Three Years After Soybean-Cover-Crop Rotation in Conventional and No-Till Practices: What Are the Consequences on Soil Nitrous Oxide Emissions?
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Treatments
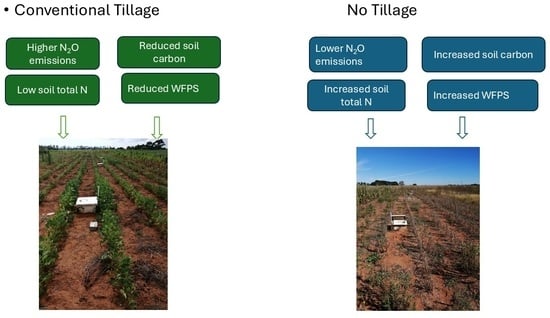
- Conventional Tillage: Soybean-Cover crop-Soybean (CT: SB-CC-SB) In these plots, there was crop rotation; soybean was followed by cover crop, then soybean again under conventional tillage; each crop was grown for 12 months, and the soil was cleared before the next crop was planted. The plant density was 270,000 plants per ha.
- No-tillage: Soybean–Cover crop–Soybean (NT: SB-CC-SB). In these plots, crop rotation took place; soybean was followed by cover crop, then soybean again under no-tillage; each crop was grown for 12 months and the soil was cleared before the next crop was planted. The plant density was 270,000 plants per ha.
2.4. Gas Sampling and Flux Measurements
2.5. Soil Sampling and Analysis
2.6. N Loss
2.7. Estimated Emissions per Product
2.8. Data Analysis Trials
3. Results
3.1. Meteorological Data
3.1.1. Rainfall Patterns and Atmospheric Temperature During the Experimental Period
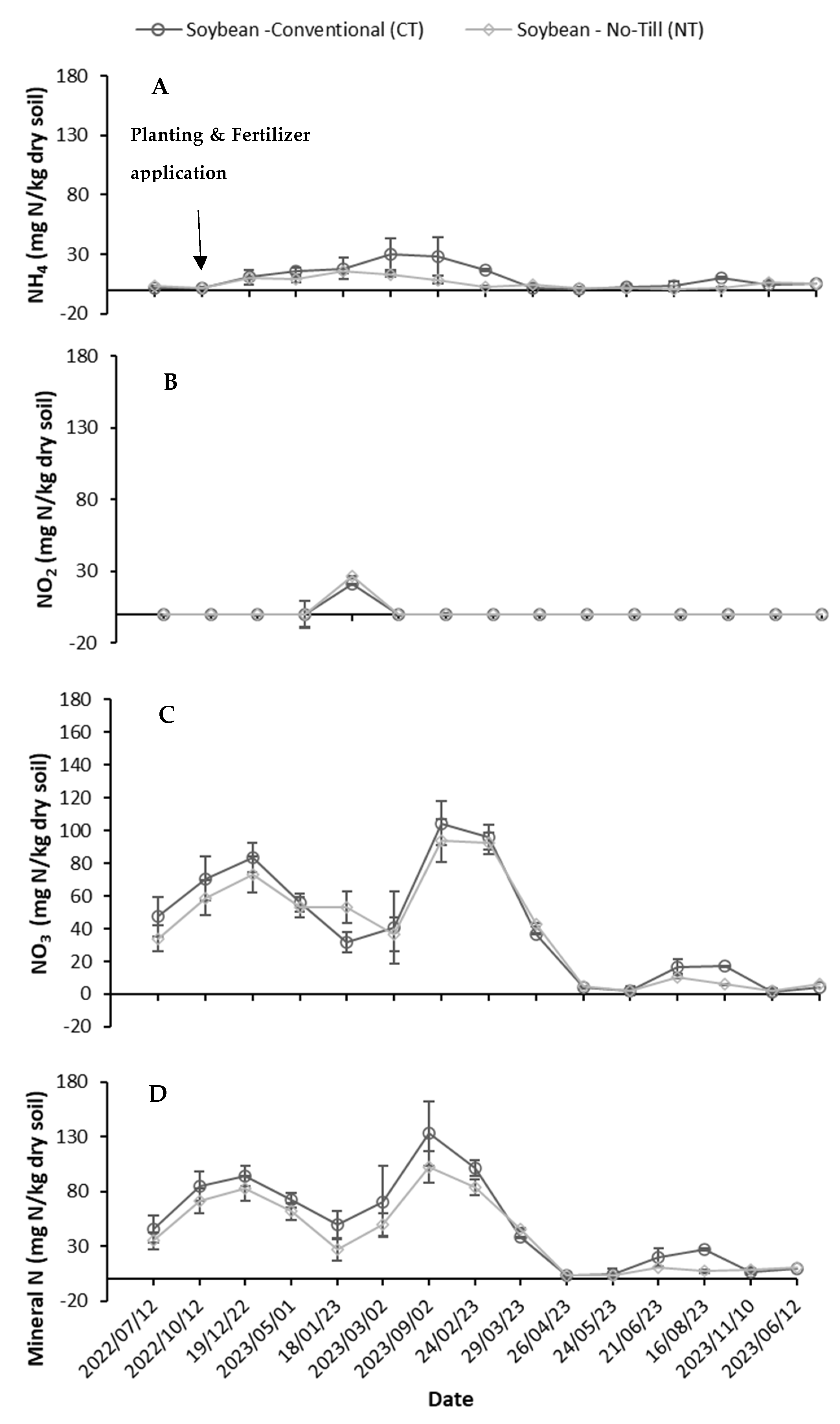
3.1.2. Soil Variables
General Soil Characteristics Before and After the Experiment
Soil Measurements
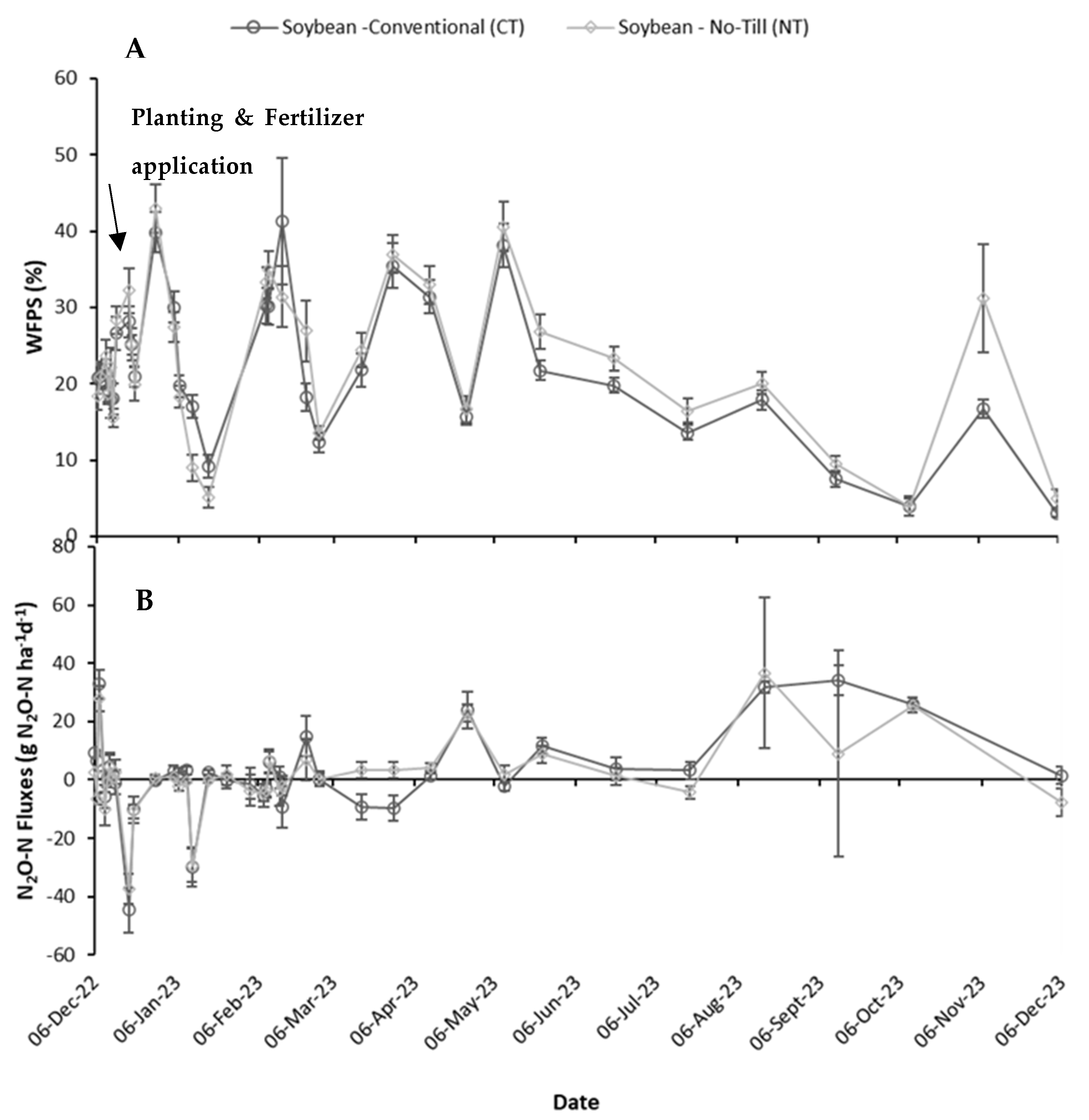
3.1.3. Nitrous Oxide Emissions
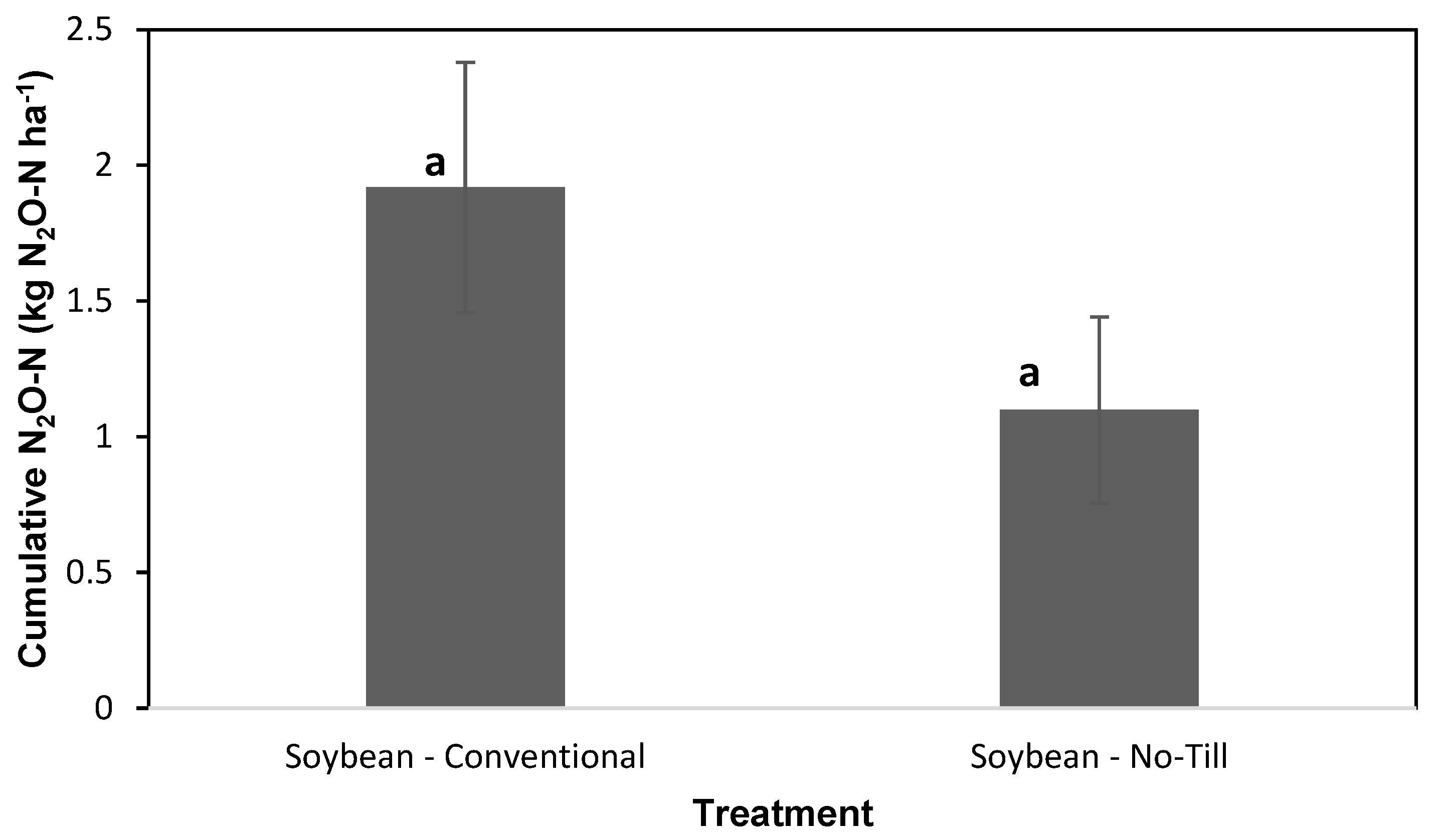
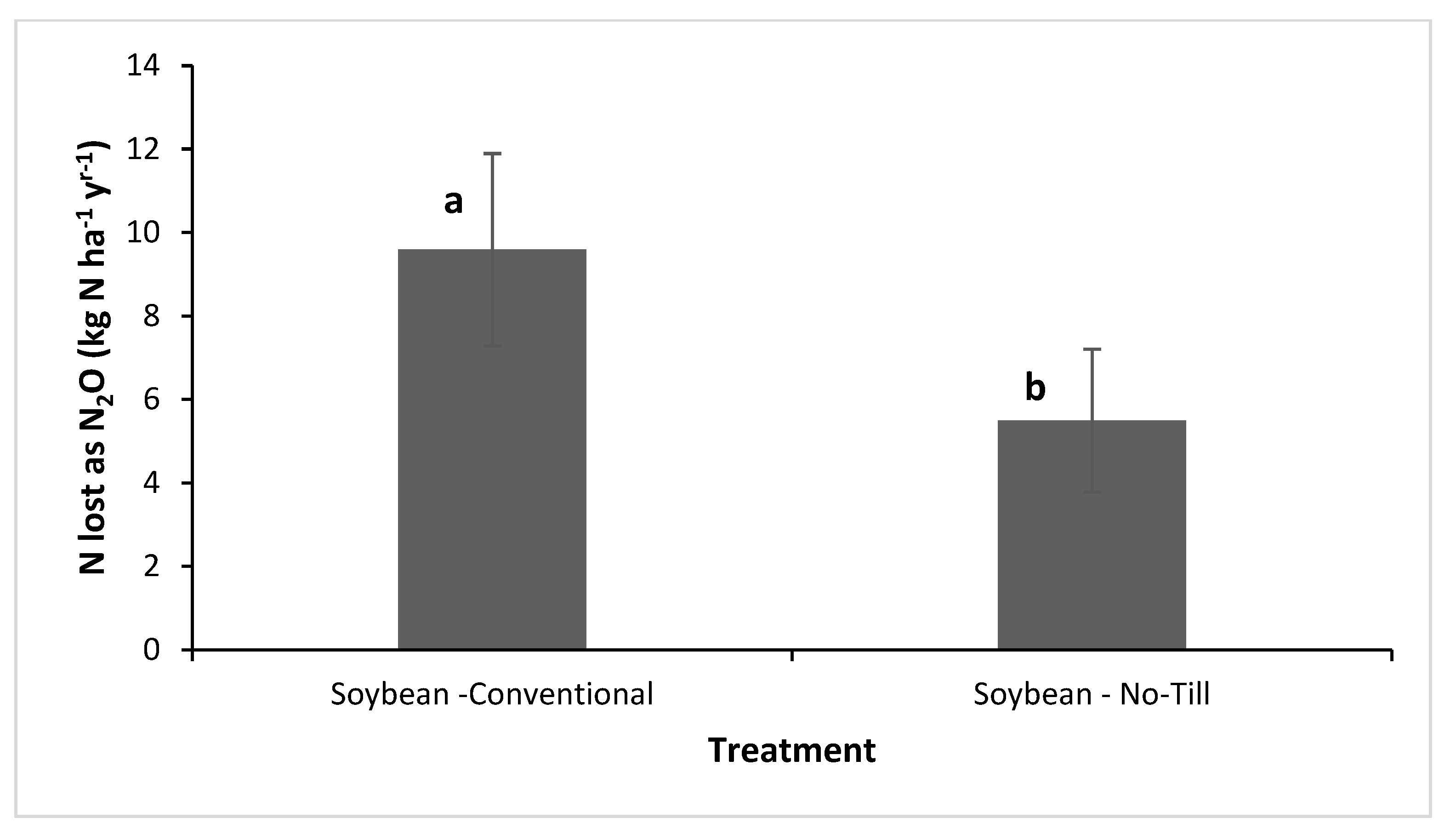
3.1.4. Cumulative N2O Emissions and N Lost as N2O
3.1.5. NT and CT Practices on Yields
4. Discussion
4.1. The Role of Soil Variables in Daily N2O Fluxes
4.2. NT and CT Practices on Cumulative N2O Emissions and N Lost as N2O
4.3. NT and CT Practices on Yields
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramzan, S.; Rasool, T.; Bhat, R.A.; Ahmad, P.; Ashraf, I.; Rashid, N.; ul Shafiq, M.; Mir, I.A. Agricultural soils a trigger to nitrous oxide: A persuasive greenhouse gas and its management. Environ. Monit. Assess. 2020, 192, 436. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr. Opin. Environ. Sustain. 2011, 3, 389–395. [Google Scholar] [CrossRef]
- Sajjad, M.; Hussain, K.; Wajid, S.A.; Saqib, Z.A. The Impact of Split Nitrogen Fertilizer Applications on the Productivity and Nitrogen Use Efficiency of Rice. Nitrogen 2024, 6, 1. [Google Scholar] [CrossRef]
- Ciarlo, E.A.; Llorente, M.M.; Conti, M.E.; Giardina, E.B. Soybean residue effect on carbon fractions and gaseous nitrogen losses at contrasting moisture contents. Commun. Soil Sci. Plant Anal. 2009, 40, 2756–2772. [Google Scholar] [CrossRef]
