Exploring Dry Salmon Sludge as an Organic Nitrogen Source for Hazelnut (Corylus avellana L.) Orchard
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Material
2.3. Treatments
2.4. Physiological Parameters
2.5. Soil Physicochemical and Biochemical Parameters
2.6. Statistical Analysis
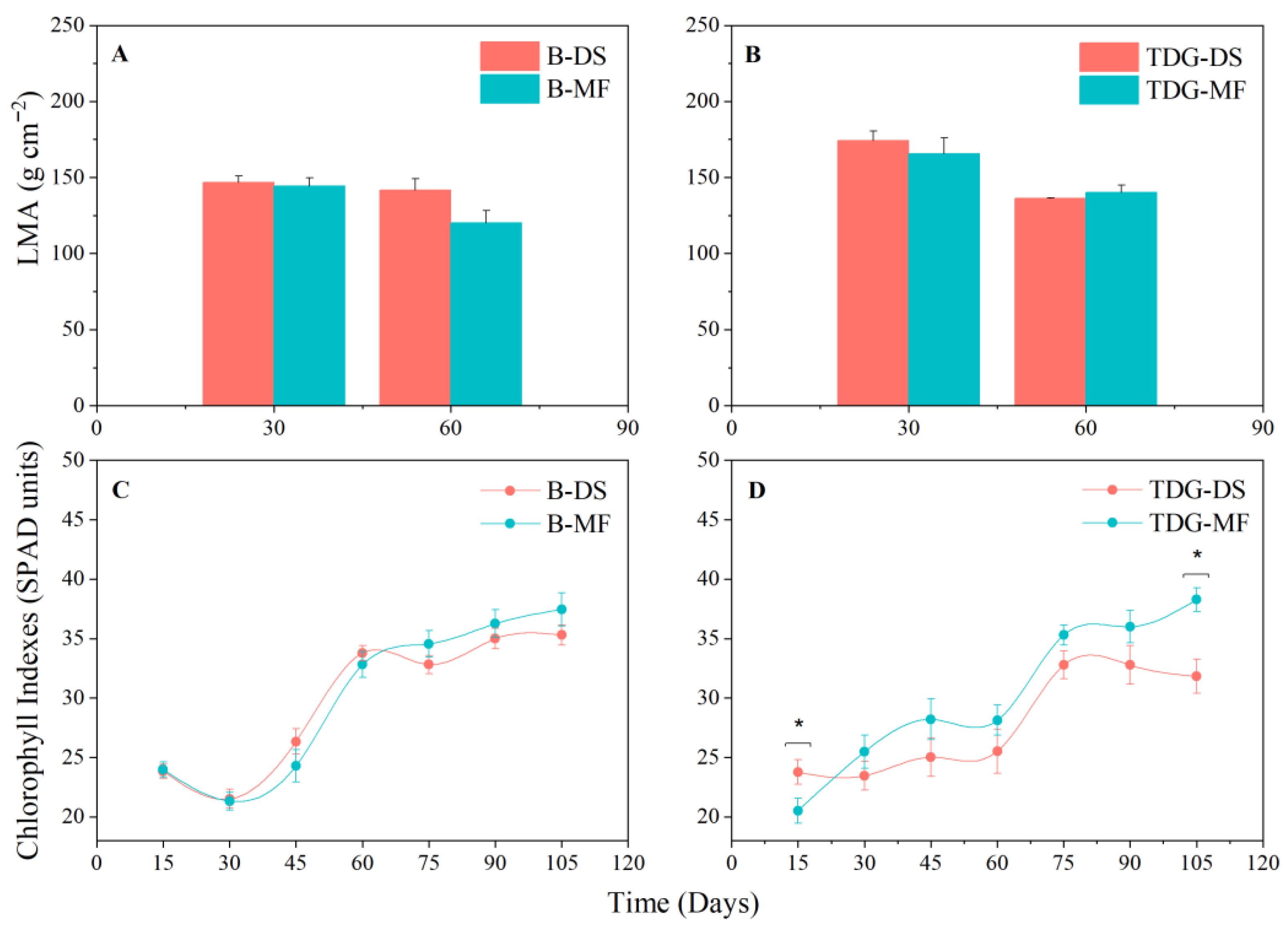
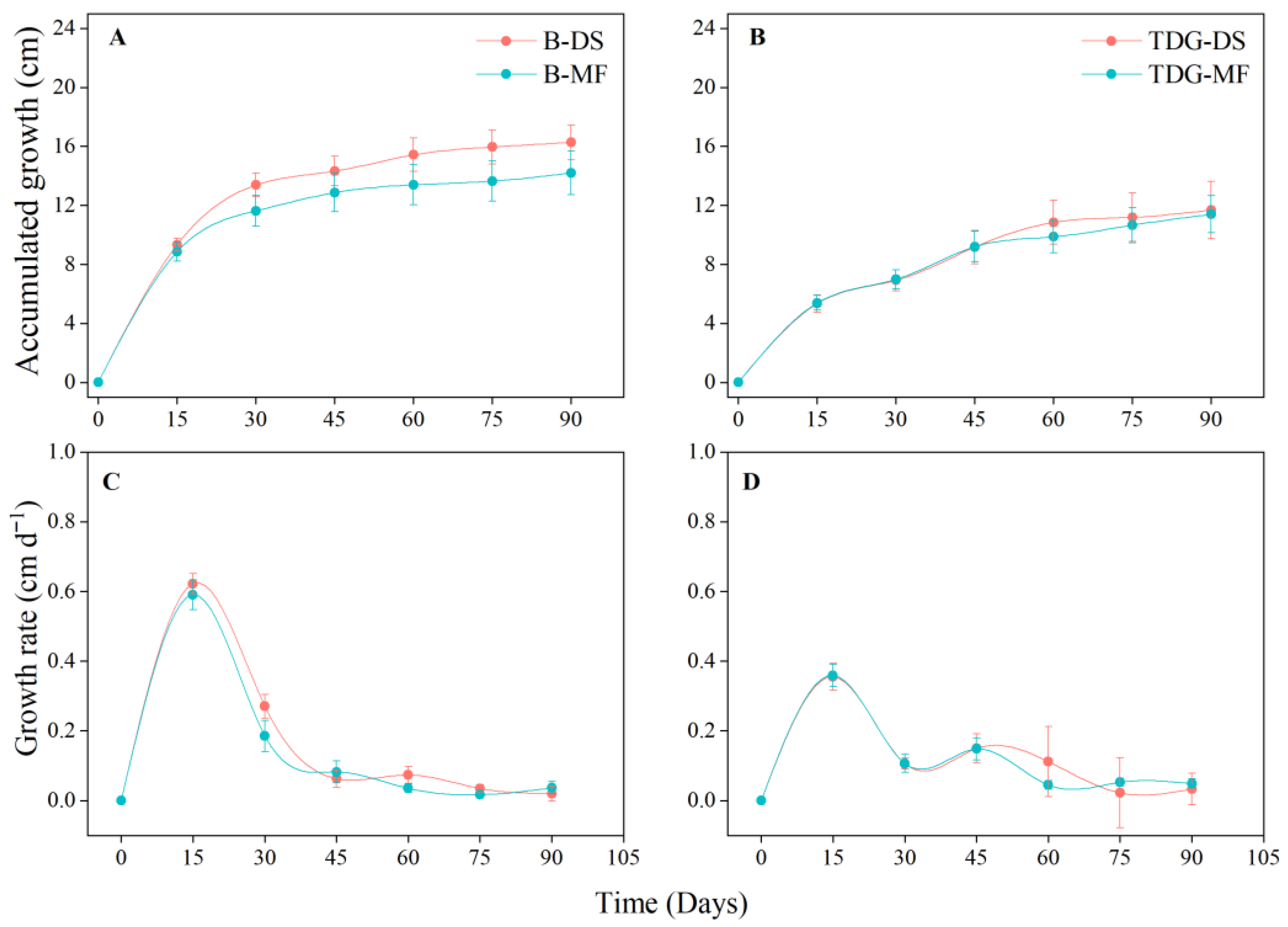
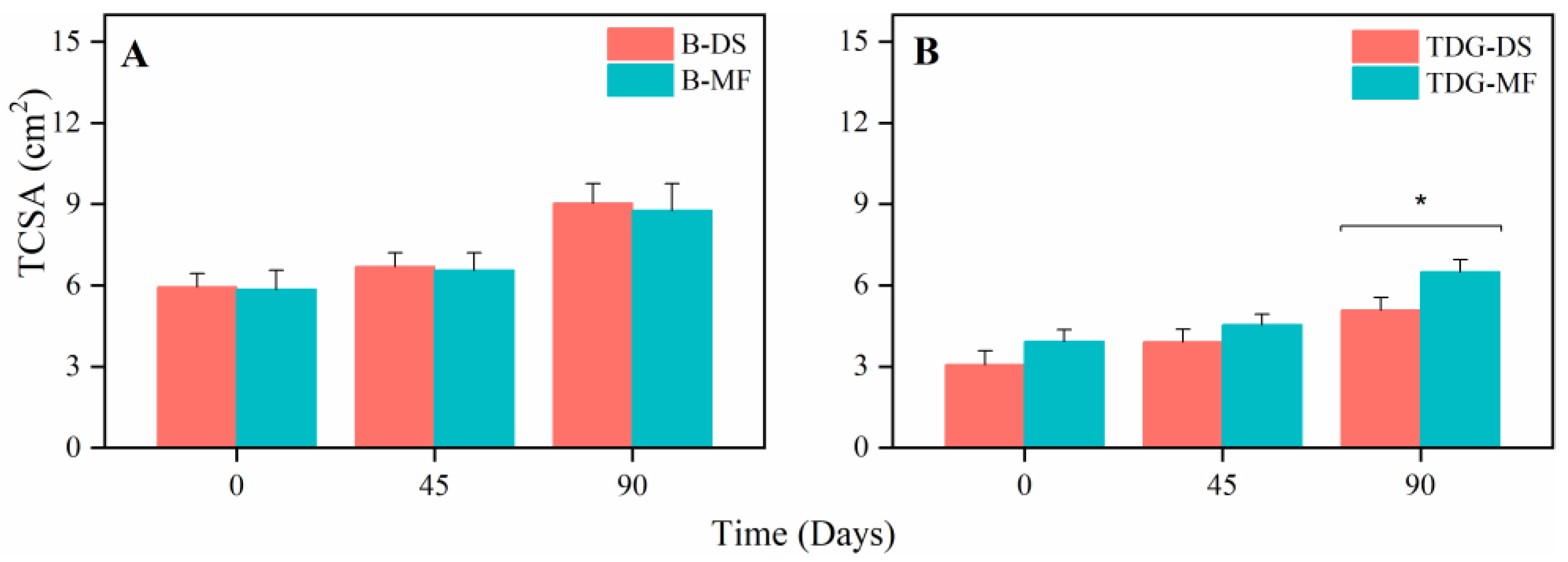
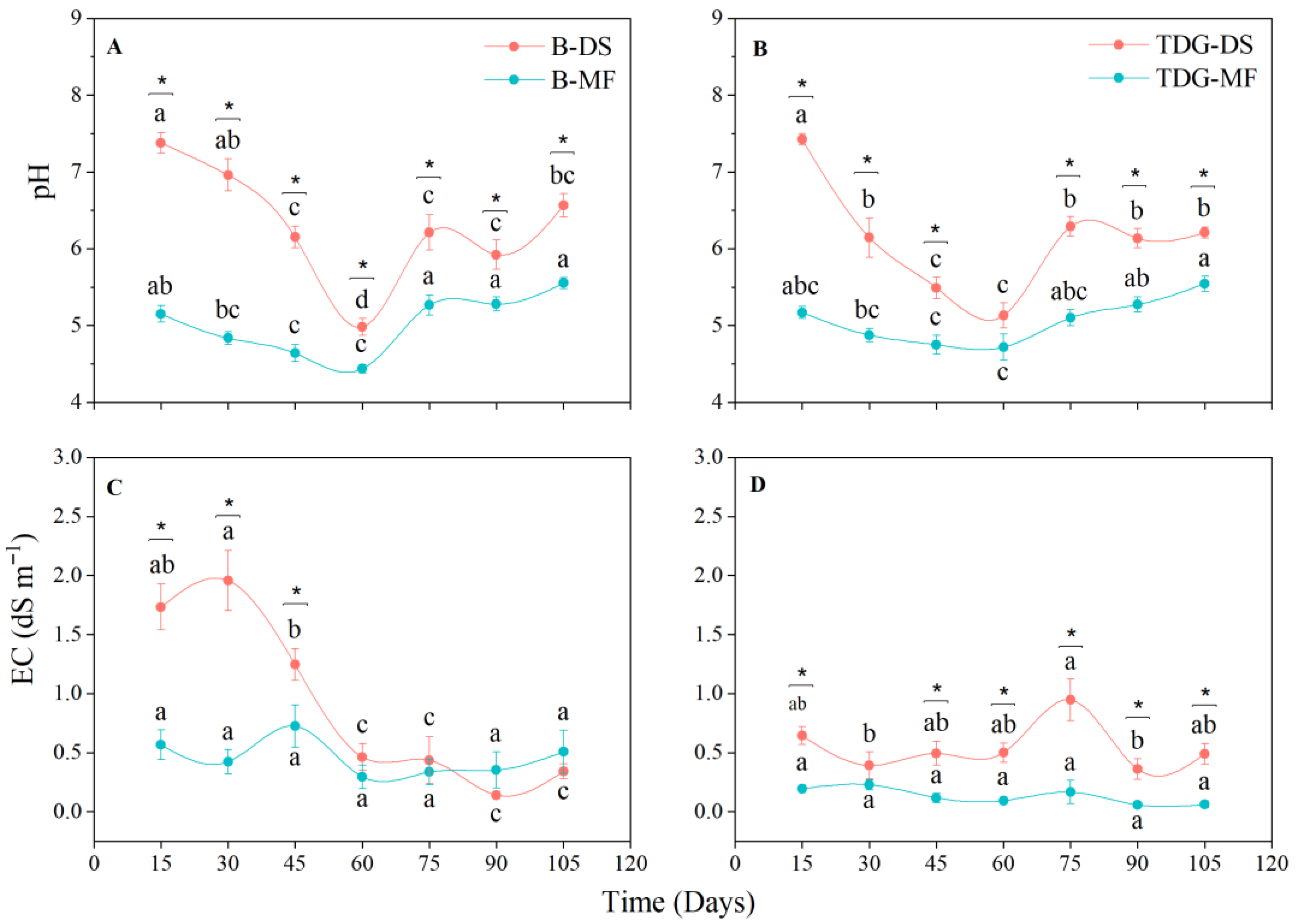
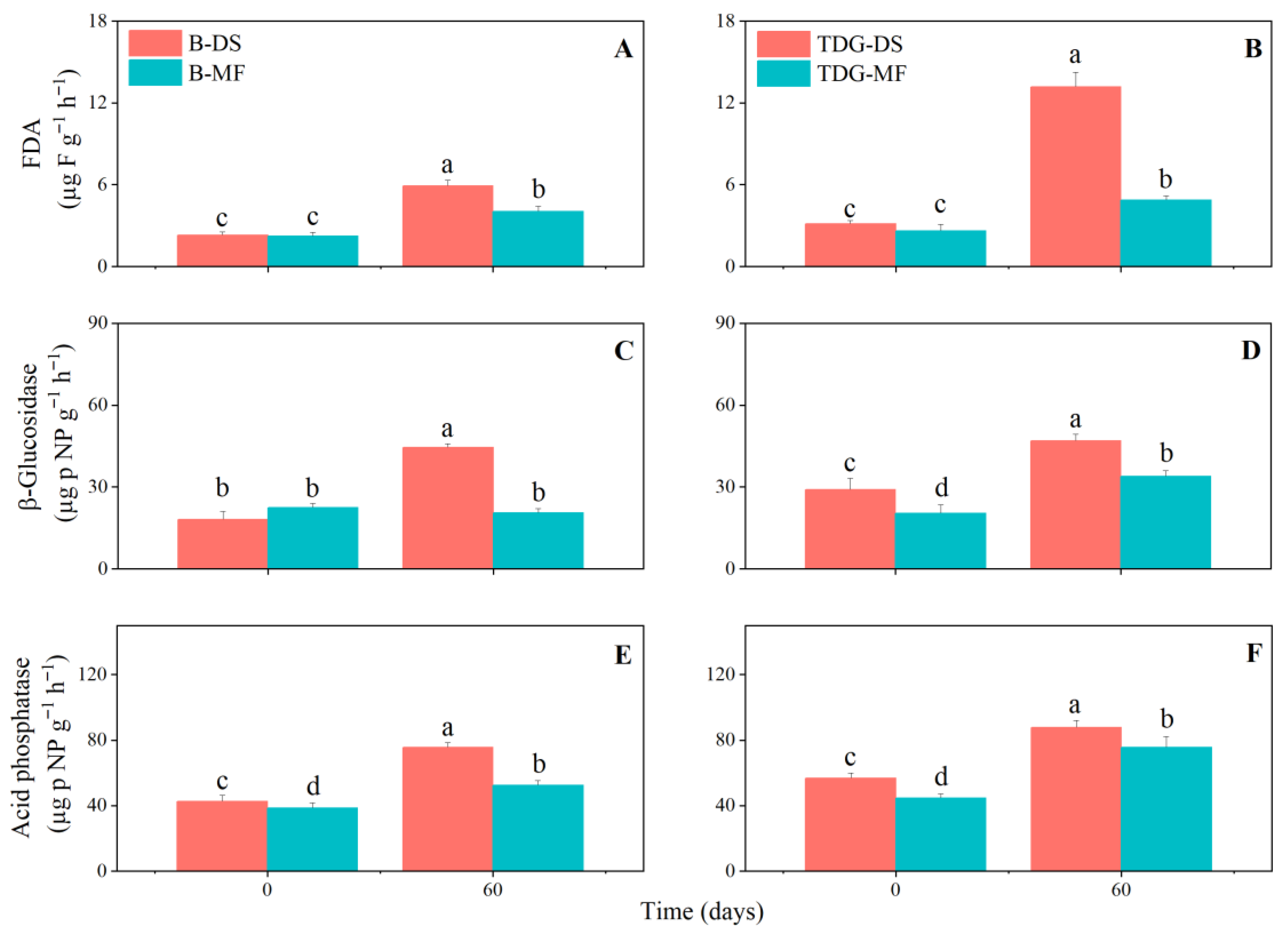
