Fermented Nettles: Bioactive Profile and Seasonal Variability
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Nettles
2.2. Total Nitrogen, Carbon, and Sulfur Content
2.3. Free Amino Acids, Primary Amines and Inorganic Ions
2.4. Protein and Peptide Content
2.5. Microbial Activity Detection
2.6. Hydrolytic Enzyme Activities
2.7. Phytohormone Analysis
2.8. Volatile Metabolite Detection
2.9. Chlorophyll and Carotenoid Contents
2.10. Phenolics and Flavonoids
2.11. Antioxidant System
2.12. Determination of Macro- and Microelements
2.13. Statistics and Data Processing
3. Results and Discussion
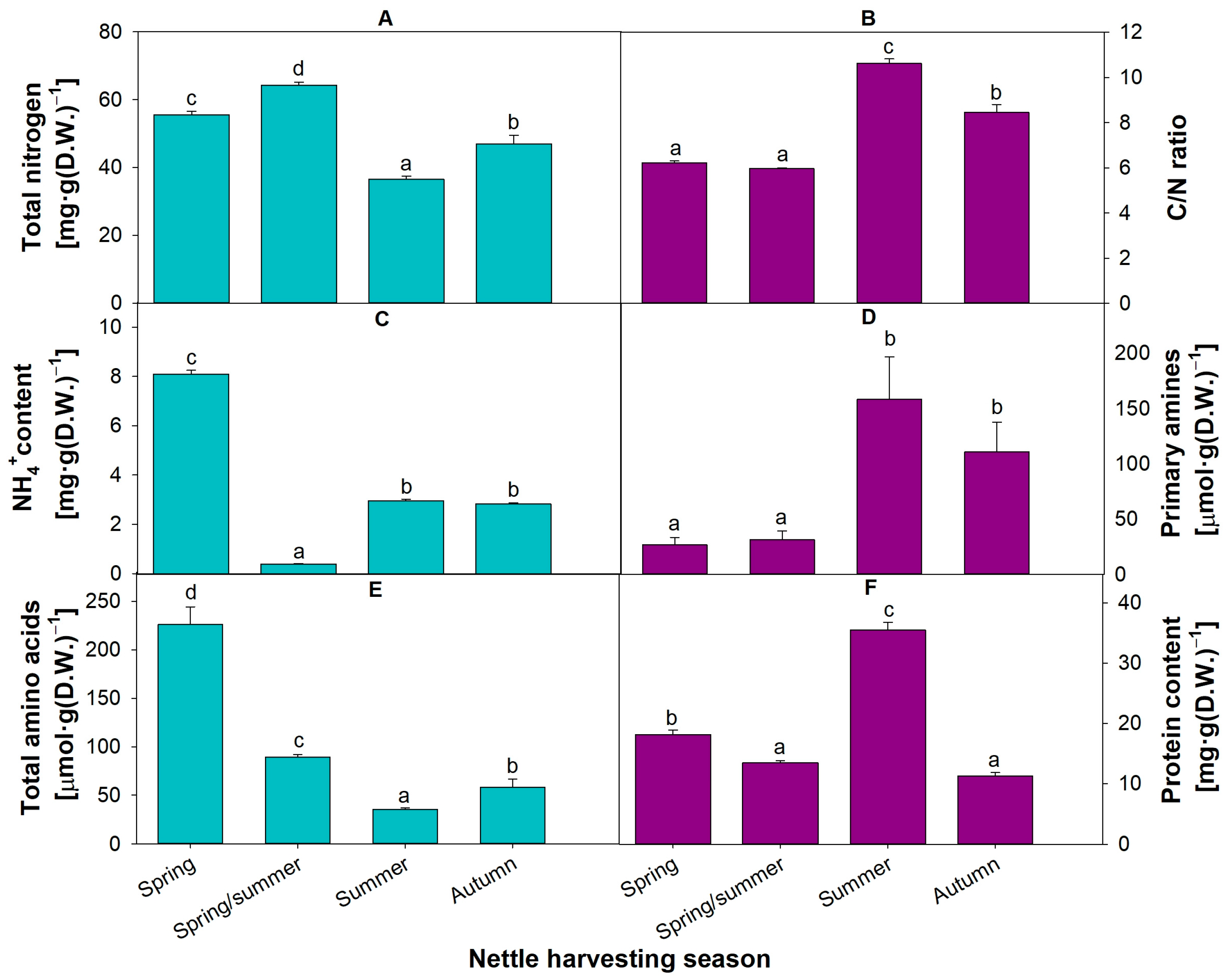
3.1. Fermented Nettles Contain Several Types of Nitrogen Compounds
3.2. Microbial Activity Peaks in the Summer Harvest
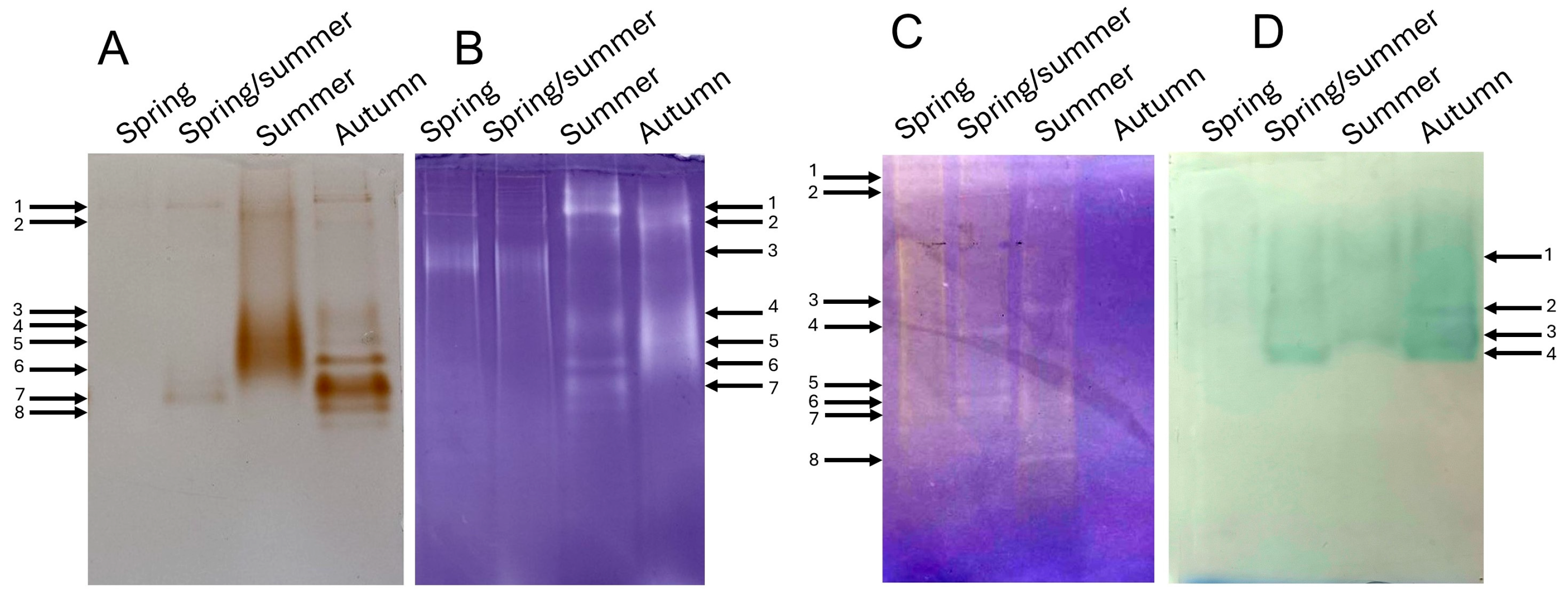
3.3. Degradation Processes Are Catalyzed by Various Hydrolytic Enzymes
3.4. Phytohormones May Contribute to Plant Growth, Seed Germination, and Defense
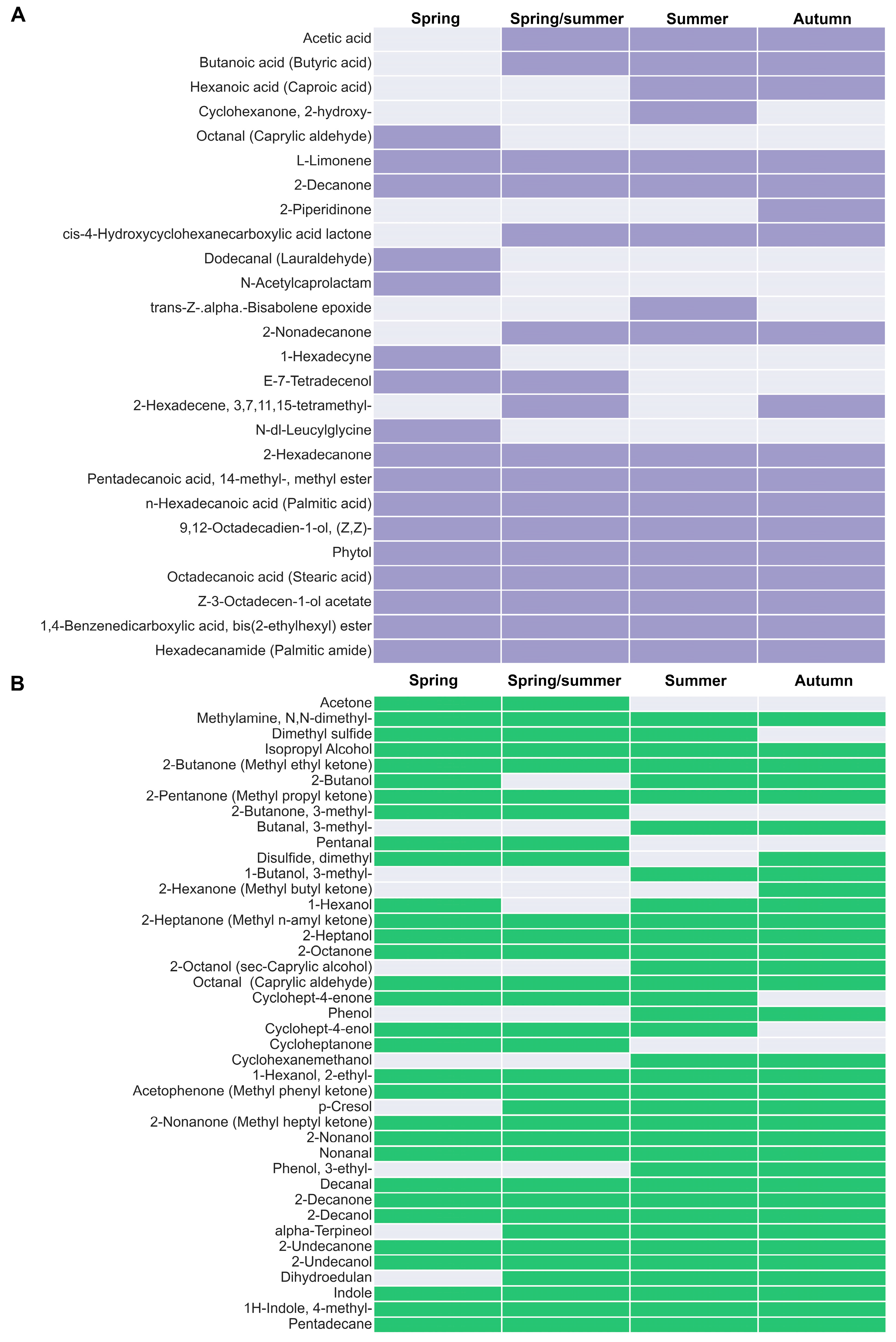
3.5. Presence of Volatile Compounds with Antimicrobial Effects
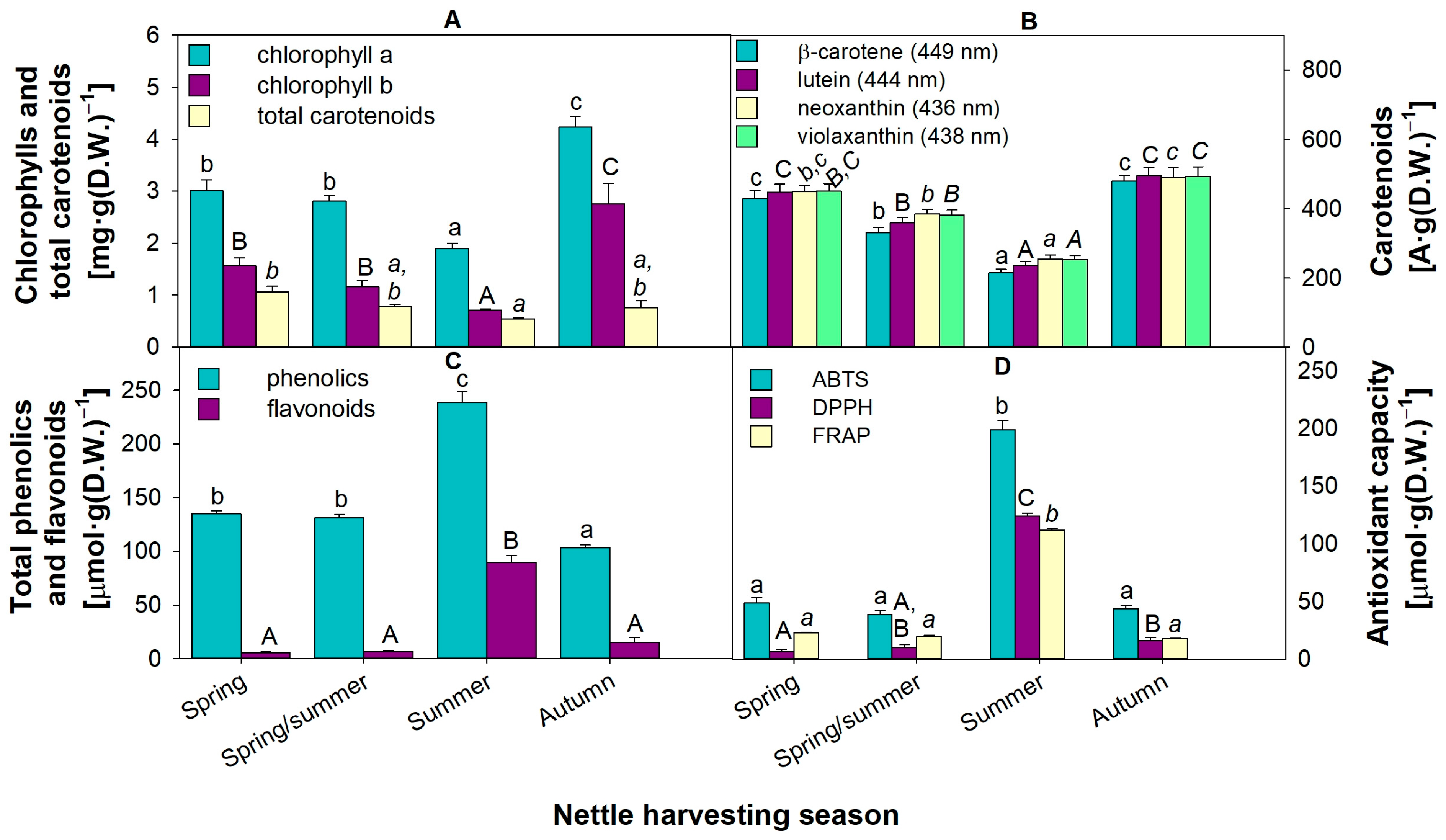
3.6. High Contents of Phenolics and Flavonoids May Serve as Beneficial Antioxidants
3.7. Fermented Nettles Are a Rich Source of Several Macro- and Micronutrients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | amino acid |
| ABA | abscisic acid |
| ABTS | 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) |
| ANOVA | one-way analysis of variance |
| C | carbon |
| CE | capillary electrophoresis |
| CKs | cytokinins |
| cZ | cis-zeatin |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DZ | dihydrozeatin |
| FN | fermented nettles |
| FRAP | ferric reducing antioxidant power assay |
| GA | gibberelic acid (gibberelin) |
| GC×GC-MS | comprehensive two-dimensional gas chromatography mass spectrometry |
| IAA | indole-3-acetic acid |
| ICP-MS | inductively coupled plasma mass spectrometry |
| INT | 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride |
| INTF | 1-(4-iodophenyl)-5-(4-nitrophenyl)-3-phenylformazan |
| iP | isopentenyl adenine |
| JA | jasmonic acid |
| JA-Ile | jasmonic acid-isoleucine |