- Aulakh, M.; Walters, D.; Doran, J.; Francis, D.; Mosier, A. Crop residue type and placement effects on denitrification and mineralization. Soil Sci. Soc. Am. J. 1991, 55, 1020–1025. [Google Scholar] [CrossRef]
- Lai, T.V.; Farquharson, R.; Denton, M.D. High soil temperatures alter the rates of nitrification, denitrification and associated N2O emissions. J. Soils Sediments 2019, 19, 2176–2189. [Google Scholar] [CrossRef]
- Russell, C.; Fillery, I.; Bootsma, N.; McInnes, K. Effect of temperature and nitrogen source on nitrification in a sandy soil. Commun. Soil Sci. Plant Anal. 2002, 33, 1975–1989. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Van Groenigen, J.; Huygens, D.; Boeckx, P.; Kuyper, T.W.; Lubbers, I.; Rütting, T.; Groffman, P. The soil N cycle: New insights and key challenges. Soil 2015, 1, 235–256. [Google Scholar] [CrossRef]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecuária Trop. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Bizimana, F.; Dong, W.; Timilsina, A.; Raseduzzaman, M.; Li, X.; Zhang, Y.; Hu, C. Effects of Straw Amendment in Combination with Synthetic N Fertilizer Addition on N2O, N2, and Their Stoichiometric Ratios in Three Different Agro-Ecosystems. Agronomy 2024, 14, 887. [Google Scholar] [CrossRef]
- Powlson, D.; Saffigna, P.; Kragt-Cottaar, M. Denitrification at sub-optimal temperatures in soils from different climatic zones. Soil Biol. Biochem. 1988, 20, 719–723. [Google Scholar] [CrossRef]
- Canion, A.; Kostka, J.E.; Gihring, T.; Huettel, M.; Van Beusekom, J.; Gao, H.; Lavik, G.; Kuypers, M.M. Temperature response of denitrification and anammox reveals the adaptation of microbial communities to in situ temperatures in permeable marine sediments that span 50 in latitude. Biogeosciences 2014, 11, 309–320. [Google Scholar] [CrossRef]
- Adouani, N.; Limousy, L.; Lendormi, T.; Sire, O. N2O and NO emissions during wastewater denitrification step: Influence of temperature on the biological process. Comptes Rendus. Chim. 2015, 18, 15–22. [Google Scholar] [CrossRef]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- Senbayram, M.; Chen, R.; Budai, A.; Bakken, L.; Dittert, K. N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric. Ecosyst. Environ. 2012, 147, 4–12. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef]
