Towards Nanostructured ITO-Based Electrochemical Sensors: Fabrication, Characterization and Functionalization †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth of ITO Electrodes
2.2. Electrochemical Characterization
2.3. Substrates Functionalization
2.4. X-ray and Infrared Spectroscopies
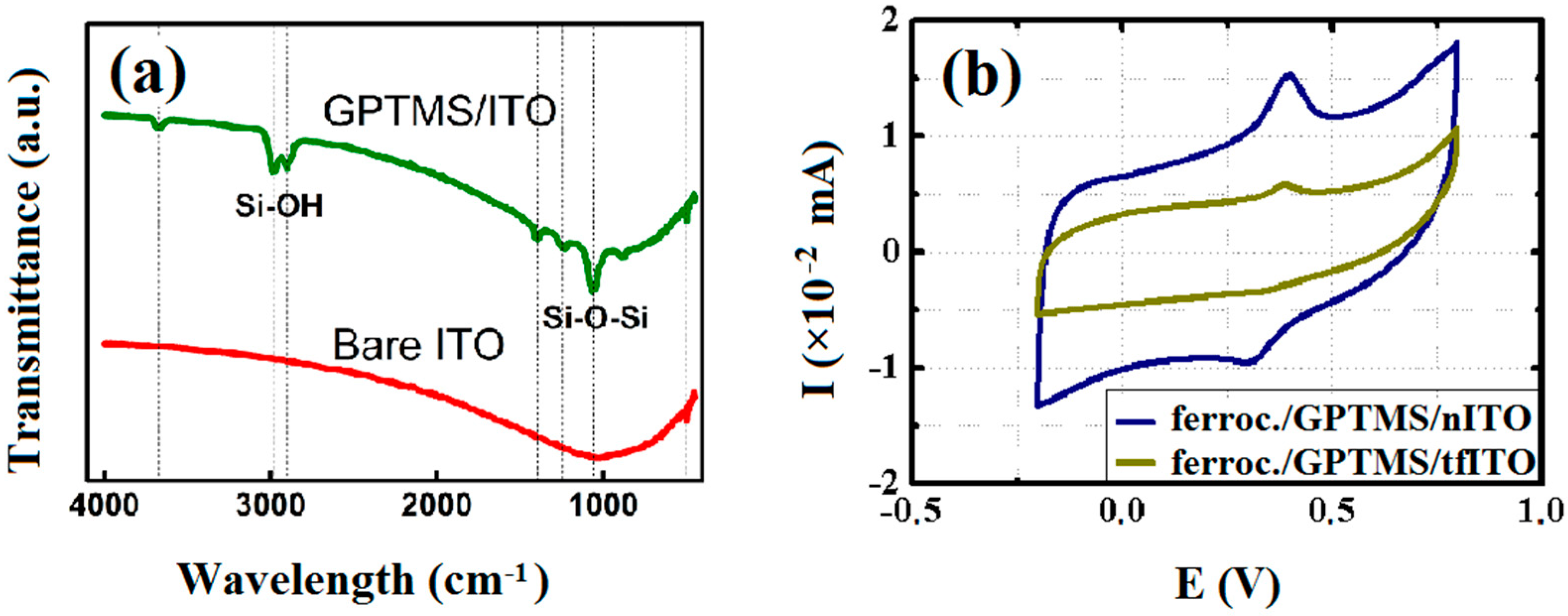
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.P.; Duesberg, G.S.; Hoenlein, W.; Kreupl, F.; Liebau, M.; Martin, R.; Rajasekharan, B.; Pamler, W.; Seidel, R.; Steinhoegl, W.; et al. How do carbon nanotubes fit into the semiconductor roadmap? Appl. Phys. A 2005, 80, 1141–1151. [Google Scholar] [CrossRef]
- Wu, M.S.; Yuan, D.J.; Xu, J.J.; Chen, H.Y. Sensitive electrochemiluminescence biosensor based on Au-ITO hybrid bipolar electrode amplification system for cell surface protein detection. Anal. Chem. 2013, 85, 11960–11965. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Kihm, K.D.; English, A.E. Optoelectronic biosensor using indium-tin-oxide electrodes. Opt. Lett. 2007, 32, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Margraves, C.H.; Jun, S.I.; English, A.E.; Rack, P.D.; Kihm, K.D. Optoelectronic cellular biosensor using optically transparent indium tin oxide (ITO) electrodes. Sensors 2008, 8, 3257–3270. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Yuan, D.J.; Xu, J.J.; Chen, H.Y. Sensitive electrochemiluminiscence biosensor based on Au-ITO hybrid bipolar electrode amplification system for cell surface protein detection. Anal. Chem. 2013, 85, 11960–11965. [Google Scholar] [CrossRef] [PubMed]
- Pruna, R.; Palacio, F.; López, M.; Pérez, J.; Mir, M.; Blázquez, O.; Hernández, S.; Garrido, B. Electrochemical characterization of organosilane-functionalized nanostructured ITO surfaces. Appl. Phys. Lett. 2016, 109, 063109. [Google Scholar] [CrossRef]
- Pruna, R.; Palacio, F.; Martínez, M.; Blázquez, O.; Hernández, S.; Garrido, B.; López, M. Organosilane-functionalization of nanostructured indium tin oxide films. Interface Focus 2016, 6, 20160056. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruna, R.; Palacio, F.; López, M. Towards Nanostructured ITO-Based Electrochemical Sensors: Fabrication, Characterization and Functionalization. Proceedings 2017, 1, 288. https://doi.org/10.3390/proceedings1040288
Pruna R, Palacio F, López M. Towards Nanostructured ITO-Based Electrochemical Sensors: Fabrication, Characterization and Functionalization. Proceedings. 2017; 1(4):288. https://doi.org/10.3390/proceedings1040288
Chicago/Turabian StylePruna, Raquel, Francisco Palacio, and Manel López. 2017. "Towards Nanostructured ITO-Based Electrochemical Sensors: Fabrication, Characterization and Functionalization" Proceedings 1, no. 4: 288. https://doi.org/10.3390/proceedings1040288
APA StylePruna, R., Palacio, F., & López, M. (2017). Towards Nanostructured ITO-Based Electrochemical Sensors: Fabrication, Characterization and Functionalization. Proceedings, 1(4), 288. https://doi.org/10.3390/proceedings1040288




