Effects of Nitrogen Fertilizer Application on Growth, Vegetation Indices, and Ammonia Volatilization in Korean Radish (Raphanus sativus L.)
Abstract
1. Introduction
2. Materials and Methods
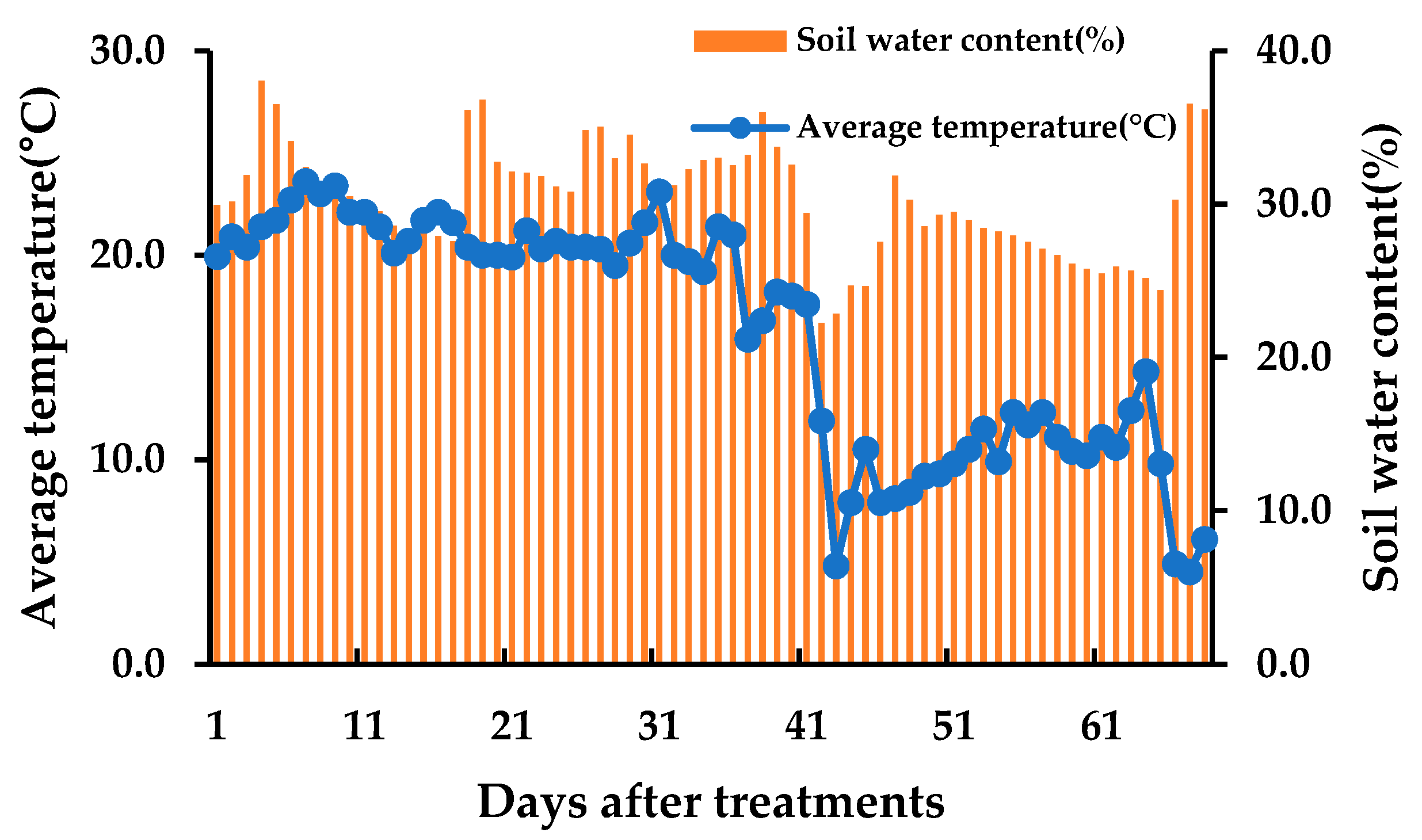
2.1. Field Experiment
2.2. Measurements
2.2.1. Growth Measurements
2.2.2. Analysis of Vegetation Indices
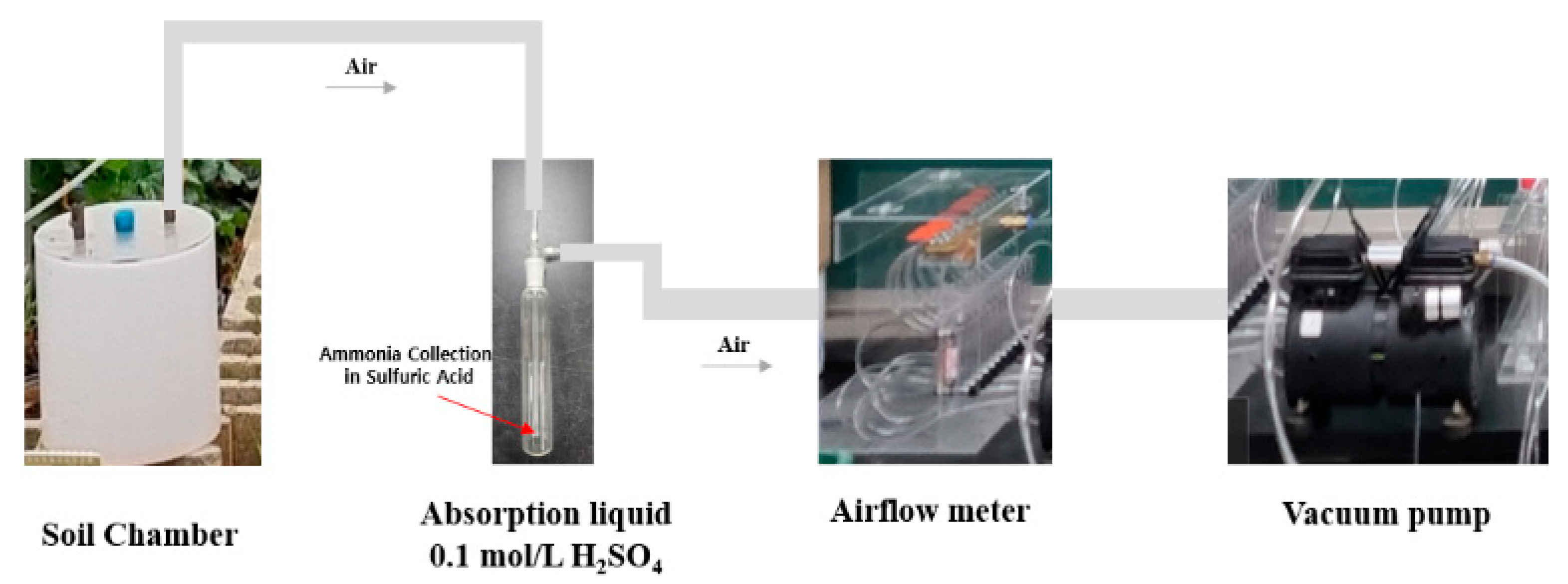
2.2.3. Measurement of Ammonia Volatilization
2.3. Statistical Analysis
3. Results and Discussions
3.1. Analysis of Plant Growth with Different Concentrations of Urea Fertilizer
3.2. Comparison of Vegetation Indices Among the Groups Treated with Different Concentrations of Urea
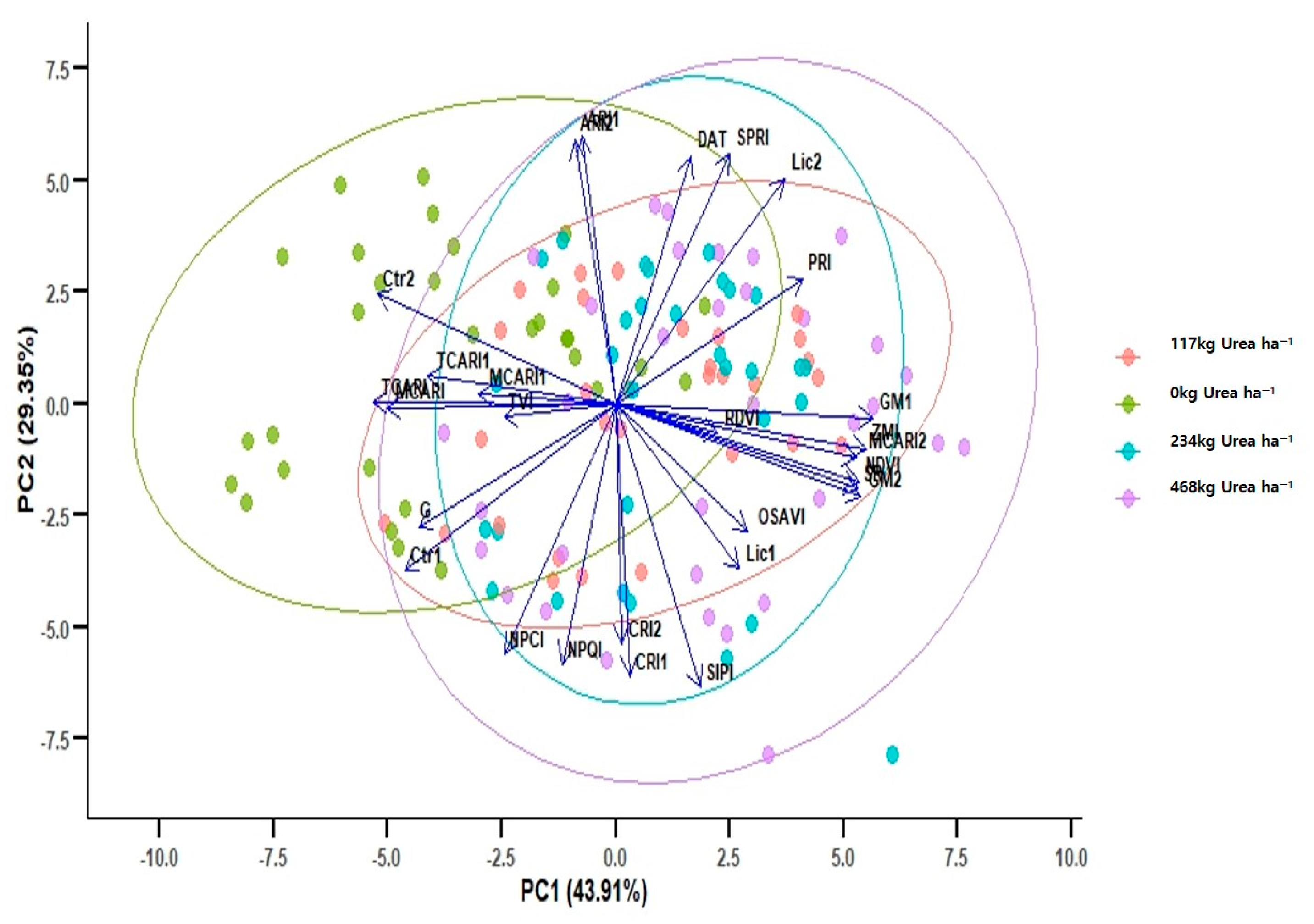
3.3. Comparison of PCA Among Treatments with Different Urea Concentrations
3.4. Comparison of Ammonia Gas Emissions as Byproduct of Treatment with Different Urea Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olivera Viciedo, D.; de Mello Prado, R.; Lizcano Toledo, R.; Nascimento dos Santos, L.C.; Peña Calzada, K.J.A.C. Response of radish seedlings (Raphanus sativus L.) to different concentrations of ammoniacal nitrogen in absence and presence of silicon. Agron. Colomb. 2017, 35, 198–204. [Google Scholar] [CrossRef]
- Bakhsh, K.; Ahmad, B.; Gill, Z.A.; Hassan, S. Estimating indicators of higher yield in radish cultivation. Int. J. Agric. Biol. 2006, 8, 783–787. [Google Scholar]
- Yousaf, M.; Bashir, S.; Raza, H.; Shah, A.N.; Iqbal, J.; Arif, M.; Bukhari, M.A.; Muhammad, S.; Hashim, S.; Alkahtani, J.; et al. Role of nitrogen and magnesium for growth, yield and nutritional quality of radish. Saudi J. Biol. Sci. 2021, 28, 3021–3030. [Google Scholar] [CrossRef]
- Srinivas, K.; Naik, L. Growth and yield of radish (Raphanus sativus L.) in relation to nitrogen and potash fertilization. Indian J. Hort. 1990, 47, 114–119. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates: Sunderland, CT, USA, 2015; 761p. [Google Scholar]
