Optimizing Rice Yield and Heat Stress Resilience Through Nitrogen Top Dressing Before Panicle Emergence
Abstract
1. Introduction
2. Materials and Methods
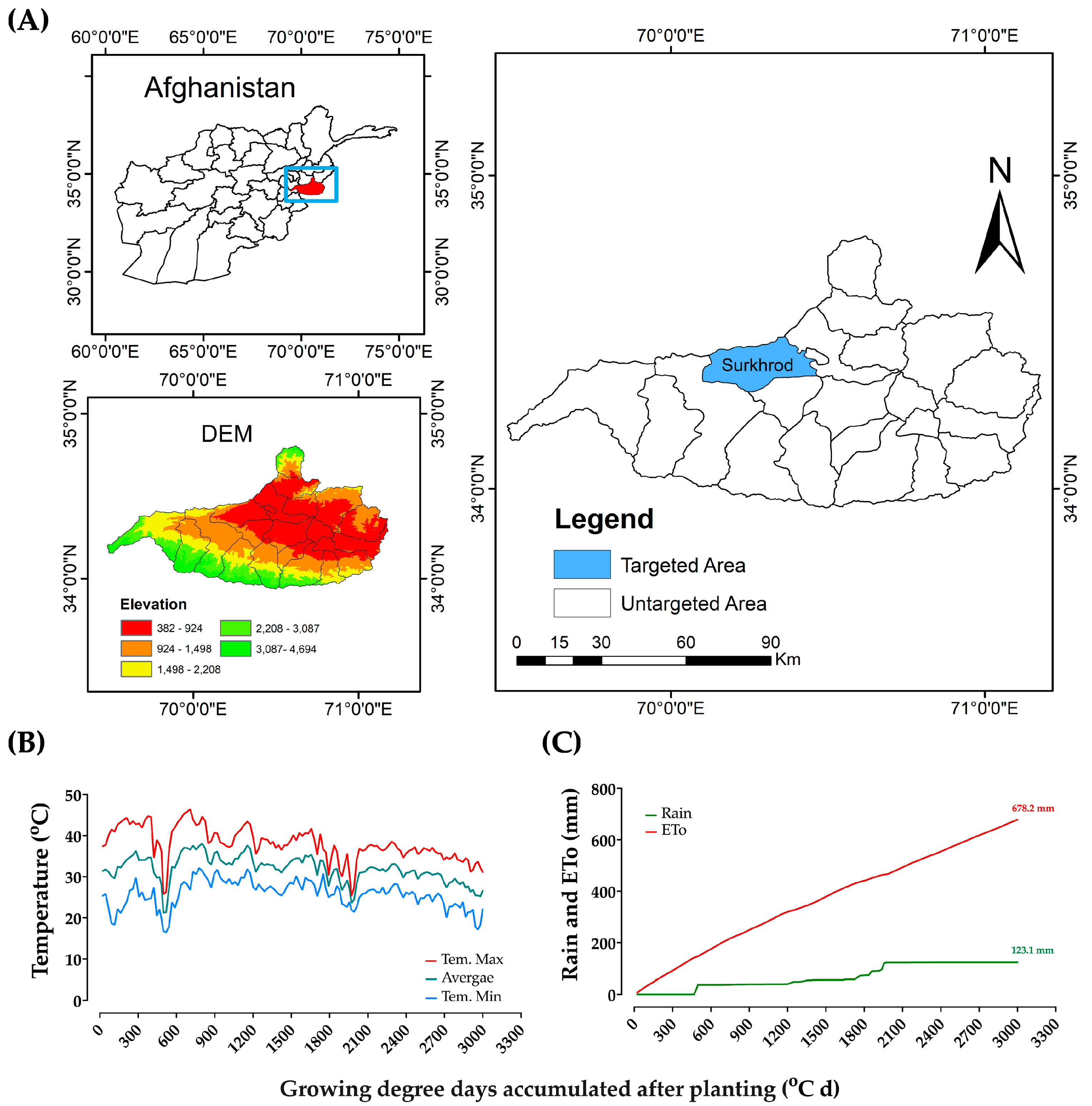
2.1. Cultivation and Heat Treatment
2.2. Physiological Attributes and SPAD Value
2.3. Spikelet Fertility
2.4. Yield and Its Components
2.5. Statistical Analysis
3. Results
3.1. Effects of High Temperature on Rice at the Anthesis Stage
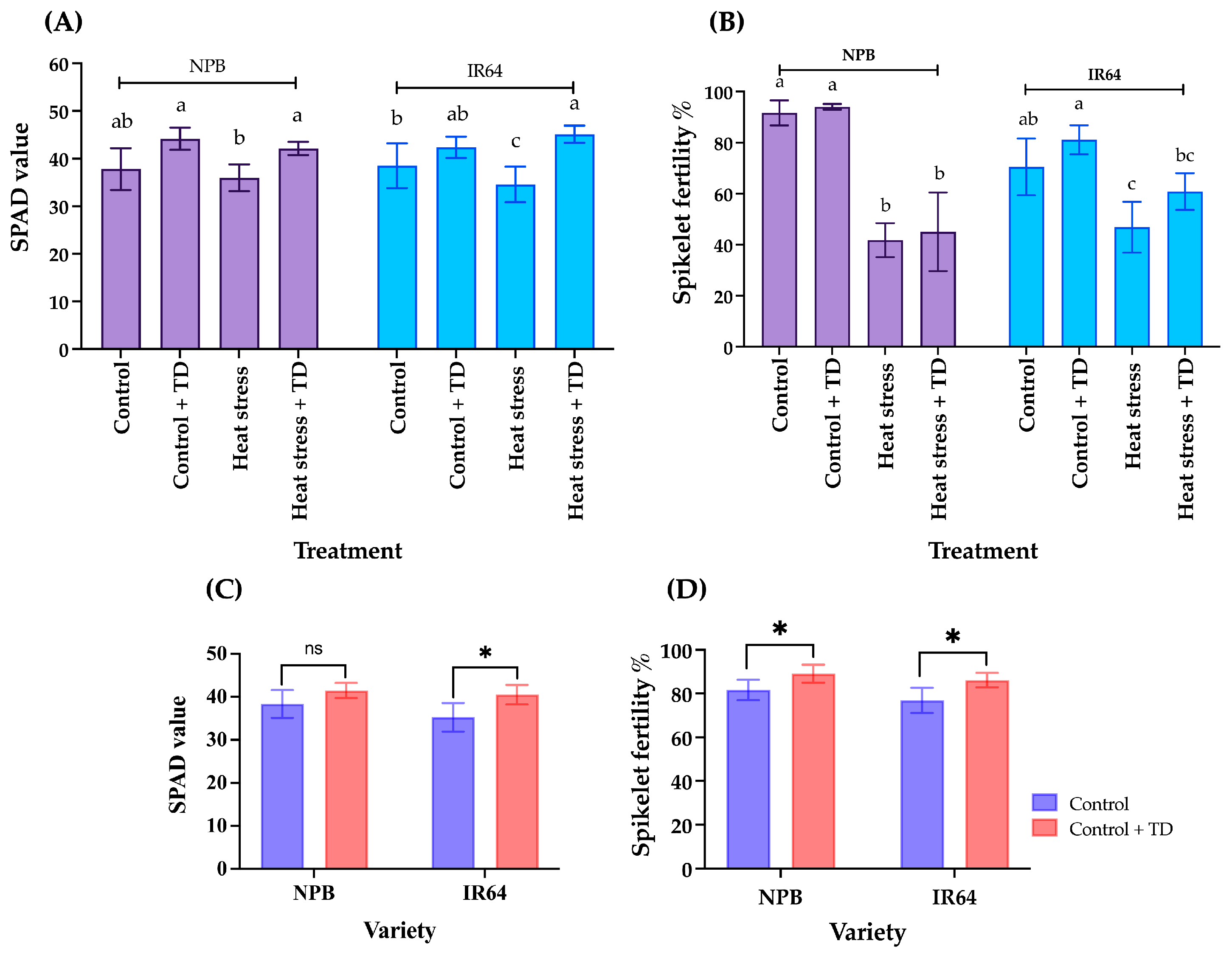
3.2. Response of N Top Dressing to Heat Stress, SPAD Value, and Spikelet Fertility
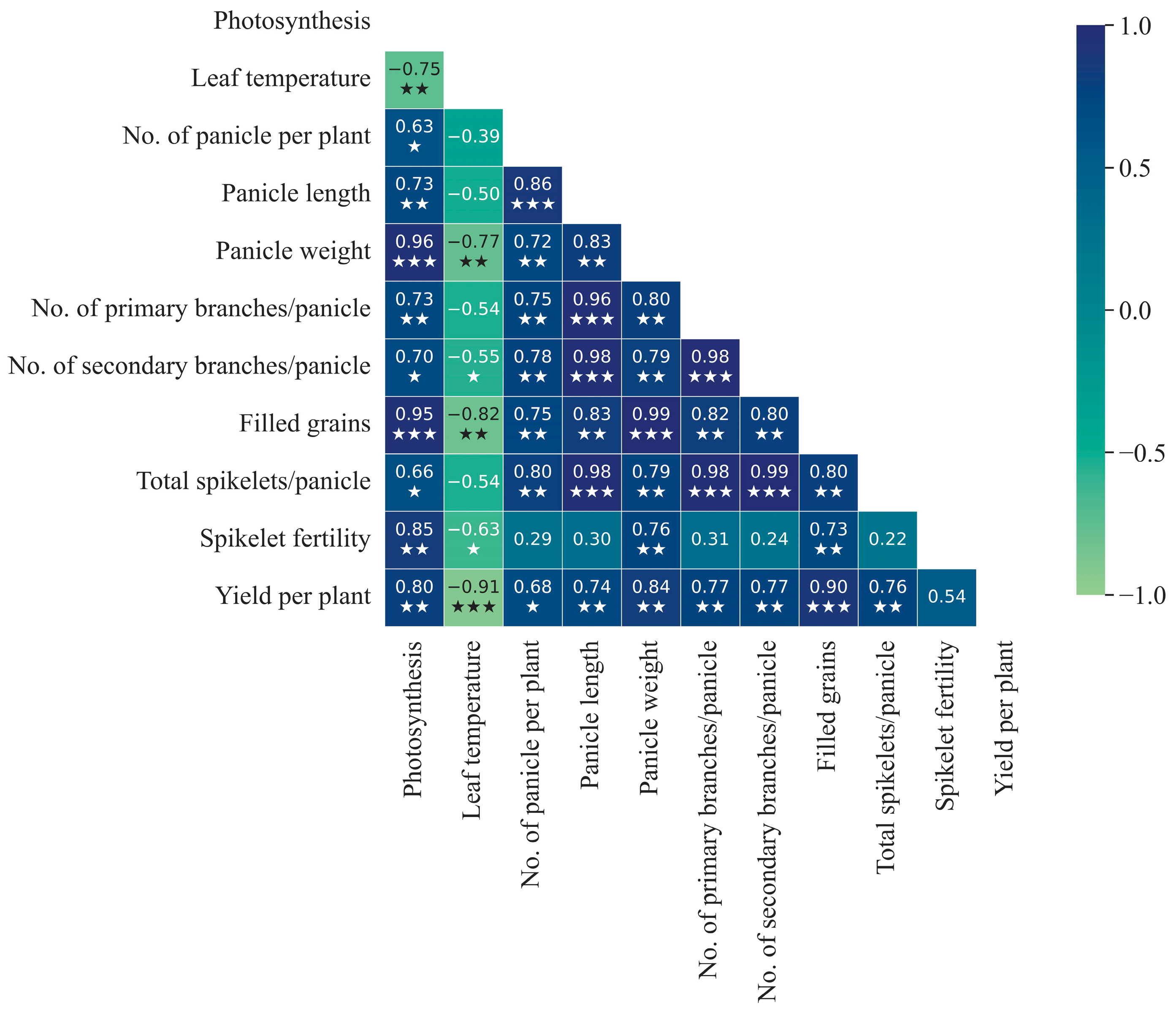
3.3. Relationship of SPAD Value with Spikelet Fertility
