Microscale Gas Chromatography with Microsensor Array Detection: Challenges and Prospects †
Abstract
:1. Introduction
2. Materials and Methods
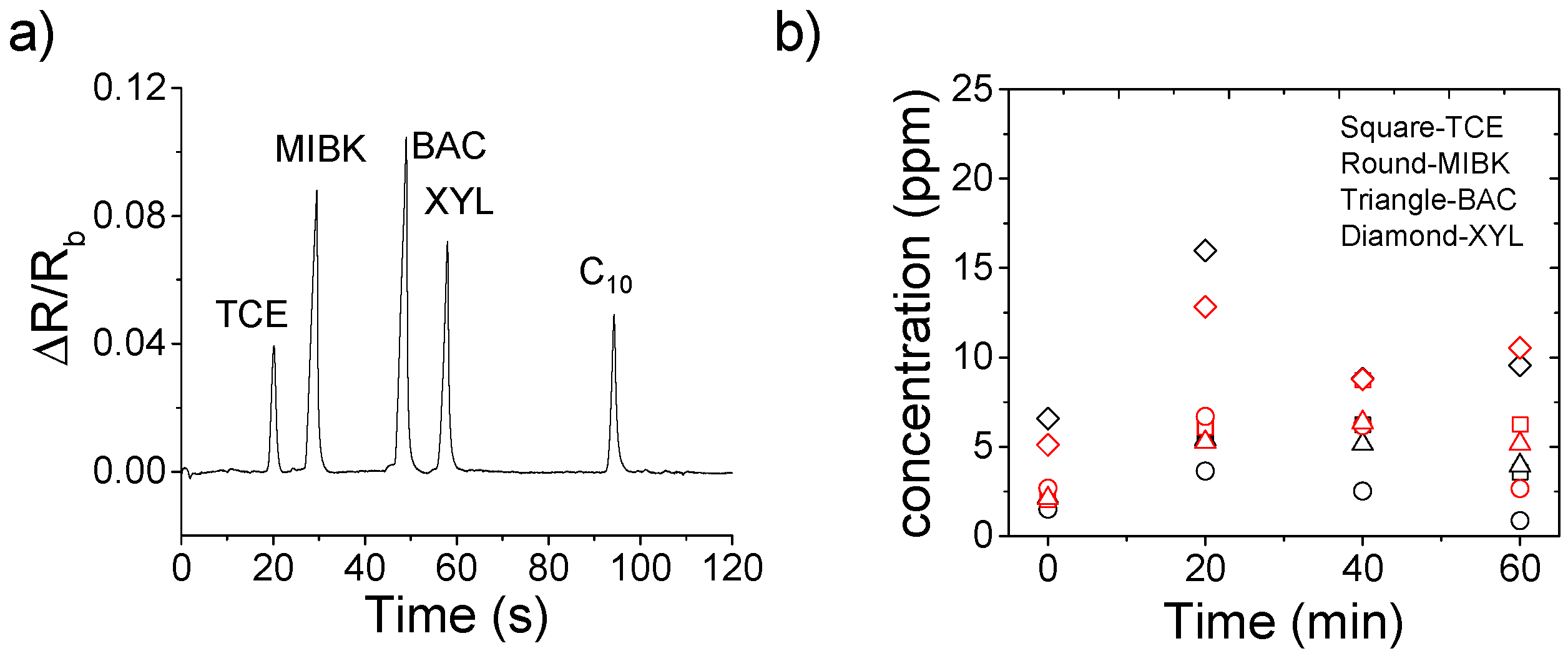
3. Results and Discussion
Acknowledgments
Conflicts of Interest
References
- Lu, C.J.; Steinecker, W.H.; Tian, W.C.; Agah, M.; Potkay, J.A.; Chan, H.K.; Sacks, R.D.; Wise, K.D.; Pang, S.; Zellers, E.T. First-generation hybrid MEMS gas chromatograph. Lab Chip 2005, 5, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.R.; Manginell, R.P.; Adkins, D.R.; Kottenstette, R.J.; Wheeler, D.R.; Sokolowski, S.S.; Trudell, D.E.; Bymes, J.E.; Okandan, M.; Bauer, J.M.; et al. Recent advancements in the gas-phase µChem Lab. IEEE Sens. J. 2006, 6, 784–795. [Google Scholar]
- Akbar, M.; Restaino, M.; Agah, M. Chip-scale gas chromatography: From injection through detection. Microsyst. Nanoeng. 2015, 1, 15039–15046. [Google Scholar] [CrossRef]
- Wang, J.; Bryant-Genevier, J.; Nuñovero, N.; Zhan, C.; Kraay, B.; Scholten, K.; Zhang, C.; Buggaveeti, S.; Nidetz, R.; Zellers, E.T. Compact prototype microfabricated gas chromatographic analyzer for autonomous determinations of VOC mixtures at typical workplace concentrations. under review.
- Wang, J.; Nuñovero, N.; Lin, Z.; Nidetz, R.; Buggaveeti, S.; Zhan, C.; Kurabayashi, K.; Steinecker, W.; Zellers, E.T. Wearable MEMS GC for multi-vapor determinations. Procedia Eng. 2016, 168, 1398–1401. [Google Scholar] [CrossRef]
- Bryant-Genevier, J.; Zellers, E.T. Toward a microfabricated preconcentrator-focuser for a wearable μGC. J. Chrom. A. 2015, 1422, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Nuñovero, N.; Wang, J.; Nidetz, R.; Buggaveeti, S.; Kurabayashi, K.; Zellers, E.T. A zone-heated gas chromatographic microcolumn: Energy efficiency. Sens. Actuators B Chem. accepted for publication. [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Nuñovero, N.; Zhan, C.; Nidetz, R.; Steinecker, W.H.; Peterson, S.J.; Brookover, B.M.; Zellers, E.T. Microscale Gas Chromatography with Microsensor Array Detection: Challenges and Prospects. Proceedings 2017, 1, 633. https://doi.org/10.3390/proceedings1040633
Wang J, Nuñovero N, Zhan C, Nidetz R, Steinecker WH, Peterson SJ, Brookover BM, Zellers ET. Microscale Gas Chromatography with Microsensor Array Detection: Challenges and Prospects. Proceedings. 2017; 1(4):633. https://doi.org/10.3390/proceedings1040633
Chicago/Turabian StyleWang, Junqi, Nicolas Nuñovero, Changhua Zhan, Robert Nidetz, William H. Steinecker, Seth J. Peterson, Bryan M. Brookover, and Edward T. Zellers. 2017. "Microscale Gas Chromatography with Microsensor Array Detection: Challenges and Prospects" Proceedings 1, no. 4: 633. https://doi.org/10.3390/proceedings1040633
APA StyleWang, J., Nuñovero, N., Zhan, C., Nidetz, R., Steinecker, W. H., Peterson, S. J., Brookover, B. M., & Zellers, E. T. (2017). Microscale Gas Chromatography with Microsensor Array Detection: Challenges and Prospects. Proceedings, 1(4), 633. https://doi.org/10.3390/proceedings1040633