- Carmo-Silva, E.; Scales, J.C.; Madgwick, P.J.; Parry, M.A.J. Optimizing R ubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 2015, 38, 1817–1832. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Sun, J.; Tian, Z.; Hu, H.; Jiang, S.; Luo, Q.; Xu, Y.; Jiang, D.; Cao, W.; et al. Enhanced Rubisco activation associated with maintenance of electron transport alleviates inhibition of photosynthesis under low nitrogen conditions in winter wheat seedlings. J. Exp. Bot. 2018, 69, 5477–5488. [Google Scholar] [CrossRef]
- Harbinson, J.; Genty, B.; Baker, N.R.J.P.R. The relationship between CO2 assimilation and electron transport in leaves. Photosynth. Res. 1990, 25, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral nutrition of higher plants. J. Ecol. 1995, 76, 1250. [Google Scholar]
- Lefsrud, M.G.; Kopsell, D.A.; Kopsell, D.E. Nitrogen levels influence biomass, elemental accumulations, and pigment concentrations in spinach. J. Plant Nutr. 2007, 30, 171–185. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid pigments in kale are influenced by nitrogen concentration and form. Sci. Food Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J.; Zhang, Q.; Li, L.; Kuang, T. Modification of photosystem II photochemistry in nitrogen deficient maize and wheat plants. J. Plant Physiol. 2001, 158, 1423–1430. [Google Scholar] [CrossRef]
- OECD.stat. Data and Metadata for OECD Countries and Selected Non-Member Economies. Organization for Economic Cooperation and Development. Available online: https://stats.oecd.org (accessed on 22 October 2024).
- Lee, J.-H.; Yoon, Y.-M. Comparison of nutrient balance and nutrient loading index for cultivated land nutrient management. Environ. Biol. Res. 2019, 37, 554–567. [Google Scholar] [CrossRef]