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation: Rome, Italy, 2022; ISBN 9789251363645. [Google Scholar]
- Bohnes, F.A.; Hauschild, M.Z.; Schlundt, J.; Nielsen, M.; Laurent, A. Environmental Sustainability of Future Aquaculture Production: Analysis of Singaporean and Norwegian Policies. Aquaculture 2022, 549, 737717. [Google Scholar] [CrossRef]
- Choudhury, A.; Lepine, C.; Good, C. Methane and Hydrogen Sulfide Production from the Anaerobic Digestion of Fish Sludge from Recirculating Aquaculture Systems: Effect of Varying Initial Solid Concentrations. Fermentation 2023, 9, 94. [Google Scholar] [CrossRef]
- Nenciu, F.; Voicea, I.; Cocarta, D.M.; Vladut, V.N.; Matache, M.G.; Arsenoaia, V.N. “Zero-Waste” Food Production System Supporting the Synergic Interaction between Aquaculture and Horticulture. Sustainability 2022, 14, 13396. [Google Scholar] [CrossRef]
- Brod, E.; Øgaard, A.F. Closing Global P Cycles: The Effect of Dewatered Fish Sludge and Manure Solids as P Fertiliser. Waste Manag. 2021, 135, 190–198. [Google Scholar] [CrossRef]
- Hepp, C. Resultados Preliminares Sobre El Uso de Lodos de Pisciculturas Sobre Suelos Agropecuarios de Origen Volcánico de La Patagonia Occidental (Aysén). Inst. Investig. Agropecu. Cent. Investig. INIA Tamel Aike 2012, 82, 223. [Google Scholar]
- Uzundumlu, A.S.; Kurtoğlu, S.; Şerefoğlu, C.; Algur, Z. The Role of Turkey in the World Hazelnut Production and Exporting. Emir. J. Food Agric. 2022, 34, 117–127. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 October 2025).
