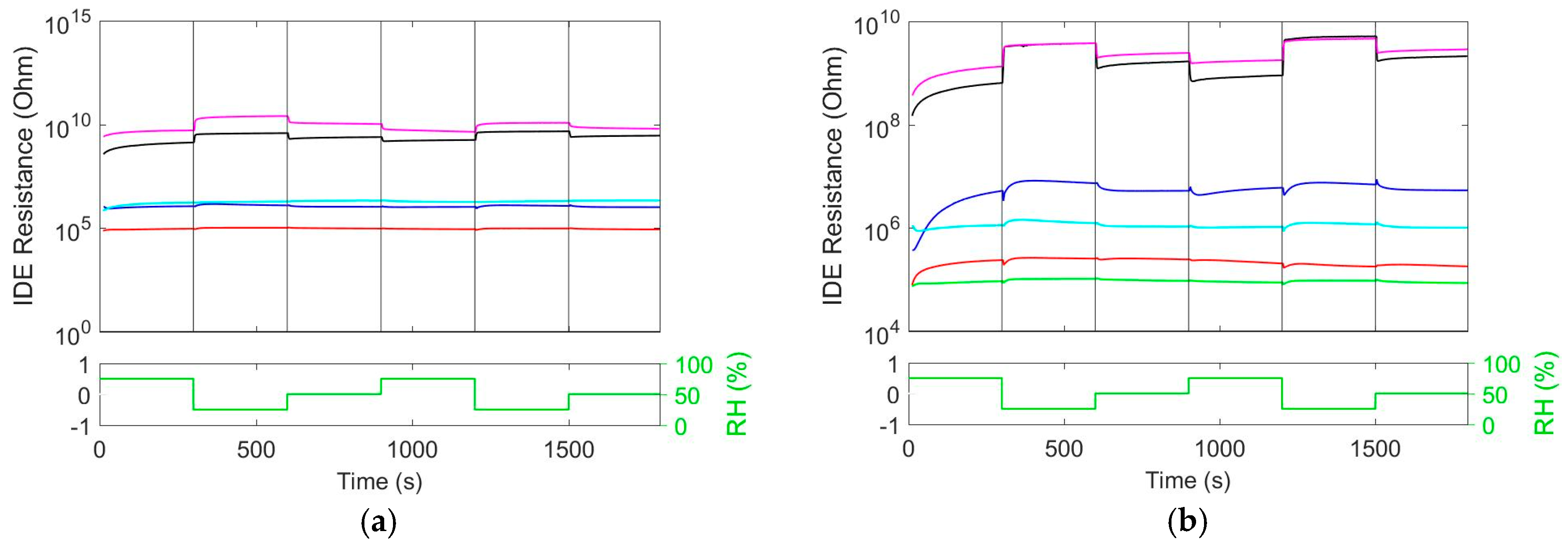
Since the ionic liquid employed in our composite is hygroscopic, an interference of humidity on the CO
2 sensitivity was expected. From
Figure 2a it can be seen that the pure Ionogel reacts almost instantaneously to changes in relative humidity. While In
2O
3 doesn’t show significant humidity response, the SnO
2 film reacts relatively strong to changes in humidity. After coating of MOX with Ionogel, the influence of humidity on the baseline resistance becomes smaller.
By increasing the temperature, less interference from humidity should be expected due to less favorable adsorption of water. The result of increasing the temperature from 30 to 50 °C is shown in
Figure 2b. In case of all three depicted materials, the sensitivity to humidity changes decreases upon heating to higher temperature.