- Lim, J.Y.; Bhuiyan, M.S.I.; Lee, S.B.; Lee, J.G.; Kim, P.J. Agricultural nitrogen and phosphorus balances of Korea and Japan: Highest nutrient surplus among OECD member countries. Environ. Pollut. 2021, 286, 117353. [Google Scholar] [CrossRef] [PubMed]
- Houlton, B.Z.; Almaraz, M.; Aneja, V.; Austin, A.T.; Bai, E.; Cassman, K.G.; Compton, J.E.; Davidson, E.A.; Erisman, J.W.; Galloway, J.N.; et al. A world of cobenefits: Solving the global nitrogen challenge. Earth’s Futur. 2019, 7, 865–872. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. The fate of nitrogen from soil to plants: Influence of agricultural practices in modern agriculture. Agriculture 2021, 11, 944. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Hua, Z.; Li, Z.; He, X.; Yan, R.; Li, Y.; Zhi, Z.; Tian, C.J.S.; Chemical, A.B. A low cost and high performance NH3 detection system for a harsh agricultural environment. Sens. Actuators B Chem. 2022, 361, 131675. [Google Scholar] [CrossRef]
- Ricardo, A.P. Evaluation and Application of a Dynamic Emissions Chamber for Quantifying Gaseous Emissions from Laying Hen Manure. Master’s Thesis, Iowa State University, Ames, IA, USA, 2011. [Google Scholar]
- Xiao, J.; Wang, Q.; Ge, X.; Zhu, L.; Li, X.; Yang, X.; Ouyang, H.; Wu, J. Defining the ecological efficiency of nitrogen use in the context of nitrogen cycling. Ecol. Indic. 2019, 107, 105493. [Google Scholar] [CrossRef]
- Kavanagh, I.; Fenton, O.; Healy, M.; Burchill, W.; Lanigan, G.; Krol, D. Mitigating ammonia and greenhouse gas emissions from stored cattle slurry using agricultural waste, commercially available products and a chemical acidifier. J. Clean. Prod. 2021, 294, 126251. [Google Scholar] [CrossRef]
- Klimczyk, M.; Siczek, A.; Schimmelpfennig, L. Improving the efficiency of urea-based fertilization leading to reduction in ammonia emission. Sci. Total. Environ. 2021, 771, 145483. [Google Scholar] [CrossRef]