| LC-MS | liquid chromatography mass spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MBTH | 3-methyl-2-benzothiazolinone hydrazone |
| N | nitrogen |
| OPAME | o-phthaldialdehyde and β-mercaptoethanol |
| OxIAA | oxindole-3-acetic acid |
| PAA | phenylacetic acid |
| PMS | 5-methylphenazinium methyl sulfate |
| SA | salicylic acid |
| SD | standard deviation |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TN | total nitrogen |
| tZ | trans-zeatin |
| UHPLC | ultra-high performance liquid chromatography |
References
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Systematic Investigation of the Effects of Seven Plant Extracts on the Physiological Parameters, Yield, and Nutritional Quality of Radish (Raphanus sativus Var. Sativus). Front. Plant Sci. 2021, 12, 651152. [Google Scholar] [CrossRef]
- Johnson, R.; Joel, J.M.; Puthur, J.T. Biostimulants: The Futuristic Sustainable Approach for Alleviating Crop Productivity and Abiotic Stress Tolerance. J. Plant Growth Regul. 2024, 43, 659–674. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A Sufficiently Effective Tool for Sustainable Agriculture in the Era of Climate Change? Plant Physiol. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica dioica L.): A Review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Đurović, S.; Micić, D.; Šorgić, S.; Popov, S.; Gašić, U.; Tosti, T.; Kostić, M.; Smyatskaya, Y.A.; Blagojević, S.; Zeković, Z. Recovery of Polyphenolic Compounds and Vitamins from the Stinging Nettle Leaves: Thermal and Behavior and Biological Activity of Obtained Extracts. Molecules 2023, 28, 2278. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica Spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef]
- Grauso, L.; De Falco, B.; Lanzotti, V.; Motti, R. Stinging Nettle, Urtica Dioica L.: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Rutto, L.K.