- Broadbent, F.; Clark, F. Denitrification. Soil Nitrogen 1965, 10, 344–359. [Google Scholar]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Coyne, M.S. Denitrification in soil. Soil Nitrogen Uses Environ. Impacts 2018, 1, 95–139. [Google Scholar]
- Sumner, D.R. Crop rotation and plant productivity. In Handbook of Agricultural Productivity; CRC Press: Boca Raton, FL, USA, 2018; pp. 273–314. [Google Scholar]
- Ghosh, P.K.; Hazra, K.K.; Venkatesh, M.S.; Praharaj, C.S.; Kumar, N.; Nath, C.P.; Singh, U.; Singh, S.S. Grain legume inclusion in cereal–cereal rotation increased base crop productivity in the long run. Exp. Agric. 2020, 56, 142–158. [Google Scholar] [CrossRef]
- Drury, C.; Yang, X.; Reynolds, W.; McLaughlin, N. Nitrous oxide and carbon dioxide emissions from monoculture and rotational cropping of corn, soybean and winter wheat. Can. J. Soil Sci. 2008, 88, 163–174. [Google Scholar] [CrossRef]
- Jeuffroy, M.; Baranger, E.; Carrouée, B.; De Chezelles, E.; Gosme, M.; Hénault, C.; Schneider, A.; Cellier, P. Nitrous oxide emissions from crop rotations including wheat, oilseed rape and dry peas. Biogeosciences 2013, 10, 1787–1797. [Google Scholar] [CrossRef]
- Gomes, J.; Bayer, C.; de Souza Costa, F.; de Cássia Piccolo, M.; Zanatta, J.A.; Vieira, F.C.B.; Six, J. Soil nitrous oxide emissions in long-term cover crops-based rotations under subtropical climate. Soil Tillage Res. 2009, 106, 36–44. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Shi, Z.; Wang, S.; Wang, Z.; Liu, Y.; Wen, X.; Mo, F.; Han, J.; Liao, Y. Crop development has more influence on shaping rhizobacteria of wheat than tillage practice and crop rotation pattern in an arid agroecosystem. Appl. Soil Ecol. 2021, 165, 104016. [Google Scholar] [CrossRef]
- Kakraliya, S.; Singh, U.; Bohra, A.; Choudhary, K.; Kumar, S.; Meena, R.S.; Jat, M. Nitrogen and legumes: A meta-analysis. In Legumes for Soil Health and Sustainable Management; Springer: Delhi, India, 2018; pp. 277–314. [Google Scholar]
- Fagodiya, R.K.; Sharma, G.; Verma, K.; Rai, A.K.; Prajapat, K.; Singh, R.; Chandra, P.; Sheoran, P.; Yadav, R.K.; Biswas, A. Computation of soil quality index after fifteen years of long-term tillage and residue management experiment (LT&RE) under rice wheat system. Agric. Syst. 2024, 219, 104039. [Google Scholar]
- Zhang, B.; Wang, G.; Liu, X. Research on corn and soybean rotation: A review of ecological benefits and economic potential. Resour. Data J. 2024, 3, 208–222. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Bailey, B.A. Role of cover crops in improving soil and row crop productivity. Commun. Soil Sci. Plant Anal. 2005, 36, 2733–2757. [Google Scholar] [CrossRef]