- Adegoke, T.O.; Moon, T.-I.; Ku, H.-H. Ammonia emission from sandy loam soil amended with manure compost and urea. Appl. Biol. Chem. 2022, 65, 83. [Google Scholar] [CrossRef]
- Zhao, B.; Duan, A.; Ata-Ul-Karim, S.T.; Liu, Z.; Chen, Z.; Gong, Z.; Zhang, J.; Xiao, J.; Liu, Z.; Qin, A.J.; et al. Exploring new spectral bands and vegetation indices for estimating nitrogen nutrition index of summer maize. Eur. J. Agron. 2018, 93, 113–125. [Google Scholar] [CrossRef]
- Ali, M.; Al-Ani, A.; Eamus, D.; Tan, D.K. Leaf nitrogen determination using non-destructive techniques—A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Marti, J.; Bort, J.; Slafer, G.; Araus, J. Can wheat yield be assessed by early measurements of Normalized Difference Vegetation Index? Ann. Appl. Biol. 2007, 150, 253–257. [Google Scholar] [CrossRef]
- Chen, P. A comparison of two approaches for estimating the wheat nitrogen nutrition index using remote sensing. Remote. Sens. 2015, 7, 4527–4548. [Google Scholar] [CrossRef]
- Blackmer, T.M.; Schepers, J.S.; Varvel, G.E.; Walter-Shea, E.A. Nitrogen deficiency detection using reflected shortwave radiation from irrigated corn canopies. Agron. J. 1996, 88, 1–5. [Google Scholar] [CrossRef]
- Elmetwalli, A.H.; Tyler, A.N. Estimation of maize properties and differentiating moisture and nitrogen deficiency stress via ground–based remotely sensed data. Agric. Water Manag. 2020, 242, 106413. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Deering, D.; Schell, J.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Texas A&M University: College Station, TX, USA, 1974. Available online: https://ntrs.nasa.gov/citations/19750020419 (accessed on 1 June 2025).