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral Properties and Dietary Value of Raw and Processed Stinging Nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013, 857120. [Google Scholar] [CrossRef]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The Potential of Stinging Nettle (Urtica dioica L.) as a Crop with Multiple Uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Klepacka, J.; Starowicz, M.; Lesińska, P. Functional Properties and Sensory Quality of Kombucha Analogs Based on Herbal Infusions. Antioxidants 2024, 13, 1191. [Google Scholar] [CrossRef]
- Pawluś, P.; Kolniak-Ostek, J. Innovative Analogs of Unpasteurized Kombucha Beverages: Comparative Analysis of Mint/Nettle Kombuchas, Considering Their Health-Promoting Effect, Polyphenolic Compounds and Chemical Composition. Int. J. Mol. Sci. 2024, 25, 7572. [Google Scholar] [CrossRef] [PubMed]
- Nygaard Sorensen, J.; Thorup-Kristensen, K. Plant-based Fertilizers for Organic Vegetable Production. Z. Pflanzenernähr. Bodenk. 2011, 174, 321–332. [Google Scholar] [CrossRef]
- Maričić, B.; Radman, S.; Romić, M.; Perković, J.; Major, N.; Urlić, B.; Palčić, I.; Ban, D.; Zorić, Z.; Ban, S.G. Stinging Nettle (Urtica dioica L.) as an Aqueous Plant-Based Extract Fertilizer in Green Bean (Phaseolus vulgaris L.) Sustainable Agriculture. Sustainability 2021, 13, 4042. [Google Scholar] [CrossRef]
- Peterson, R.; Jensén, P. Effects of Nettle Water on Growth and Mineral Nutrition of Plants. I. Composition and Properties of Nettle Water. Biol. Agric. Hortic. 1985, 2, 303–314. [Google Scholar] [CrossRef]
- Garmendia, A.; Raigón, M.D.; Marques, O.; Ferriol, M.; Royo, J.; Merle, H. Effects of Nettle Slurry (Urtica dioica L.) Used as Foliar Fertilizer on Potato (Solanum tuberosum L.) Yield and Plant Growth. PeerJ 2018, 6, e4729. [Google Scholar] [CrossRef]
- Butcher, J.; Villette, C.; Zumsteg, J.; Maurer, L.; Barchietto, T.; Rigo, R.; Floch, K.; Cseh, A.; Buchet, S.; Stintzi, A.; et al. Microbial Bioremediation of Persistent Organic Pollutants in Plant Tissues Provides Crop Growth Promoting Liquid Fertilizer. Nat. Commun. 2025, 16, 5768. [Google Scholar] [CrossRef]
- Hodek, O.; Křížek, T.; Coufal, P.; Ryšlavá, H. Design of Experiments for Amino Acid Extraction from Tobacco Leaves and Their Subsequent Determination by Capillary Zone Electrophoresis. Anal. Bioanal. Chem. 2017, 409, 2383–2391. [Google Scholar] [CrossRef]