- Singh, G.; Bhattacharyya, R.; Das, T.; Sharma, A.; Ghosh, A.; Das, S.; Jha, P. Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the Indo-Gangetic Plains. Soil Tillage Res. 2018, 184, 291–300. [Google Scholar] [CrossRef]
- Drury, C.; Yang, X.; Reynolds, W.; Tan, C. Influence of crop rotation and aggregate size on carbon dioxide production and denitrification. Soil Tillage Res. 2004, 79, 87–100. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Del Grosso, S.J.; Reule, C.A. Nitrogen, tillage, and crop rotation effects on nitrous oxide emissions from irrigated cropping systems. J. Environ. Qual. 2008, 37, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Z.; Zhao, D.; Zhang, B.; Zhu, B.; Yao, Z.; Kiese, R.; Butterbach-Bahl, K.; Zhou, M. Effects of Conservation Agriculture on Soil N2O Emissions and Crop Yield in Global Cereal Cropping Systems. Glob. Change Biol. 2025, 31, e70048. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Menheere, N.; Gao, Y.; Brown, S.; Van Eerd, L.L.; Lauzon, J.D.; Bruun, S.; Wagner-Riddle, C. Increased N2O emissions by cover crops in a diverse crop rotation can be mediated with dual nitrification and urease inhibitors. Agric. Ecosyst. Environ. 2024, 374, 109178. [Google Scholar] [CrossRef]
- Kumar, T.; Kundu, M.S.; Jha, R.K. Impact of crop rotation and tillage operations on mitigating greenhouse gas emissions and evaluation of sustainability index in rice-wheat-green gram cropping system of north Bihar. J. Environ. Manag. 2024, 366, 121689. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yang, S.; Luan, C.-s.; Wu, Q.; Lin, L.-l.; Li, X.-x.; Che, Z.; Zhou, D.-b.; Dong, Z.-r.; Song, H. Partial organic substitution for synthetic fertilizer improves soil fertility and crop yields while mitigating N2O emissions in wheat-maize rotation system. Eur. J. Agron. 2024, 154, 127077. [Google Scholar] [CrossRef]
- Braker, G.; Matthies, D.; Hannig, M.; Brandt, F.B.; Brenzinger, K.; Gröngröft, A. Impact of land use management and soil properties on denitrifier communities of namibian savannas. Microb. Ecol. 2015, 70, 981–992. [Google Scholar] [CrossRef]
- Desloover, J.; Vlaeminck, S.E.; Clauwaert, P.; Verstraete, W.; Boon, N. Strategies to mitigate N2O emissions from biological nitrogen removal systems. Curr. Opin. Biotechnol. 2012, 23, 474–482. [Google Scholar] [CrossRef]