- Daughtry, C.S.; Walthall, C.; Kim, M.; De Colstoun, E.B.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; Lopez-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.; De Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Estimation of chlorophyll fluorescence under natural illumination from hyperspectral data. Int. J. Appl. Earth Obs. Geoinf. 2001, 3, 321–327. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Pen Uelas, J.; Filella, I.; Lloret, P.; Mun Oz, F.; Vilajeliu, M. Reflectance assessment of mite effects on apple trees. Int. J. Remote. Sens. 1995, 16, 2727–2733. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.; Fredeen, A.; Merino, J.; Field, C. Reflectance indices associated with physiological changes in nitrogen-and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Gamon, J.; Penuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Lang, M.; Sowinska, M.; Heisel, F.; Miehe, J. Detection of vegetation stress via a new high resolution fluorescence imaging system. J. Plant Physiol. 1996, 148, 599–612. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Carter, G.A.; Cibula, W.G.; Miller, R.L. Narrow-band reflectance imagery compared with thermalimagery for early detection of plant stress. J. Plant Physiol. 1996, 148, 515–522. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Dashdondov, K.; Jo, K.; Kim, M.-H. Linear interpolation and Machine Learning Methods for Gas Leakage Prediction Base on Multi-source Data Integration. J. Korea Converg. Soc. 2022, 13, 33–41. [Google Scholar] [CrossRef]
- Pacholski, A.; Cai, G.; Nieder, R.; Richter, J.; Fan, X.; Zhu, Z.; Roelcke, M. Calibration of a simple method for determining ammonia volatilization in the field–comparative measurements in Henan Province, China. Nutr. Cycl. Agroecosystems 2006, 74, 259–273. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: https://www.R-project.org/ (accessed on 21 November 2024).