- FIA. Resultados y Lecciones Aprendidas. In Resultados y Lecciones en Mejoramiento de Competitividad del Cultivo de Avellano Europeo: Proyecto de Innovación en Región de La Araucanía; Salinas, M., Casanova, G., Feuerhake, G., Eds.; Fundación para la Innovación Agraria (FIA), Ministerio de Agricultura: Santiago, Chile, 2021; pp. 11–41. [Google Scholar]
- Ellena, M. Historia, Origen, Distribución, Geográfica, Taxonomía y Características Botánicas del Avellano Europeo; Colección Libros INIA—Instituto de Investigaciones Agropecuarias: Temuco, Chile, 2018; p. 44. [Google Scholar]
- Issock Issock, P.B.; Mpinganjira, M.; Roberts-Lombard, M. Beyond Sustainable Consumption Practices: Linking Organic Food Consumption to Hedonic and Eudaimonic Well-Being. Appetite 2023, 188, 106633. [Google Scholar] [CrossRef]
- Pérez-San Martín, A.; Tortosa, G.; González, A.; Cayunao, S.; Curaqueo, G. Drying Treatment for Sludges of the Chilean Salmon Farming Industry and Its Potential as an Agricultural Soil Amendment. Arch. Agron. Soil Sci. 2024, 70, 1–18. [Google Scholar] [CrossRef]
- Orrego-Verdugo, R.; Abarca-Del-rio, R.; Lara-Uribe, C. Spatial Dynamics and Consistency of Agroclimatic Trends in Chile during 1985-2015 to the Köppen-Geiger Climate Classification. Chil. J. Agric. Res. 2021, 81, 618–629. [Google Scholar] [CrossRef]
- USDA, Natural Resources Conservation Service. Soil Survey Staff. In Key to Soil Taxonomy, 8th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 1998. [Google Scholar]
- CIREN. Descripciones de Suelos y Materiales y Simbolos. Estudio Agrologico IX Región; Publicación CIREN N° 122; CIREN: Santiago, Chile, 2002; 338p. [Google Scholar]
- Jorquera-Fontena, E.; Pastenes, C.; Meriño-Gergichevich, C.; Franck, N. Effect of Source/Sink Ratio on Leaf and Fruit Traits of Blueberry Fruiting Canes in the Field. Sci. Hortic. 2018, 241, 51–56. [Google Scholar] [CrossRef]
- O’Neal, M.E.; Landis, D.A.; Isaacs, R. An Inexpensive, Accurate Method for Measuring Leaf Area and Defoliation Through Digital Image Analysis. J. Econ. Entomol. 2002, 95, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.; Peña-Fleitas, M.T.; Thompson, R.B.; Gallardo, M.; Grasso, R.; Padilla, F.M. The Use of Chlorophyll Meters to Assess Crop N Status and Derivation of Sufficiency Values for Sweet Pepper. Sensors 2019, 19, 2949. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, A.; Venturi, M.; Gutiérrez-gordillo, S.; Manfrini, L.; Corelli-grappadelli, L.; Morandi, B. Vascular and Transpiration Flows Affecting Apricot (Prunus armeniaca L.) Fruit Growth. Agronomy 2022, 12, 989. [Google Scholar] [CrossRef]
- Torres, A.; Héctor, E.; Hernández, G.; Cué, J.; Fosado, O. Efectos Del BIOSTAN® En Los Índices de Crecimiento y Los Pigmentos Fotosintéticos de Phaseolus vulgaris L. Técnica Rev. Agrocienc. 2017, 18, 25–35, ISSN 2477-8982. [Google Scholar] [CrossRef]
- Alvear, M.; Reyes, F.; Morales, A.; Arriagada, C.; Reyes, M. Actividad Biológica y Agregados Estables Al Agua En Dos Tipos de Formaciones Vegetales de Un Bosque Templado Del Centro- Sur de Chile Con Perturbación Antrópica. Ecol. Austral. 2007, 17, 113–122. [Google Scholar]
- Yap, B.W.; Sim, C.H. Comparisons of Various Types of Normality Tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The Impact of Levene’s Test of Equality of Variances on Statistical Theory and Practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Nikipelova, O.; Pyliak, N.; Yarochevsky, V.; Rucins, A.; Bulgakov, V. Study of the Influence of Different Organic Fertilizers on Soil Agrochemical Parameters in Hazelnut Plantations in Ukrainian Southern Steppe. Agron. Res. 2024, 22, 1241–1253. [Google Scholar] [CrossRef]
- Catoni, R.; Bracco, F.; Gratani, L.; Granata, M.U. Physiological, Morphological and Anatomical Leaf Traits Variation across Leaf Development in Corylus Avellana. Mediterr. Bot. 2019, 40, 185–192. [Google Scholar] [CrossRef]
- Rovira, M.; Hermoso, J.F.; Rufat, J.; Cristofori, V.; Silvestri, C.; Romero, A. Agronomical and Physiological Behavior of Spanish Hazelnut Selection “Negret-N9” Grafted on Non-Suckering Rootstocks. Front. Plant Sci. 2022, 12, 813902. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Ji, C.; Xie, L.; Liu, Q.; Zhang, Z. Dynamic Simulation of the Leaf Mass per Area (LMA) in Multilayer Crowns of Young Larix Principis-Rupprechtii. Plants 2024, 13, 1223. [Google Scholar] [CrossRef]