- Darrouzet-Nardi, A.; Ladd, M.P.; Weintraub, M.N. Fluorescent Microplate Analysis of Amino Acids and Other Primary Amines in Soils. Soil Biol. Biochem. 2013, 57, 78–82. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Schägger, H. Tricine–SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]
- Petiti, J.; Revel, L.; Divieto, C. Standard Operating Procedure to Optimize Resazurin-Based Viability Assays. Biosensors 2024, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- ISO 23753-2:2019; Soil Quality—Determination of Dehydrogenases Activity in Soils Part 2: Method Using Iodotetrazolium Chloride (INT). ISO: Geneva, Switzerland, 2019.
- Babson, A.L.; Phillips, G.E. A Rapid Colorimetric Assay for Serum Lactic Dehyurogenase. Clin. Chim. Acta 1965, 12, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Bělonožníková, K.; Vaverová, K.; Vaněk, T.; Kolařík, M.; Hýsková, V.; Vaňková, R.; Dobrev, P.; Křížek, T.; Hodek, O.; Čokrtová, K.; et al. Novel Insights into the Effect of Pythium Strains on Rapeseed Metabolism. Microorganisms 2020, 8, 1472. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Barrett, D.M. Determination of Reducing Sugars with 3-Methyl-2-Benzothiazolinonehydrazone. Anal. Biochem. 2002, 305, 287–289. [Google Scholar] [CrossRef]
- Coêlho, D.F.; Saturnino, T.P.; Fernandes, F.F.; Mazzola, P.G.; Silveira, E.; Tambourgi, E.B. Azocasein Substrate for Determination of Proteolytic Activity: Reexamining a Traditional Method Using Bromelain Samples. Biomed. Res. Int. 2016, 2016, 8409183. [Google Scholar] [CrossRef]
- Heussen, C.; Dowdle, E.B. Electrophoretic Analysis of Plasminogen Activators in Polyacrylamide Gels Containing Sodium Dodecyl Sulfate and Copolymerized Substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Leber, T.M.; Balkwill, F.R. Zymography: A Single-Step Staining Method for Quantitation of Proteolytic Activity on Substrate Gels. Anal. Biochem. 1997, 249, 24–28. [Google Scholar] [CrossRef]
- Lazar, I., Sr.; Lazar, I., Jr. GelAnalyzer 19.1. Available online: http://www.gelanalyzer.com/ (accessed on 20 August 2025).