- Jilani, M.S.; Burki, T.; Waseem, K. Effect of nitrogen on growth and yield of radish. J. Agric. Res. 2010, 48, 219–225. [Google Scholar] [CrossRef]
- Kirkby, E.A. Plant growth in relation to nitrogen supply. Ecol. Bull. 1981, 33, 249–267. [Google Scholar]
- Bojović, B.; Marković, A.J. Correlation between nitrogen and chlorophyll content in wheat (Triticum aestivum L.). Kragujev. J. Sci. 2009, 31, 69–74. [Google Scholar]
- Samarina, L.; Malyukova, L.; Koninskaya, N.; Malyarovskaya, V.; Ryndin, A.; Tong, W.; Xia, E.; Khlestkina, E.J.H. Efficient vegetation indices for phenotyping of abiotic stress tolerance in tea plant (Camellia sinensis (L.) Kuntze). Heliyon 2024, 10, e35522. [Google Scholar] [CrossRef]
- Kakani, V.; Reddy, K. Mineral deficiency stress: Reflectance properties, leaf photosynthesis and growth of nitrogen deficient big bluestem (Andropogon gerardii). J. Agron. Crop. Sci. 2010, 196, 379–390. [Google Scholar] [CrossRef]
- Myneni, R.B.; Hall, F.G.; Sellers, P.J.; Marshak, A.L. The interpretation of spectral vegetation indexes. IEEE Trans. Geosci. Remote Sens. 1995, 33, 481–486. [Google Scholar] [CrossRef]
- Rubio-Delgado, J.; Pérez, C.J.; Vega-Rodríguez, M.A. Predicting leaf nitrogen content in olive trees using hyperspectral data for precision agriculture. Precis. Agric. 2021, 22, 1–21. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Narrow-band reflectance indices for mapping the combined effects of water and nitrogen stress in field grown tomato crops. Biosyst. Eng. 2020, 192, 133–143. [Google Scholar] [CrossRef]
- Ren, B.; Huang, Z.; Liu, P.; Zhao, B.; Zhang, J. Urea ammonium nitrate solution combined with urease and nitrification inhibitors jointly mitigate NH3 and N2O emissions and improves nitrogen efficiency of summer maize under fertigation. Field Crop. Res. 2023, 296, 108909. [Google Scholar] [CrossRef]
- Groenestein, C.; Hutchings, N.; Haenel, H.; Amon, B.; Menzi, H.; Mikkelsen, M.; Misselbrook, T.; Van Bruggen, C.; Kupper, T.; Webb, J. Comparison of ammonia emissions related to nitrogen use efficiency of livestock production in Europe. J. Clean. Prod. 2019, 211, 1162–1170. [Google Scholar] [CrossRef]

| Soil Texture | pH (1:5) | O.M 1 (g kg−1) | TK-N (mg kg−1) | Av. SiO2 (mg kg−1) | K+ | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|
| (cmolc kg−1) | |||||||
| Loam | 5.33 | 9.80 | 839.3 | 398.7 | 0.66 | 3.62 | 0.22 |
| Total | Compost (kg) | Basal Application (kg) | First Additional Fertilizer DAT 21 (kg) | Second Additional Fertilizer DAT 35 (kg) |
|---|---|---|---|---|
| 0 kg urea ha−1 (0 N) | 1500 | 0 | 0 | 0 |
| 117 kg urea ha−1 (0.5 N) | 1500 | 42 | 37.5 | 37.5 |
| 234 kg urea ha−1 (1 N) | 1500 | 84 | 75 | 75 |
| 468 kg urea ha−1 (2 N) | 1500 | 168 | 150 | 150 |
| Vegetation Index | Equation |
|---|---|