- De La Riva, E.G.; Olmo, M.; Poorter, H.; Ubera, J.L.; Villar, R. Leaf Mass per Area (LMA) and Its Relationship with Leaf Structure and Anatomy in 34 Mediterranean Woody Species along a Water Availability Gradient. PLoS ONE 2016, 11, e0148788. [Google Scholar] [CrossRef]
- Bostan, S.Z.; Karakaya, O. Morphological, Chemical, and Molecular Characterization of a New Late-Leafing and High Fruit Quality Hazelnut (Corylus avellana L.) Genotype. Genet. Resour. Crop Evol. 2024, 71, 5113–5126. [Google Scholar] [CrossRef]
- Fiorentini, M.; Zenobi, S.; Giorgini, E.; Basili, D.; Conti, C.; Pro, C.; Monaci, E.; Id, R.O. Nitrogen and Chlorophyll Status Determination in Durum Wheat as Influenced by Fertilization and Soil Management: Preliminary Results. PLoS ONE 2019, 14, e0225126. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.C.; Gillman, J.H.; Hoover, E.E.; Russelle, M.P. Nitrogen Fertilization for Young Established Hybrid Hazelnuts in the Upper Midwest of the United States of America. Can. J. Plant Sci. 2011, 91, 907–918. [Google Scholar] [CrossRef]
- Picariello, E.; Pucci, L.; Carotenuto, M.; Libralato, G.; Lofrano, G.; Baldantoni, D. Compost and Sewage Sludge for the Improvement of Soil Chemical and Biological Quality of Mediterranean Agroecosystems. Sustainability 2020, 13, 26. [Google Scholar] [CrossRef]
- Valarini, P.J.; Curaqueo, G.; Seguel, A.; Manzano, K.; Rubio, R.; Cornejo, P.; Borie, F. Effect of Compost Application on Some Properties of a Volcanic Soil from Central South Chile. Chil. J. Agric. Res. 2009, 69, 416–425. [Google Scholar] [CrossRef]
- Hardin, J.A.; Smith, M.W.; Weckler, P.R.; Cheary, B.S. In Situ Measurement of Pecan Leaf Nitrogen Concentration Using a Chlorophyll Meter and Vis-near Infrared Multispectral Camera. HortScience 2012, 47, 955–960. [Google Scholar] [CrossRef]
- Hernández, S.; Pérez, J.; Masaguer, A.; Eymar, E. Caracterización Físico-Química de Residuos Orgánicos Compostados, Evaluación de Su Potencial Nutritivo y Aprovechamiento Agrícola. III Jorn. Grupo Fertil. SECH 2009, 56, 165–171. [Google Scholar]
- Silvestri, C.; Caceres, M.E.; Ceccarelli, M.; Pica, A.L.; Rugini, E.; Cristofori, V. Influence of Continuous Spectrum Light on Morphological Traits and Leaf Anatomy of Hazelnut Plantlets. Front. Plant Sci. 2019, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Grau, P. Avellano Europeo, Manual de Plantación y Manejo; Boletin INIAN° 108; Instituto de Investigaciones Agropecuarias: Chillán, Chile, 2003; 90p. [Google Scholar]
- Ellena, M.; González, A.; Sandoval, P.; Marchant, F. The Effects of Single-Axis and Multi-Axis Training Systems on Cumulative Yield and Nut Quality of ‘Barcelona’ and ‘Tonda Di Giffoni’ in Two Different Agroecological Zones in Southern Chile. Acta Hortic. 2018, 1226, 255–260. [Google Scholar] [CrossRef]
- Nikipelova, O.; Pyliak, N.; Hodorchuk, V. Organic Fertilizers in Increase of Hazelnut Yield. Phytosanit. Saf. 2023, 69, 118–128. [Google Scholar] [CrossRef]
- Du, T.; He, H.; Zhang, Q.; Lu, L.; Mao, W.; Zhai, M. Positive Effects of Organic Fertilizers and Biofertilizers on Soil Microbial Community Composition and Walnut Yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar] [CrossRef]
- Naserian, E.S.; Cheraghi, M.; Lorestani, B.; Sobhanardakani, S.; Sadr, M.K. Qualitative Investigation of Sewage Sludge Composting: Effect of Aerobic/Anaerobic Pretreatments. Arab. J. Geosci. 2021, 14, 836. [Google Scholar] [CrossRef]
- Cardoso, P.H.S.; Gonçalves, P.W.B.; Alves, G.d.O.; Pegoraro, R.F.; Fernandes, L.A.; Frazão, L.A.; Sampaio, R.A. Improving the Quality of Organic Compost of Sewage Sludge Using Grass Cultivation Followed by Composting. J. Environ. Manag. 2022, 314, 115076. [Google Scholar] [CrossRef]
- Pérez, A. Mineralización de Nitrógeno en un Suelo Franco Arcilloso de la Región Metropolitana, Tratado Con Biosólidos Urbanos; Universidad de Chile: Santiago, Chile, 2012. [Google Scholar]
- Wong, J.W.C.; Lai, K.M.; Fang, M.; Ma, K.K. Effect of Sewage Sludge Amendment on Soil Microbial Activity and Nutrient Mineralization. Environ. Int. 1998, 24, 935–943. [Google Scholar] [CrossRef]