- Maseko, S.T.; Dakora, F.D. Rhizosphere Acid and Alkaline Phosphatase Activity as a Marker of P Nutrition in Nodulated Cyclopia and Aspalathus Species in the Cape Fynbos of South Africa. S. Afr. J. Bot. 2013, 89, 289–295. [Google Scholar] [CrossRef]
- Pawar, V.S.; Bhande, D.; Pawar, S.D.; Mudila, H.; Kaushik, A.; Kumar, A. Investigating Purification and Activity Analysis of Urease Enzyme Extracted from Jack Bean Source: A Green Chemistry Approach. Anal. Biochem. 2022, 659, 114925. [Google Scholar] [CrossRef] [PubMed]
- Hýsková, V.; Jakl, M.; Jaklová Dytrtová, J.; Ćavar Zeljković, S.; Vrobel, O.; Bělonožníková, K.; Kavan, D.; Křížek, T.; Šimonová, A.; Vašková, M.; et al. Triazoles as a Potential Threat to the Nutritional Quality of Tomato Fruits. Metabolites 2023, 13, 988. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 978-0-12-182048-0. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and Antiproliferative Activities of Raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Tupec, M.; Hýsková, V.; Bělonožníková, K.; Hraníček, J.; Červený, V.; Ryšlavá, H. Characterization of Some Potential Medicinal Plants from Central Europe by Their Antioxidant Capacity and the Presence of Metal Elements. Food Biosci. 2017, 20, 43–50. [Google Scholar] [CrossRef]
- Hýsková, V.; Jakl, M.; Jaklová Dytrtová, J.; Ćavar Zeljković, S.; Vrobel, O.; Bělonožníková, K.; Kavan, D.; Křížek, T.; Šimonová, A.; Vašková, M.; et al. Antifungal Triazoles Affect Key Non-Target Metabolic Pathways in Solanum lycopersicum L. Plants. Ecotoxicol. Environ. Saf. 2023, 268, 115729. [Google Scholar] [CrossRef]
- Egbewale, S.O.; Kumar, A.; Mokoena, M.P.; Olaniran, A.O. Purification, Characterization and Three-Dimensional Structure Prediction of Multicopper Oxidase Laccases from Trichoderma lixii FLU1 and Talaromyces pinophilus FLU12. Sci. Rep. 2024, 14, 13371. [Google Scholar] [CrossRef]
- Parre, E.; De Virville, J.; Cochet, F.; Leprince, A.-S.; Richard, L.; Lefebvre-De Vos, D.; Ghars, M.A.; Bordenave, M.; Zachowski, A.; Savouré, A. A New Method for Accurately Measuring Δ1-Pyrroline-5-Carboxylate Synthetase Activity. In Plant Stress Tolerance; Sunkar, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 333–340. ISBN 978-1-60761-701-3. [Google Scholar]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant Biostimulants: A Categorical Review, Their Implications for Row Crop Production, and Relation to Soil Health Indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A. Chemical and Biochemical Properties of Common Nettle (Urtica dioica L.) Depending on Various Nitrogen Fertilization Doses in Crop Production. Sustainability 2025, 17, 6394. [Google Scholar] [CrossRef]
- De Guardia, A.; Petiot, C.; Rogeau, D.; Druilhe, C. Influence of Aeration Rate on Nitrogen Dynamics during Composting. Waste Manag. 2008, 28, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Deppermann, P.; Mühling, K.H. Ammonium Fertilization Enhances Nutrient Uptake, Specifically Manganese and Zinc, and Growth of Maize in Unlimed and Limed Acidic Sandy Soil. Nitrogen 2023, 4, 239–252. [Google Scholar] [CrossRef]
- Marino, D.; Moran, J.F. Can Ammonium Stress Be Positive for Plant Performance? Front. Plant Sci. 2019, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.F.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Mosa, W.F.A. Growth Performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine. Horticulturae 2022, 8, 1110. [Google Scholar] [CrossRef]
- Ye, P.; Li, X.; Cui, B.; Song, S.; Shen, F.; Chen, X.; Wang, G.; Zhou, X.; Deng, Y. Proline Utilization A Controls Bacterial Pathogenicity by Sensing Its Substrate and Cofactors. Commun. Biol. 2022, 5, 496. [Google Scholar] [CrossRef]
- Goswami, G.; Hazarika, D.J.; Chowdhury, N.; Bora, S.S.; Sarmah, U.; Naorem, R.S.; Boro, R.C.; Barooah, M. Proline Confers Acid Stress Tolerance to Bacillus megaterium G18. Sci. Rep. 2022, 12, 8875. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Zhu, W.; Wu, G. Amino Acids in Microbial Metabolism and Function. In Recent Advances in Animal Nutrition and Metabolism; Wu, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2022; Volume 1354, pp. 127–143. ISBN 978-3-030-85685-4. [Google Scholar]
- Henderson, B.C.R.; Sanderson, J.M.; Fowles, A. A review of the foliar application of individual amino acids as biostimulants in plants. Discov. Agric. 2025, 3, 69. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of Plant Growth Promoting Rhizobacteria in Sustainable Production of Vegetables: Current Perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Fusco, G.M.; Burato, A.; Pentangelo, A.; Cardarelli, M.; Nicastro, R.; Carillo, P.; Parisi, M. Can Microbial Consortium Applications Affect Yield and Quality of Conventionally Managed Processing Tomato? Plants 2022, 12, 14. [Google Scholar] [CrossRef]