| Normalized difference vegetation index [30] | |
| Simple ratio index [30] | |
| Modified chlorophyll absorption in reflectance index [31,32] | |
| Transformed chlorophyll absorption in reflectance index [33] | |
| Optimized soil-adjusted vegetation index [34] | |
| Greenness index [35] | |
| Triangular vegetation index [36] | )] |
| Zarco-Tejada & Miller index [37] | |
| Simple ratio pigment index [38] | |
| Normalized phaeophytinization index [39] | ) |
| Normalized pigment chlorophyll index [40] | ) |
| Photochemical reflectance index [41] | ) |
| Structure intensive pigment index [38] | ) |
| Lichtenthaler indices [42] | ) |
| Carter indices [43,44] | |
| Gitelson and Merzlyak Indices [45] | |
| Anthocyanin reflectance indices [46] | |
| Carotenoid reflectance indices [47] | |
| Renormalized difference vegetation index [48] | )0.5) |
| Treatment (kg urea ha−1) | Height * (cm/plant) | Leaf Width *** (cm/plant) | Root Length *** (cm/plant) | Root Diameter *** (cm/plant) | Fresh Shoot Weight (g/plant) *** | Fresh Root Weight (g/plant) *** |
|---|---|---|---|---|---|---|
| 0 | 28.0 ± 1.0 b | 11.8 ± 0.6 c | 25.8 ± 1.3 b | 7.4 ± 0.5 b | 65.7 ± 14.1 b | 406.3 ± 74.5 b |
| 117 | 42.0 ± 1.5 a | 16.1 ± 0.6 b | 34.3 ± 1.2 a | 10.1 ± 0.4 a | 248.7 ± 60.1 a | 1338.1 ± 209.2 a |
| 234 | 44.0 ± 0.6 a | 16.6 ± 0.8 b | 33.0 ± 1.2 a | 10.3 ± 0.3 a | 250.6 ± 60.8 a | 1327.9 ± 60.8 a |
| 468 | 44.9 ± 1.5 a | 18.5 ± 0.1 a | 32.3 ± 2.0 a | 10.5 ± 0.3 a | 217.1 ± 20.5 a | 1246.4 ± 28.1 a |
| Vegetation Index | 0 kg urea ha−1 | 117 kg urea ha−1 | 234 kg urea ha−1 | 468 kg urea ha−1 |
|---|---|---|---|---|
| NDVI *** | 0.680 ± 0.037 b | 0.731 ± 0.027 a | 0.733 ± 0.023 a | 0.739 ± 0.031 a |
| SR *** | 5.330 ± 0.737 b | 6.515 ± 0.757 a | 6.551 ± 0.660 a | 6.754 ± 0.894 a |
| MCARI (700, 670 nm) *** | 0.432 ± 0.099 a | 0.307 ± 0.075 b | 0.281 ± 0.074 bc | 0.256 ± 0.088 c |
| MCARI (750, 705 nm) *** | 0.669 ± 0.125 b | 0.881 ± 0.140 a | 0.915 ± 0.131 a | 0.973 ± 0.183 a |
| TCARI *** | 0.505 ± 0.083 a | 0.390 ± 0.062 b | 0.369 ± 0.062 b | 0.345 ± 0.078 b |
| OSAVI ** | 0.751 ± 0.019 b | 0.768 ± 0.015 a | 0.765 ± 0.014 a | 0.766 ± 0.024 a |
| G *** | 3.112 ± 0.367 a | 2.787 ± 0.336 b | 2.692 ± 0.351 b | 2.625 ± 0.457 b |
| TVI ** | 44.373 ± 1.811 a | 43.221 ± 1.883 a | 42.673 ± 2.088 b | 42.220 ± 3.254 b |
| ZMI *** | 1.783 ± 0.142 c | 2.026 ± 0.156 b | 2.076 ± 0.162 ab | 2.143 ± 0.202 a |
| SPRIns | 1.117 ± 0.061 | 1.160 ± 0.058 | 1.147 ± 0.064 | 1.146 ± 0.060 |
| NPQIns | 0.061 ± 0.003 | 0.064 ± 0.005 | 0.073 ± 0.007 | 0.071 ± 0.006 |
| NPCIns | −0.047 ± 0.091 | −0.067 ± 0.088 | −0.061 ± 0.087 | −0.059 ± 0.095 |
| PRI * | −0.002 ± 0.025 b | 0.011 ± 0.024 a | 0.014 ± 0.022 a | 0.010 ± 0.024 a |
| SIPI ** | 0.748 ± 0.019 b | 0.771 ± 0.019 a | 0.771 ± 0.027 a | 0.772 ± 0.032 a |