- Hu, H.-W.; Chen, D.; He, J.-Z. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates. FEMS Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J. Management strategies to mitigate N2O emissions in agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.; Carozzi, M.; Massad, R.S.; Butterbach-Bahl, K.; Scheer, C. Long term impact of residue management on soil organic carbon stocks and nitrous oxide emissions from European croplands. Sci. Total Environ. 2022, 836, 154932. [Google Scholar] [CrossRef]
- Otchere, O. The Impact of Cover Cropping on Soil Nitrogen Availability, Crop Nitrogen Use, and Nitrous Oxide Emissions in a Prairie Potato—Grain Crop Rotation. Doctoral Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2022. [Google Scholar]
- Yoo, J.; Woo, S.-H.; Park, K.-D.; Chung, K.-Y. Effect of no-tillage and conventional tillage practices on the nitrous oxide (N2O) emissions in an upland soil: Soil N2O emission as affected by the fertilizer applications. Appl. Biol. Chem. 2016, 59, 787–797. [Google Scholar] [CrossRef]
- Monteiro, R.C.; Franchini, J.C.; Jantalia, C.P.; Urquiaga, S.; Alves, B.J.R.; Boddey, R.M. Soil nitrous oxide emissions from a soybean-wheat succession under different tillage systems in Southern Brazil. Rev. Bras. De Ciência Do Solo 2023, 47, e0220135. [Google Scholar] [CrossRef]
- Smith, K.; Thomson, P.; Clayton, H.; McTaggart, I.; Conen, F. Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos. Environ. 1998, 32, 3301–3309. [Google Scholar] [CrossRef]
- Chadwick, D.; Cardenas, L.; Misselbrook, T.; Smith, K.; Rees, R.; Watson, C.; McGeough, K.; Williams, J.; Cloy, J.; Thorman, R. Optimizing chamber methods for measuring nitrous oxide emissions from plot-based agricultural experiments. Eur. J. Soil Sci. 2014, 65, 295–307. [Google Scholar] [CrossRef]
- West, T.; Griffith, D.; Steinhardt, G.; Kladivko, E.; Parsons, S. Effect of tillage and rotation on agronomic performance of corn and soybean: Twenty-year study on dark silty clay loam soil. J. Prod. Agric. 1996, 9, 241–248. [Google Scholar] [CrossRef]
- Connor, B.F.; Rose, D.L.; Noriega, M.C.; Murtaugh, L.K.; Abney, S.R. Methods of Analysis by the US Geological Survey National Water Quality Laboratory-Determination of 86 Volatile Organic Compounds in Water by Gas Chromatography/Mass Spectrometry, Including Detections Less Than Reporting Limits; US Geological Survey: Denver, CO, USA, 1998.