- Marchut-Mikołajczyk, O.; Chlebicz, M.; Kawecka, M.; Michalak, A.; Prucnal, F.; Nielipinski, M.; Filipek, J.; Jankowska, M.; Perek, Z.; Drożdżyński, P.; et al. Endophytic Bacteria Isolated from Urtica dioica L.- Preliminary Screening for Enzyme and Polyphenols Production. Microb. Cell Fact. 2023, 22, 169. [Google Scholar] [CrossRef]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant Growth Promotion Using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef]
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging Roles of β-Glucanases in Plant Development and Adaptative Responses. Plants 2022, 11, 1119. [Google Scholar] [CrossRef]
- Tamburino, R.; Docimo, T.; Sannino, L.; Gualtieri, L.; Palomba, F.; Valletta, A.; Ruocco, M.; Scotti, N. Enzyme-Based Biostimulants Influence Physiological and Biochemical Responses of Lactuca sativa L. Biomolecules 2023, 13, 1765. [Google Scholar] [CrossRef]
- Cook, S.D. An Historical Review of Phenylacetic Acid. Plant Cell Physiol. 2019, 60, 243–254. [Google Scholar] [CrossRef]
- Syrova, D.S.; Shaposhnikov, A.I.; Yuzikhin, O.S.; Belimov, A.A. Destruction and Transformation of Phytohormones by Microorganisms. Appl. Biochem. Microbiol. 2022, 58, 1–18. [Google Scholar] [CrossRef]
- Gao, J.; Zhuang, S.; Zhang, W. Advances in Plant Auxin Biology: Synthesis, Metabolism, Signaling, Interaction with Other Hormones, and Roles under Abiotic Stress. Plants 2024, 13, 2523. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Selvaraj, P.; Naqvi, N.I. Functional Analysis of Auxin Derived from a Symbiotic Mycobiont. Front. Plant Sci. 2023, 14, 1216680. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; De Paiva Gonçalves, J.; Siqueira, J.A.; Martins, A.O.; Ribeiro, D.M.; Nunes-Nesi, A.; Zsögön, A.; Araújo, W.L. Auxin Metabolism and the Modulation of Plant Growth. Environ. Exp. Bot. 2024, 226, 105917. [Google Scholar] [CrossRef]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP Isopentenyltransferases and tRNA Isopentenyltransferases in Cytokinin Biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J. Interactions of Gibberellins with Phytohormones and Their Role in Stress Responses. Horticulturae 2022, 8, 241. [Google Scholar] [CrossRef]
- Verma, K.; Kumari, K.; Rawat, M.; Devi, K.; Joshi, R. Crosstalk of Jasmonic Acid and Salicylic Acid with Other Phytohormones Alleviates Abiotic and Biotic Stresses in Plants. J. Soil Sci. Plant Nutr. 2025, 25, 4997–5019. [Google Scholar] [CrossRef]
- Gilroy, E.; Breen, S. Interplay between Phytohormone Signalling Pathways in Plant Defence–Other than Salicylic Acid and Jasmonic Acid. Essays Biochem. 2022, 66, 657–671. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Liu, L.; Zhao, L.; Shen, X.; Rao, G.; Li, T. Effects of Exogenous Salicylic Acid and Abscisic Acid on Growth, Photosynthesis and Antioxidant System of Rice. Chil. J. Agric. Res. 2022, 82, 21–32. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance Effect of Plant Growth-Promoting Bacillus Cereus SA1 on Soybean during Heat Stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial Volatiles as Plant Growth Inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef]
- Dos Reis, C.M.; Da Rosa, B.V.; Da Rosa, G.P.; Do Carmo, G.; Morandini, L.M.B.; Ugalde, G.A.; Kuhn, K.R.; Morel, A.F.; Jahn, S.L.; Kuhn, R.C. Antifungal and Antibacterial Activity of Extracts Produced from Diaporthe Schini. J. Biotech. 2019, 294, 30–37. [Google Scholar] [CrossRef]
- Abdelalatif, A.M.; Elwakil, B.H.; Mohamed, M.Z.; Hagar, M.; Olama, Z.A. Fungal Secondary Metabolites/Dicationic Pyridinium Iodide Combinations in Combat against Multi-Drug Resistant Microorganisms. Molecules 2023, 28, 2434. [Google Scholar] [CrossRef]
- Soledad, C.-P.T.; Paola, H.-C.; Carlos Enrique, O.-V.; Israel, R.-L.I.; GuadalupeVirginia, N.-M.; Raúl, Á.-S. Avocado Seeds (Persea americana Cv. Criollo Sp.): Lipophilic Compounds Profile and Biological Activities. Saudi J. Biol. Sci. 2021, 28, 3384–3390. [Google Scholar] [CrossRef]
- Boubakri, H. Induced Resistance to Biotic Stress in Plants by Natural Compounds: Possible Mechanisms. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 79–99. ISBN 978-0-12-817892-8. [Google Scholar]