| Lic1 ** | 0.778 ± 0.025 b | 0.794 ± 0.019 a | 0.792 ± 0.016 a | 0.793 ± 0.022 a |
| Lic2 ** | 0.845 ± 0.163 b | 0.936 ± 0.160 a | 0.935 ± 0.158 a | 0.956 ± 0.179 a |
| Ctr1 *** | 1.642 ± 0.310 a | 1.395 ± 0.245 b | 1.359 ± 0.233 b | 1.340 ± 0.291 b |
| Ctr2 *** | 0.232 ± 0.028 a | 0.191 ± 0.019 b | 0.187 ± 0.018 b | 0.180 ± 0.022 b |
| GM1 *** | 2.646 ± 0.331 c | 3.197 ± 0.352 b | 3.267 ± 0.308 ab | 3.408 ± 0.453 a |
| GM2 *** | 2.988 ± 0.338 c | 3.596 ± 0.350 b | 3.693 ± 0.386 ab | 3.861 ± 0.464 a |
| ARI1 * | −0.486 ± 0.154 a | −0.564 ± 0.181 a | −0.613 ± 0.282 a | −0.651 ± 0.232 b |
| ARI2 * | −0.341 ± 0.103 a | −0.398 ± 0.126 a | −0.426 ± 0.177 a | −0.454 ± 0.157 b |
| CRI1 ns | 4.516 ± 0.633 | 4.954 ± 0.683 | 4.869 ± 1.020 | 4.952 ± 1.227 |
| CRI2 ns | 4.030 ± 0.556 | 4.390 ± 0.650 | 4.256 ± 0.808 | 4.301 ± 1.100 |
| RDVI * | 0.694 ± 0.021 b | 0.704 ± 0.018 a | 0.707 ± 0.017 a | 0.703 ± 0.032 a |
| Positive | Negative | ||
|---|---|---|---|
| Vegetation Index | Relative Contribution | Vegetation Index | Relative Contribution |
| GM1 | 0.287 | Ctr2 | −0.269 |
| ZMI | 0.279 | TCARI | −0.266 |
| NDVI | 0.276 | MCARI(670) | −0.252 |
| SR | 0.274 | Ctr1 | −0.243 |
| MCARI(705) | 0.274 | G | −0.219 |
| GM2 | 0.273 | NPCI | −0.135 |
| PRI | 0.218 | TVI | −0.101 |
| Lic2 | 0.197 | NPQI | −0.067 |
| OSAVI | 0.162 | ARI2 | −0.044 |
| Lic1 | 0.144 | ARI1 | −0.032 |
| SPRI | 0.138 | CRI2 | −0.002 |
| RDVI | 0.138 | - | - |
| DAT | 0.092 | - | - |
| SIPI | 0.092 | - | - |
| CRI1 | 0.006 | - | - |
| Kg urea ha−1 | Pre-Experiment Total Soil Nitrogen (mg kg−1) | Post-Harvest Total Soil Nitrogen *** (mg kg−1) | Kg NH3 ha−1 Season−1 ** |
|---|---|---|---|
| 0 | 839.3 | 515.8 ± 8.53 c | 16.57 b |
| 117 | 461.4 ± 26.4 c | 13.61 c | |
| 234 | 784.8 ± 46.6 b | 18.27 a | |
| 468 | 971.9 ± 24.3 a | 26.41 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, T.; Kim, T.; Yoo, S. Effects of Nitrogen Fertilizer Application on Growth, Vegetation Indices, and Ammonia Volatilization in Korean Radish (Raphanus sativus L.). Nitrogen 2025, 6, 42. https://doi.org/10.3390/nitrogen6020042
Eom T, Kim T, Yoo S. Effects of Nitrogen Fertilizer Application on Growth, Vegetation Indices, and Ammonia Volatilization in Korean Radish (Raphanus sativus L.). Nitrogen. 2025; 6(2):42. https://doi.org/10.3390/nitrogen6020042
Chicago/Turabian StyleEom, TaeSeon, TaeWan Kim, and SungYung Yoo. 2025. "Effects of Nitrogen Fertilizer Application on Growth, Vegetation Indices, and Ammonia Volatilization in Korean Radish (Raphanus sativus L.)" Nitrogen 6, no. 2: 42. https://doi.org/10.3390/nitrogen6020042
APA StyleEom, T., Kim, T., & Yoo, S. (2025). Effects of Nitrogen Fertilizer Application on Growth, Vegetation Indices, and Ammonia Volatilization in Korean Radish (Raphanus sativus L.). Nitrogen, 6(2), 42. https://doi.org/10.3390/nitrogen6020042