- Cardenas, L.; Thorman, R.; Ashlee, N.; Butler, M.; Chadwick, D.; Chambers, B.; Cuttle, S.; Donovan, N.; Kingston, H.; Lane, S. Quantifying annual N2O emission fluxes from grazed grassland under a range of inorganic fertiliser nitrogen inputs. Agric. Ecosyst. Environ. 2010, 136, 218–226. [Google Scholar] [CrossRef]
- Louro, A.; Sawamoto, T.; Chadwick, D.; Pezzolla, D.; Bol, R.; Báez, D.; Cardenas, L. Effect of slurry and ammonium nitrate application on greenhouse gas fluxes of a grassland soil under atypical South West England weather conditions. Agric. Ecosyst. Environ. 2013, 181, 1–11. [Google Scholar] [CrossRef]
- McLean, E. Soil pH and lime requirement. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 199–224. [Google Scholar]
- Wheatley, R.; MacDonald, R.; Smith, A.M. Extraction of nitrogen from soils. Biol. Fertil. Soils 1989, 8, 189–190. [Google Scholar] [CrossRef]
- Sims, J.T. Soil test phosphorus: Bray and Kurtz P-1. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; Researchgatenet: Newark, NJ, USA, 2000; Volume 13. [Google Scholar]
- Dickey, G.; Allen, R.; Wright, J.; Murray, N.; Stone, J.; Hunsaker, D. Soil bulk density sampling for neutron gauge calibration. Irrig. Drain. Syst. 1993, 1103–1111. [Google Scholar]
- Amirinejad, A.A.; Kamble, K.; Aggarwal, P.; Chakraborty, D.; Pradhan, S.; Mittal, R.B. Assessment and mapping of spatial variation of soil physical health in a farm. Geoderma 2011, 160, 292–303. [Google Scholar] [CrossRef]
- Gasche, R.; Papen, H. A 3-year continuous record of nitrogen trace gas fluxes from untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany: 2. NO and NO2 fluxes. J. Geophys. Res. Atmos. 1999, 104, 18505–18520. [Google Scholar] [CrossRef]
- Roelle, P.A.; Aneja, V.P.; Gay, B.; Geron, C.; Pierce, T. Biogenic nitric oxide emissions from cropland soils. Atmos. Environ. 2001, 35, 115–124. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Luo, J.; Tillman, R.; Ball, P. Grazing effects on denitrification in a soil under pasture during two contrasting seasons. Soil Biol. Biochem. 1999, 31, 903–912. [Google Scholar] [CrossRef]
- Drury, C.; Reynolds, W.; Tan, C.; Welacky, T.; Calder, W.; McLaughlin, N. Emissions of nitrous oxide and carbon dioxide: Influence of tillage type and nitrogen placement depth. Soil Sci. Soc. Am. J. 2006, 70, 570–581. [Google Scholar] [CrossRef]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar] [CrossRef]
- Coppens, F.; Garnier, P.; De Gryze, S.; Merckx, R.; Recous, S. Soil moisture, carbon and nitrogen dynamics following incorporation and surface application of labelled crop residues in soil columns. Eur. J. Soil Sci. 2006, 57, 894–905. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, S.; Zhang, Z.; Du, Y.; Yao, B.; Xie, H.; Liu, P.; Zhang, Y.; Wang, W.; Zhou, H. Soil moisture but not warming dominates nitrous oxide emissions during freeze–thaw cycles in a Qinghai–Tibetan plateau alpine meadow with discontinuous permafrost. Front. Ecol. Evol. 2021, 9, 676027. [Google Scholar] [CrossRef]
- Russow, R.; Sich, I.; Neue, H.-U. The formation of the trace gases NO and N2O in soils by the coupled processes of nitrification and denitrification: Results of kinetic 15N tracer investigations. Chemosphere-Glob. Change Sci. 2000, 2, 359–366. [Google Scholar] [CrossRef]
- Wu, D.; Dong, W.; Oenema, O.; Wang, Y.; Trebs, I.; Hu, C. N2O consumption by low-nitrogen soil and its regulation by water and oxygen. Soil Biol. Biochem. 2013, 60, 165–172. [Google Scholar] [CrossRef]
- Bateman, E.; Baggs, E. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Iqbal, J.; Mitchell, D.C.; Barker, D.W.; Miguez, F.; Sawyer, J.E.; Pantoja, J.; Castellano, M.J. Does nitrogen fertilizer application rate to corn affect nitrous oxide emissions from the rotated soybean crop? J. Environ. Qual. 2015, 44, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, P.; Yang, P.; Ren, S. Effects of fertilizer and biochar applications on the relationship among soil moisture, temperature, and N2O emissions in farmland. PeerJ 2021, 9, e11674. [Google Scholar] [CrossRef]
- Del Prado, A.; Merino, P.; Estavillo, J.; Pinto, M.; González-Murua, C. N2O and NO emissions from different N sources and under a range of soil water contents. Nutr. Cycl. Agroecosyst. 2006, 74, 229–243. [Google Scholar] [CrossRef]