- Wang, L.; Fan, R.; Ma, H.; Sun, Y.; Huang, Y.; Wang, Y.; Guo, Q.; Ren, X.; Xu, L.; Zhao, J.; et al. Genomic and Metabolomic Insights into the Antimicrobial Compounds and Plant Growth-Promoting Potential of Bacillus velezensis Q-426. BMC Genom. 2023, 24, 589. [Google Scholar] [CrossRef] [PubMed]
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of Bioactive Volatiles by Different Burkholderia ambifaria Strains. J. Chem. Ecol. 2013, 39, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, M.; Tong, Y.; Xia, Z.; Tong, Y.; Sun, Y.; Cao, J.; Zhang, J.; Liu, J.; Zhao, S.; et al. A Review of Volatile Compounds in Edible Macroalgae. Food Res. Int. 2023, 165, 112559. [Google Scholar] [CrossRef]
- Lomans, B.P.; Van Der Drift, C.; Pol, A.; Op Den Camp, H.J.M. Microbial Cycling of Volatile Organic Sulfur Compounds. Cell. Mol. Life Sci. CMLS 2002, 59, 575–588. [Google Scholar] [CrossRef]
- Zoubiri, S.; Baaliouamer, A.; Seba, N.; Chamouni, N. Chemical Composition and Larvicidal Activity of Algerian Foeniculum Vulgare Seed Essential Oil. Arab. J. Chem. 2014, 7, 480–485. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Salvage, R.; Cannon, T.; Kingsmill, P.; Liu, F.; Fleming, C.C. A Complex Biostimulant Based on Plant Flavonoids Enhances Potato Growth and Commercial Yields. Front. Sustain. Food Syst. 2024, 8, 1368423. [Google Scholar] [CrossRef]
- Yu, G.-H.; Kuzyakov, Y. Fenton Chemistry and Reactive Oxygen Species in Soil: Abiotic Mechanisms of Biotic Processes, Controls and Consequences for Carbon and Nutrient Cycling. Earth Sci. Rev. 2021, 214, 103525. [Google Scholar] [CrossRef]
- Bai, Y.; Ali, S.; Liu, S.; Zhou, J.; Tang, Y. Characterization of Plant Laccase Genes and Their Functions. Gene 2023, 852, 147060. [Google Scholar] [CrossRef] [PubMed]
- Eichlerová, I.; Šnajdr, J.; Baldrian, P. Laccase Activity in Soils: Considerations for the Measurement of Enzyme Activity. Chemosphere 2012, 88, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; Broadley, M.R.; White, P.J. Plant Macro- and Micronutrient Minerals. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2016; pp. 1–6. ISBN 978-0-470-01617-6. [Google Scholar]
- Santos-Torres, M.; Romero-Perdomo, F.; Mendoza-Labrador, J.; Gutiérrez, A.Y.; Vargas, C.; Castro-Rincon, E.; Caro-Quintero, A.; Uribe-Velez, D.; Estrada-Bonilla, G.A. Genomic and Phenotypic Analysis of Rock Phosphate-Solubilizing Rhizobacteria. Rhizosphere 2021, 17, 100290. [Google Scholar] [CrossRef]
- Hii, Y.S.; Yen San, C.; Lau, S.W.; Danquah, M.K. Isolation and Characterisation of Phosphate Solubilizing Microorganisms from Peat. Biocatal. Agric. Biotechnol. 2020, 26, 101643. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The Effect of Global Change on Soil Phosphatase Activity. Glob. Change Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary Control of Leaf Element Composition in Plants. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef]
- Tack, F.; Verloo, M. Metal Contents in Stinging Nettle (Urtica dioica L.) as Affected by Soil Characteristics. Sci. Total Environ. 1996, 192, 31–39. [Google Scholar] [CrossRef]
- Gazulla, M.F.; Orduña, M.; Rodrigo, M.; Ventura, M.J. A New Methodology for the Determination of Silicon in Plants by Wavelength Dispersive X-ray Fluorescence. X-Ray Spectrom. 2019, 48, 78–84. [Google Scholar] [CrossRef]
- Garg, K.; Dhar, S.; Jinger, D. Silicon Nutrition in Rice (Oryza sativa L.)—A Review. Ann. Agric. Res. 2020, 41, 221–229. [Google Scholar]
- Savant, N.K.; Datnoff, L.E.; Snyder, G.H. Depletion of Plant-available Silicon in Soils: A Possible Cause of Declining Rice Yields. Commun. Soil Sci. Plant Anal. 1997, 28, 1245–1252. [Google Scholar] [CrossRef]
- Zhao, S.; Huq, M.E.; Fahad, S.; Kamran, M.; Riaz, M. Boron Toxicity in Plants: Understanding Mechanisms and Developing Coping Strategies; a Review. Plant Cell Rep. 2024, 43, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, H.; Wei, Y. Supplemental Silicon and Boron Alleviates Aluminum-Induced Oxidative Damage in Soybean Roots. Plants 2024, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Viktorova, J.; Jandova, Z.; Madlenakova, M.; Prouzova, P.; Bartunek, V.; Vrchotova, B.; Lovecka, P.; Musilova, L.; Macek, T. Native Phytoremediation Potential of Urtica dioica for Removal of PCBs and Heavy Metals Can Be Improved by Genetic Manipulations Using Constitutive CaMV 35S Promoter. PLoS ONE 2016, 11, e0167927. [Google Scholar] [CrossRef] [PubMed]



| Amino Acids [μmol·g (D.W.)−1] | Spring | Spring/Summer | Summer | Autumn |
|---|---|---|---|---|
| Ala | 56.1 ± 0.6 b | 13.3 ± 0.1 a | 9.0 ± 0.2 a | 11.2 ± 1.4 a |