3.3. Cross-Sensitivity to Nitrogen Dioxide (NO2) and Carbon Monoxide (CO)
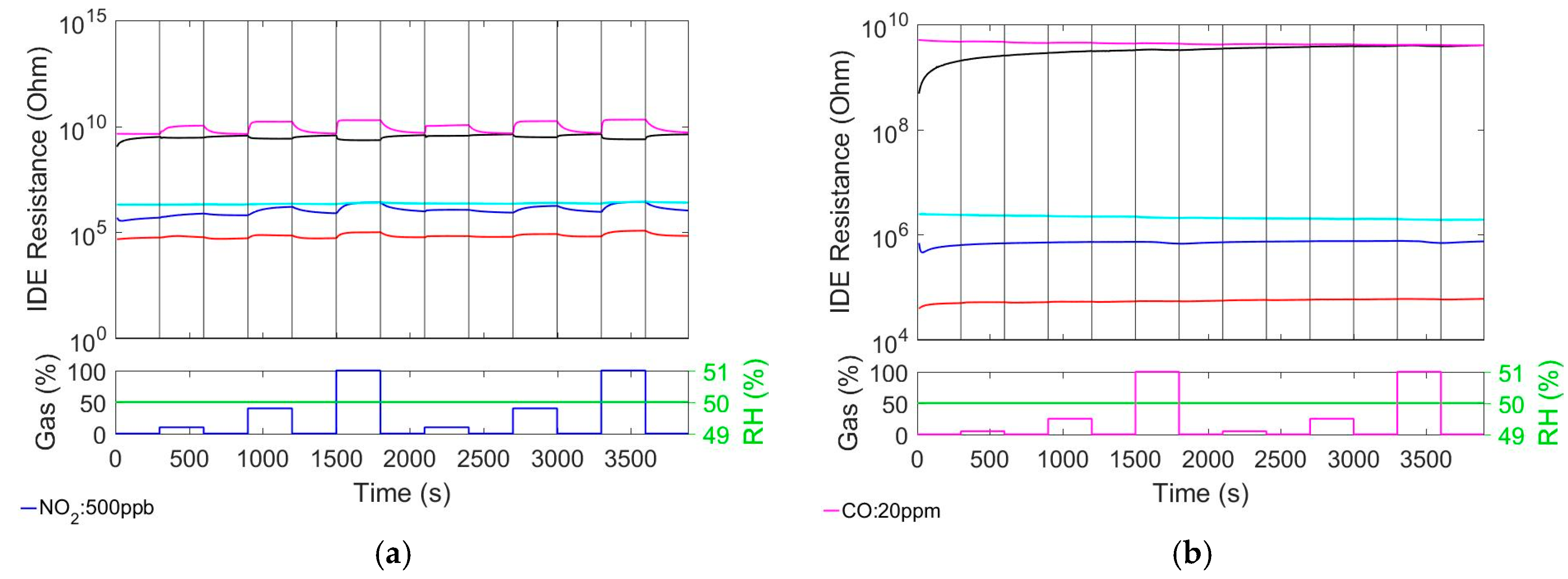
As it can be seen from
Figure 3a, only In
2O
3 doesn’t react to NO
2. SnO
2 shows the strongest response with fast response time but slower recovery time. Compared to pure MOX, by coating both MOX with Ionogel, the NO
2 sensitivity of SnO
2@Ionogel and In
2O
3@Ionogel decreases and increases, respectively. The pure Ionogel shows low but significant NO
2 response, which causes a decrease in resistance. This is in contrast to the increasing resistance in the case of MOX and MOX@Ionogel materials.
From
Figure 3b it can be seen that none of the materials exhibits significant CO response.
4. Discussion
While CO
2 storage capability of ionic liquids is well explored, the sensing behavior is less investigated. Extending the works on Ionogel [
4] and on composites of MOX with PILs [
3], our investigations revealed interesting phenomena occurring upon combining both approaches. Namely, CO
2 response which is stronger than reported in literature and also is manifested by increasing resistance, has been observed. The change of resistance in opposite direction makes clearly indicates a different sensing mechanism. Since SnO
2 and In
2O
3 do not react with CO
2, a pre-concentration effect as suggested by Willa et al. is probably not responsible for the CO
2 response of our composite. Since the baseline resistance of MOX@Ionogel is much lower than for Ionogel or MOX alone, the sensing mechanism is believed to occur at the interface of both phases, maybe even at the three-phase interface with the electrode.
Since the cross-sensitivity to RH is low and gets even lower at higher temperatures, the operating conditions of the sensors (temperature and DC bias) can be optimized with respect to increased CO2 sensitivity and decreased NO2 cross-sensitivity. The small interference originating from RH variations are thought to be compensated easily by knowledge of the current RH. Maybe the contribution of NO2 to the total resistance can be compensated by using a selective NO2 sensor.
5. Conclusions
The material in this work was anticipated to be used as a CO2 sensors. However cross-sensitivity to NO2 is significant and may be compensated.
It seems like our approach is not limited to a certain metal oxide phase. It can be seen as a universal approach to obtain gas sensors with tunable sensitivity towards desired analytes and elimination of cross-sensitivity to interfering agents. In order to prove this claim, further investigation on combining different MOX phases with various ionic liquids will be undertaken.
Additionally, future characterization of this class of composite material by advanced methods should reveal the mechanism of sensing. This should allow systematic approach in combining MOX with ionic liquid in order to obtain a selective sensor.
Furthermore, investigation on the reliability has to be performed since ionic liquids still have a finite vapor pressure and can be dislocated from the IDE upon mechanical impact or applied electrical field.