- Li, Y.; Barton, L.; Chen, D. Simulating response of N2O emissions to fertiliser N application and climatic variability from a rain-fed and wheat-cropped soil in Western Australia. J. Sci. Food Agric. 2012, 92, 1130–1143. [Google Scholar] [CrossRef]
- Carmo, J.B.d.; Neill, C.; Garcia-Montiel, D.C.; de Cássia Piccolo, M.; Cerri, C.C.; Steudler, P.A.; de Andrade, C.A.; Passianoto, C.C.; Feigl, B.J.; Melillo, J.M. Nitrogen dynamics during till and no-till pasture restoration sequences in Rondônia, Brazil. Nutr. Cycl. Agroecosyst. 2005, 71, 213–225. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Zanatta, J.A.; Bayer, C.; Vieira, F.C.; Gomes, J.; Tomazi, M. Nitrous oxide and methane fluxes in South Brazilian Gleysol as affected by nitrogen fertilizers. Rev. Bras. De Ciência Do Solo 2010, 34, 1653–1665. [Google Scholar] [CrossRef]
- Mosier, A.R. Exchange of gaseous nitrogen compounds between agricultural systems and the atmosphere. Plant Soil 2001, 228, 17–27. [Google Scholar] [CrossRef]
- Khalil, K.; Mary, B.; Renault, P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol. Biochem. 2004, 36, 687–699. [Google Scholar] [CrossRef]
- Rochette, P.; Janzen, H.H. Towards a revised coefficient for estimating N2O emissions from legumes. Nutr. Cycl. Agroecosyst. 2005, 73, 171–179. [Google Scholar] [CrossRef]
- Escobar, L.F.; Amado, T.J.C.; Bayer, C.; Chavez, L.F.; Zanatta, J.A.; Fiorin, J.E. Postharvest nitrous oxide emissions from a subtropical oxisol as influenced by summer crop residues and their management. Rev. Bras. De Ciência Do Solo 2010, 34, 507–516. [Google Scholar] [CrossRef]
- Rochette, P. No-till only increases N2O emissions in poorly-aerated soils. Soil Tillage Res. 2008, 101, 97–100. [Google Scholar] [CrossRef]
- Zschornack, T.; Bayer, C.; Zanatta, J.A.; Vieira, F.C.B.; Anghinoni, I. Mitigation of methane and nitrous oxide emissions from flood-irrigated rice by no incorporation of winter crop residues into the soil. Rev. Bras. De Ciência Do Solo 2011, 35, 623–634. [Google Scholar] [CrossRef]
- Almaraz, J.J.; Zhou, X.; Mabood, F.; Madramootoo, C.; Rochette, P.; Ma, B.-L.; Smith, D.L. Greenhouse gas fluxes associated with soybean production under two tillage systems in southwestern Quebec. Soil Tillage Res. 2009, 104, 134–139. [Google Scholar] [CrossRef]
- Nouchi, I.; Yonemura, S. CO2, CH4 and N2O fluxes from soybean and barley double-cropping in relation to tillage in Japan. Phyton 2005, 45, 327–338. [Google Scholar]
- Asakawa, S. Denitrifying ability of indigenous strains of Bradyrhizobium japonicum isolated from fields under paddy-upland rotation. Biol. Fertil. Soils 1993, 15, 196–200. [Google Scholar] [CrossRef]
- Inaba, S.; Ikenishi, F.; Itakura, M.; Kikuchi, M.; Eda, S.; Chiba, N.; Katsuyama, C.; Suwa, Y.; Mitsui, H.; Minamisawa, K. N2O emission from degraded soybean nodules depends on denitrification by Bradyrhizobium japonicum and other microbes in the rhizosphere. Microbes Environ. 2012, 27, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; Lee, H.H.; Moon, S.M.; Han, H.R.; Hong, C.O. Nitrous oxide emissions and maize yield as influenced by nitrogen fertilization and tillage operations in upland soil. Appl. Biol. Chem. 2021, 64, 1–12. [Google Scholar] [CrossRef]
- Omonode, R.A.; Halvorson, A.D.; Gagnon, B.; Vyn, T.J. Achieving lower nitrogen balance and higher nitrogen recovery efficiency reduces nitrous oxide emissions in North America’s maize cropping systems. Front. Plant Sci. 2017, 8, 1080. [Google Scholar] [CrossRef] [PubMed]
- Peigné, J.; Ball, B.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Franzluebbers, A.; Hons, F.; Zuberer, D. Tillage and crop effects on seasonal soil carbon and nitrogen dynamics. Soil Sci. Soc. Am. J. 1995, 59, 1618–1624. [Google Scholar] [CrossRef]
- Malhi, S.S.; Lemke, R.; Wang, Z.; Chhabra, B.S. Tillage, nitrogen and crop residue effects on crop yield, nutrient uptake, soil quality, and greenhouse gas emissions. Soil Tillage Res. 2006, 90, 171–183. [Google Scholar] [CrossRef]
- Pekrun, C.; Messelhäuser, M.H.; Finck, M.; Hartung, K.; Möller, K.; Gerhards, R. Yield, soil Nitrogen content and weed control in six years of conservation agriculture on-farm field trials in Southwest Germany. Soil Tillage Res. 2023, 227, 105615. [Google Scholar] [CrossRef]
- Kaspar, T.; Jaynes, D.; Parkin, T.; Moorman, T.; Singer, J. Effectiveness of oat and rye cover crops in reducing nitrate losses in drainage water. Agric. Water Manag. 2012, 110, 25–33. [Google Scholar] [CrossRef]