| Arg | <LOQ | 4.4 ± 0.3 b | 1.2 ± 0.1 a | 1.3 ± 0.1 a |
| Asn | <LOQ | <LOQ | <LOQ | <LOQ |
| Asp | 2.3 ± 0.7 a | 5.9 ± 0.1 b | 2.2 ± 0.2 a | 3.3 ± 0.7 a |
| Cys | <LOQ | 2.7 ± 0.2 b | <LOQ | 1.5 ± 0.1 a |
| Gln | 3.8 ± 0.3 b | 1.0 ± 0.2 a | 1.6 ± 0.1a | 0.8 ± 0.0 a |
| Glu | 16.3 ± 1.6 b | 8.0 ± 0.2 a | 6.2 ± 0.3 a | 4.3 ± 0.6 a |
| Gly | 28.0 ± 0.04 b | 2.5 ± 0.2 a | 2.8 ± 0.1 a | 2.0 ± 0.3 a |
| His | 2.4 ± 0.3 b | 0.8 ± 0.02 a | <LOQ | <LOQ |
| Ile | 15.1 ± 1.7 c | 5.2 ± 0.3 b | 0.8 ± 0.1 a | 3.4 ± 0.7 b |
| Leu | 28.5 ± 2.4 d | 14.6 ± 0.6 c | 2.4 ± 0.1 a | 9.0 ± 1.1 b |
| Lys | 10 ± 0 c | 5.6 ± 0.2 b | 1.8 ± 0.1 a | 1.4 ± 0.2 a |
| Met | 8.5±0.1 c | 3.3 ± 0.1 b | <LOQ | 2.1 ± 0.3 a |
| Phe | 12.6 ± 0.2 c | 4.5 ± 0.2 a | 1.0 ± 0.1 a | 5.1 ± 0.8 b |
| Pro | <LOQ | <LOQ | <LOQ | <LOQ |
| Ser | 5.0 ± 2.7 b | 3.2 ± 0.1 b | 0.7 ± 0.1 a | 2.6 ± 0.5 b |
| Thr | 8.4 ± 2.7 b | 3.9 ± 0.2 a | 1.1 ± 0.1 a | 2.4 ± 0.4 a |
| Trp | 3.2 ± 0.0 b | 0.8 ± 0.1 a | <LOQ | 0.8 ± 0.1 a |
| Tyr | 8.7 ± 0.1 c | 3.1 ± 0.1 b | 1.6 ± 0.1 a | 3.1 ± 0.4 b |
| Val | 22.3 ± 0.8 c | 6.3 ± 0.4 b | 2.1 ± 0.1 a | 3.7 ± 0.8 a |
| Phytohormones | Spring | Spring/Summer | Summer | Autumn |
|---|---|---|---|---|
| IAA [nmol·g(D.W.)−1] | 175.69 ± 30.77 a | 371.94 ± 54.08 a | 4797.34 ± 272.07 b | 315.39 ± 104.41 a |
| PAA [nmol·g(D.W.)−1] | 4095.19 ± 511 a | 8542.34 ± 474.07 b,c | 9233.88 ± 41.9 c | 6962.94 ± 870.1 b |
| OxIAA [nmol·g(D.W.)−1] | 26.82 ± 8.42 a | 307.02 ± 87.82 b | 63.34 ± 36.17 a | 20.22 ± 6.85 a |
| cZ [pmol·g(D.W.)−1] | 145.23 ± 4.54 c | 24.35 ± 10.7 a | 74.76 ± 0.58 b | 38.7 ± 13.15 a |
| DZ and iP [pmol·g(D.W.)−1] | 154.81 ± 11.89 c | 103.86 ± 3.59 b | 158.71 ± 7.36 c | 50.33 ± 11.75 a |
| Active GAs [pmol·g(D.W.)−1] | 1299.06 ± 21.38 a | 1156.62 ± 16.49 a | 1125.43 ± 26.84 a | 1882.2 ± 108.53 b |
| ABA [pmol·g(D.W.)−1] | 20.94 ± 10.87 a | 44.61 ± 0.27 a | 78.3 ± 17.22 a | 61.2 ± 16.49 a |
| JA [nmol·g(D.W.)−1] | 20.71 ± 7.87 a | 19.26 ± 4.5 a | 25.93 ± 7.03 a | 20.65 ± 6.77 a |
| JA-Ile [nmol·g(D.W.)−1] | 17.26 ± 5.42 a | 10.33 ± 1.6 a | 7.83 ± 1.5 a | 7.19 ± 1.41 a |
| SA [nmol·g(D.W.)−1] | 8.84 ± 0.1 a | 4.91 ± 0.31 a | 76.75 ± 2.69 a | 32.51 ± 10.9 b |
| Nutrient | Spring | Spring/Summer | Summer | Autumn |
|---|---|---|---|---|
| K [mg·g(D.W.)−1] | 93.7 ± 3.9 d | 72.9 ± 1.3 c | 59.7 ± 2.2 b | 32.4 ± 1.7 a |
| Ca [mg·g(D.W.)−1] | 55.9 ± 3.2 a | 50.8 ± 1.0 a | 65.6 ± 2.8 a | 87.8 ± 5 b |
| Mg [mg·g(D.W.)−1] | 8 ± 0.4 b | 7 ± 0.9 a,b | 5.5 ± 0.7 a | 6.3 ± 0.4 a,b |
| S [mg·g(D.W.)−1] | 2.8 ± 0.4 a | 5.1 ± 0.4 a | 3.5 ± 0.4 a | 4.5 ± 1.3 a |
| Phosphates [mg·g(D.W.)−1] | 30.7 ± 0.5 a | 64.8 ± 3.2 a | 479.8 ± 33.9 b | 91.4 ± 2.9 a |
| Na [µg·g(D.W.)−1] | 2633 ± 129 c | 1811 ± 222 b | 1464 ± 187 b | 805 ± 52 a |
| Si [µg·g(D.W.)−1] | 8959 ± 811 b | 5384 ± 229 a | 4482 ± 252 a | 5442 ± 599 a |
| Fe [µg·g(D.W.)−1] | 1647 ± 144 c | 1293 ± 454 b | 754 ± 101 a | 1347 ± 338 b |
| B [µg·g(D.W.)−1] | 562 ± 53 a | 1713 ± 1268 b | 1770 ± 558 b | 1429 ± 383 b |
| Zn [µg·g(D.W.)−1] | 302 ± 26 c | 103 ± 9 b | 12 ± 4 a | 501 ± 82 d |
| Mn [µg·g(D.W.)−1] | 273 ± 9 b | 307 ± 6 c | 218 ± 5 a | 351 ± 40 d |
| Cu [µg·g(D.W.)−1] | 161 ± 5 d | 108 ± 16 b | 48 ± 2 a | 142 ± 18 c |
| Mo [µg·g(D.W.)−1] | 58 ± 4 b | 51 ± 7 b | 32 ± 1 a | 53 ± 6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praženicová, R.; Larkov, A.; Hanzelková, K.; Korban, A.; Křížek, T.; Hýsková, V.; Ječmen, T.; Hraníček, J.; Vlčková, D.; Gaudinová, A.; et al. Fermented Nettles: Bioactive Profile and Seasonal Variability. Nitrogen 2025, 6, 109. https://doi.org/10.3390/nitrogen6040109
Praženicová R, Larkov A, Hanzelková K, Korban A, Křížek T, Hýsková V, Ječmen T, Hraníček J, Vlčková D, Gaudinová A, et al. Fermented Nettles: Bioactive Profile and Seasonal Variability. Nitrogen. 2025; 6(4):109. https://doi.org/10.3390/nitrogen6040109
Chicago/Turabian StylePraženicová, Romana, Andrei Larkov, Kateřina Hanzelková, Anton Korban, Tomáš Křížek, Veronika Hýsková, Tomáš Ječmen, Jakub Hraníček, Denisa Vlčková, Alena Gaudinová, and et al. 2025. "Fermented Nettles: Bioactive Profile and Seasonal Variability" Nitrogen 6, no. 4: 109. https://doi.org/10.3390/nitrogen6040109
APA StylePraženicová, R., Larkov, A., Hanzelková, K., Korban, A., Křížek, T., Hýsková, V., Ječmen, T., Hraníček, J., Vlčková, D., Gaudinová, A., Dobrev, P., Vanková, R., Ryšlavá, H., & Bělonožníková, K. (2025). Fermented Nettles: Bioactive Profile and Seasonal Variability. Nitrogen, 6(4), 109. https://doi.org/10.3390/nitrogen6040109