- De Soto, I.S.; Zamanian, K.; Urmeneta, H.; Enrique, A.; Virto, I. 25 Years of Continuous Sewage Sludge Application vs. Mineral Fertilizers on a Calcareous Soil Affected PH but Not Soil Carbonates. Front. Soil Sci. 2022, 2, 960426. [Google Scholar] [CrossRef]
- Del Pino, A.; Repetto, C.; Mori, C. Patrones de Descomposición de Estiércoles En el Suelo. Terra Latinoam. 2008, 26, 43–52. [Google Scholar]
- Ayiti, O.E.; Babalola, O.O. Factors Influencing Soil Nitrification Process and the Effect on Environment and Health. Front. Sustain. Food Syst. 2022, 6, 821994. [Google Scholar] [CrossRef]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within Composting: A Review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, M.; Dutta, S.; Sahu, N.C. Effect of Application of Lime with Vermicompost on the Activities of Microorganisms of Some Acidic Soils of West Bengal. J. Environ. Biol. 2023, 44, 299–309. [Google Scholar] [CrossRef]
- Dal Molin, S.J.; Ernani, P.R.; Gerber, J.M. Soil Acidification and Nitrogen Release Following Application of Nitrogen Fertilizers. Commun. Soil Sci. Plant Anal. 2020, 51, 2551–2558. [Google Scholar] [CrossRef]
- Merl, T.; Sedlacek, C.J.; Pjevac, P.; Fuchslueger, L.; Sandén, T.; Spiegel, H.; Koren, K.; Giguere, A.T. Visualizing Small-Scale Subsurface NH3 and PH Dynamics Surrounding Nitrogen Fertilizer Granules and Impacts on Nitrification Activity. Soil Biol. Biochem. 2024, 189, 109273. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen Transformation during Organic Waste Composting by the Rutgers System and Its Effects on PH, EC and Maturity of the Composting Mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Disale, A.S.; Undre, P.B.; Alameen, A.S.; Khirade, P.W. Influence of Soil Chemical Composition on Electrical Conductivity. Appl. Innov. Res. 2020, 2, 156–161. [Google Scholar]
- VanderGheynst, J.S.; Pettygrove, S.; Dooley, T.M.; Arnold, K.A. Estimating Electrical Conductivity of Compost Extracts At Different Extraction Ratios. Compost. Sci. Util. 2004, 12, 202–207. [Google Scholar] [CrossRef]
- Xiao, K.; Li, D.; Wen, L.; Yang, L.; Luo, P.; Chen, H.; Wang, K. Dynamics of Soil Nitrogen Availability during Post-Agricultural Succession in a Karst Region, Southwest China. Geoderma 2018, 314, 184–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Šimůnek, J.; Shi, H.; Chen, N.; Hu, Q.; Tian, T. Evaluating Soil Salt Dynamics in a Field Drip-Irrigated with Brackish Water and Leached with Freshwater during Different Crop Growth Stages. Agric. Water Manag. 2021, 244, 106601. [Google Scholar] [CrossRef]
- Delgado, M.D.M.; Mendoza, K.; González, M.; Tadeo, J.; Martín, J. Assessment of the Composting Process of Poultry Manure Using Different Mixtures of Substrates. Rev. Int. Contam. Ambient. 2019, 35, 965–977. [Google Scholar] [CrossRef]
- Ayed, F.; Boussadia, O.; Grissa, H.; Aydi Ben Abdallah, R.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Assessment of Physico-Chemical, Microbial and Phytotoxic Changes of Various Organic Wastes During Their Composting Process. J. Environ. Agric. Stud. 2021, 2, 21–35. [Google Scholar] [CrossRef]
- Maier, R.M.; Gentry, T.J. Physiological Methods; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780123946263. [Google Scholar]
- Sánchez-Monedero, M.A.; Mondini, C.; Cayuela, M.L.; Roig, A.; Contin, M.; De Nobili, M. Fluorescein Diacetate Hydrolysis, Respiration and Microbial Biomass in Freshly Amended Soils. Biol. Fertil. Soils 2008, 44, 885–890. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for Fluorescein Diacetate Hydrolytic Activity: Optimization for Soil Samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Ye, Q.; Peng, Z.; Zhu, S.; Chen, H.; Liu, D.; Li, Y.; Deng, L.; Shu, X.; et al. Positive Effects of Organic Amendments on Soil Microbes and Their Functionality in Agro-Ecosystems. Plants 2023, 12, 3790. [Google Scholar] [CrossRef]
- Elbl, J.; Maková, J.; Javoreková, S.; Medo, J.; Kintl, A.; Lošák, T.; Lukas, V. Response of Microbial Activities in Soil to Various Organic and Mineral Amendments as an Indicator of Soil Quality. Agronomy 2019, 9, 485. [Google Scholar] [CrossRef]