- Gillette, K.; Malone, R.; Kaspar, T.; Ma, L.; Parkin, T.; Jaynes, D.; Fang, Q.; Hatfield, J.; Feyereisen, G.; Kersebaum, K. N loss to drain flow and N2O emissions from a corn-soybean rotation with winter rye. Sci. Total Environ. 2018, 618, 982–997. [Google Scholar] [CrossRef]
- Toliver, D.K.; Larson, J.A.; Roberts, R.K.; English, B.C.; De La Torre Ugarte, D.G.; West, T.O. Effects of no-till on yields as influenced by crop and environmental factors. Agron. J. 2012, 104, 530–541. [Google Scholar] [CrossRef]
- Evanylo, G. No-Till Corn Response to Nitrogen Rate and Timing in the Middle Atlantic Coastal Plain. J. Prod. Agric. 1991, 4, 180–185. [Google Scholar] [CrossRef]
- Yin, X.; Al-Kaisi, M.M. Periodic response of soybean yields and economic returns to long-term no-tillage. Agron. J. 2004, 96, 723–733. [Google Scholar] [CrossRef]
- Parkin, T.B.; Kaspar, T.C. Nitrous oxide emissions from corn–soybean systems in the Midwest. J. Environ. Qual. 2006, 35, 1496–1506. [Google Scholar] [CrossRef]




| Parameter | Soybea-CT | Soybean-NT | LSD |
|---|---|---|---|
| Before 06/12/2022 | |||
| Soil Texture | |||
| Sand (%) | 91.00 ± 4.67 b | 93.67 ± 4.25 a | 1.95 |
| Silt (%) | 6.00 ± 4.82 a | 3.00 ± 3.36 b | 1.91 |
| Clay (%) | 2.92 ± 2.07 c | 3.33 ± 1.97 b | 0.8 |
| Soil pH | 6.05 ± 0.57 a | 6.30 ± 0.50 a | 0.29 |
| Bulk density (g/cm3) | 1.44 ± 0.17 b | 1.52 ± 0.12 a | 0.09 |
| Total C (g C/kg dry soil) | 1.65 ± 0.41 a | 1.44 ± 0.26 a | 0.22 |
| Total N (mg N/kg dry soil) | 342.59 ± 84.37 a | 333.08 ± 69.78 a | 49.45 |
| Organic matter (% w/w) | 13.88 ± 4.06 a | 11.96 ± 1.99 a | 1.78 |
| WFPS (%) | 21.63 ± 4.48 a | 22.91 ± 4.74 a | 2.91 |
| NH4+ (mg N/kg dry soil) | 1.24 ± 2.19 b | 3.05 ± 3.74 a | 1.58 |
| NO3 (mg N/kg dry soil) | 47.3 ± 60.06 a | 33.87 ± 21.24 a | 19.54 |
| NO2 (mg N/kg dry soil) | 0.018 ± 0.061 a | 0.038 ± 0.086 a | 0.052 |
| C/N Ratio | 4.77 ± 1.17 a | 4.86 ± 1.64 c | 1.38 |
| After 06/12/2023 | |||
| Soil Texture | |||
| Sand (%) | 88.00 ± 4.67 a | 89.00 ± 2.89 a | 0.24 |
| Silt (%) | 6.92 ± 2.88 a | 5.83 ± 2.41 a | 1.64 |
| Clay (%) | 5.08 ± 3.00 a | 5.17 ±1.59 a | 1.35 |
| Soil pH | 6.06 ± 0.25 a | 5.91 ± 0.39 b | 0.16 |
| Bulk density (g/cm3) | 1.40 ± 0.17 c | 1.50 ± 0.13 a | 0.087 |
| Total C (g C/kg dry soil) | 2.07 ± 0.77 a | 1.97 ± 0.81 a | 0.4 |
| Total N (mg N/kg dry soil) | 50.03 ± 49.61 a | 27.23 ± 31.62 a | 33.59 |
| Organic matter (% w/w) | 3.12 ± 1.19 a | 3.04 ± 1.29 a | 0.69 |
| WFPS (%) | 3.05 ± 1.88 a | 4.98 ± 4.21 a | 1.86 |
| Soil porosity (%) | 0.46 ± 0.065 a | 0.43 ± 0.044 ab | 0.033 |
| NH4+ (mg N/kg dry soil) | 5.05 ± 4.65 a | 5.21 ± 3.46 a | 2.32 |
| NO3 (mg N/kg dry soil) | 4.41 ± 2.65 b | 6.01 ± 3.97 a | 2.39 |
| NO2 (mg N/kg dry soil) | 0.015 ± 0.012 a | 0.038 ± 0.057 a | 0.19 |
| Hydraulic conductivity (cm/s) | 0.16−2 ± 0.37−3 a | 0.33−2 ± 0.39−3 a | 0.18−2 |
| C/N Ratio | 49.96 ± 31.3 a | 104.87 ± 73.93 b | 32.66 |
| Treatment | N2O kg N ha−1 | Yield t ha−1 | Kg N ha−1 Dry Matter |
|---|---|---|---|
| Soybean-CT | 2 | 2.1 | 0.95 |
| Soybean-NT | 1 | 0.6 | 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, N.O.; Banda, L.; Cardenas, L.M.; Louro-Lopez, A.; Dlamini, J.C. Three Years After Soybean-Cover-Crop Rotation in Conventional and No-Till Practices: What Are the Consequences on Soil Nitrous Oxide Emissions? Nitrogen 2025, 6, 45. https://doi.org/10.3390/nitrogen6020045
Dlamini NO, Banda L, Cardenas LM, Louro-Lopez A, Dlamini JC. Three Years After Soybean-Cover-Crop Rotation in Conventional and No-Till Practices: What Are the Consequences on Soil Nitrous Oxide Emissions? Nitrogen. 2025; 6(2):45. https://doi.org/10.3390/nitrogen6020045
Chicago/Turabian StyleDlamini, Nokwanda O., Lindsay Banda, Laura M. Cardenas, Aranzazu Louro-Lopez, and Jerry C. Dlamini. 2025. "Three Years After Soybean-Cover-Crop Rotation in Conventional and No-Till Practices: What Are the Consequences on Soil Nitrous Oxide Emissions?" Nitrogen 6, no. 2: 45. https://doi.org/10.3390/nitrogen6020045
APA StyleDlamini, N. O., Banda, L., Cardenas, L. M., Louro-Lopez, A., & Dlamini, J. C. (2025). Three Years After Soybean-Cover-Crop Rotation in Conventional and No-Till Practices: What Are the Consequences on Soil Nitrous Oxide Emissions? Nitrogen, 6(2), 45. https://doi.org/10.3390/nitrogen6020045