- Maurya, S.; Abraham, J.S.; Somasundaram, S.; Toteja, R.; Gupta, R.; Makhija, S. Indicators for Assessment of Soil Quality: A Mini-Review. Environ. Monit. Assess. 2020, 192, 604. [Google Scholar] [CrossRef]
- Tao, K.; Tian, H.; Fan, J.; Li, D.; Liu, C.; Megharaj, M.; Li, H.; Hu, M.; Jia, H.; He, W. Kinetics and Catalytic Efficiency of Soil Fluorescein Diacetate Hydrolase under the Pesticide Parathion Stress. Sci. Total Environ. 2021, 771, 144835. [Google Scholar] [CrossRef]
- Kremer, R.J. The Solar Corridor Crop System; Chapter 4—Soil Health Benefits of the Solar Corridor Crop System; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128147931. [Google Scholar]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil Labile Organic Carbon Fractions and Soil Enzyme Activities after 10 Years of Continuous Fertilization and Wheat Residue Incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- Ferraz De Almeida, R.; Naves, E.R.; Pinheiro, R.; Mota, D. Soil Quality: Enzymatic Activity of Soil β-Glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–150. [Google Scholar]
- Li, N.; Wang, Z.; Tian, H.; Megharaj, M.; He, W. Ecotoxicity of Soil Pb Pollution Reflected by Soil β-Glucosidase: Comparison of Extracellular and Intracellular Enzyme Pool. Sci. Total Environ. 2023, 882, 163364. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The Biological Activities of β-Glucosidase, Phosphatase and Urease as Soil Quality Indicators: A Review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of Soil Enzymes in Sustainable Crop Production; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128132807. [Google Scholar]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The Effect of Global Change on Soil Phosphatase Activity. Glob. Change Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, E.K. Assessment of Gross and Net Mineralization Rates of Soil Organic Phosphorus—A Review. Soil Biol. Biochem. 2015, 89, 82–98. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, J.; Chen, H.; Wang, H.; Mo, J. Responses of Soil Acid Phosphatase and Beta-Glucosidase to Nitrogen and Phosphorus Addition in Two Subtropical Forests in Southern China. Eur. J. Soil Biol. 2015, 68, 77–84. [Google Scholar] [CrossRef]
- Janes-Bassett, V.; Blackwell, M.S.A.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A Meta-Analysis of Phosphatase Activity in Agricultural Settings in Response to Phosphorus Deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
| Parameters | Mineral Fertilizer | Dry Sludge |
|---|---|---|
| Total N (%) | 16.0 ± 0.4 | 6.4 ± 0.10 |
| NO3− (mg kg−1) | 7.4 ± 1.8 | 292 ± 2.65 |
| NH4+ (mg kg−1) | 8.6 ± 0.2 | 2608 ± 34 |
| Total P2O5 (mg kg−1) | 8.0 ± 0.3 | 73.05 ± 21 |
| Total K2O (mg kg−1) | 12.0 ± 0.7 | 1.8 ± 0.1 |
| Total B (mg kg−1) | 0.02 ± 0.001 | 10 ± 0.6 |
| Total Cu (mg kg−1) | 0.05 ± 0.001 | 24 ± 0.3 |
| Total Fe (mg kg−1) | 0.40 ± 0.05 | 290 ± 5.70 |
| Total Mn (mg kg−1) | 0.06 ± 0.001 | 60 ± 1.80 |
| Total Zn (mg kg−1) | 0.02 ± 0.001 | 812 ± 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cayunao, S.; Pérez-San Martín, A.; Jorquera-Fontena, E.; Huerta-Mendoza, V.; Tortosa, G.; Alvear, M.; Ortíz, J.; Oladele, S.O.; Curaqueo, G. Exploring Dry Salmon Sludge as an Organic Nitrogen Source for Hazelnut (Corylus avellana L.) Orchard. Nitrogen 2025, 6, 110. https://doi.org/10.3390/nitrogen6040110
Cayunao S, Pérez-San Martín A, Jorquera-Fontena E, Huerta-Mendoza V, Tortosa G, Alvear M, Ortíz J, Oladele SO, Curaqueo G. Exploring Dry Salmon Sludge as an Organic Nitrogen Source for Hazelnut (Corylus avellana L.) Orchard. Nitrogen. 2025; 6(4):110. https://doi.org/10.3390/nitrogen6040110
Chicago/Turabian StyleCayunao, Susana, Andrés Pérez-San Martín, Emilio Jorquera-Fontena, Vanessa Huerta-Mendoza, Germán Tortosa, Marysol Alvear, Juan Ortíz, Segun O. Oladele, and Gustavo Curaqueo. 2025. "Exploring Dry Salmon Sludge as an Organic Nitrogen Source for Hazelnut (Corylus avellana L.) Orchard" Nitrogen 6, no. 4: 110. https://doi.org/10.3390/nitrogen6040110
APA StyleCayunao, S., Pérez-San Martín, A., Jorquera-Fontena, E., Huerta-Mendoza, V., Tortosa, G., Alvear, M., Ortíz, J., Oladele, S. O., & Curaqueo, G. (2025). Exploring Dry Salmon Sludge as an Organic Nitrogen Source for Hazelnut (Corylus avellana L.) Orchard. Nitrogen, 6(4), 110. https://doi.org/10.3390/nitrogen6040110