3.4. Effects of High Temperature on Physiological Attributes and the Response of N Top Dressing
3.5. Effects of N Top Dressing on Yield Components and Grain Yield of Rice
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. Population. Available online: https://www.un.org/en/global-issues/population (accessed on 17 December 2023).
- USDA. Food Security. Available online: https://www.usda.gov/topics/food-and-nutrition/food-security (accessed on 17 December 2023).
- Food and Agriculture Organization. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 17 December 2023).
- IRRI. International Rice Research Institute. Available online: https://www.irri.org/search?search=Importance+of+Rice (accessed on 17 December 2023).
- Kakar, K.; Xuan, T.D.; Haqani, M.I.; Rayee, R.; Wafa, I.K.; Abdiani, S.; Tran, H.-D. Current Situation and Sustainable Development of Rice Cultivation and Production in Afghanistan. Agriculture 2019, 9, 49. [Google Scholar] [CrossRef]
- Dawe, D.C.; Hardy, B.; Hettel, G.P.; Maclean, J. Rice Almanac: Source Book for the Most Important Economic Activity on Earth; CABI Publishing: Oxon, UK, 2002. [Google Scholar] [CrossRef]
- Ministry of Agriculture Irrigation and Livestock. Rice Planting and Yields Increased by 15% in 1399 Compared to 1398. Available online: https://mail.gov.af/en/node/4592 (accessed on 17 December 2023).
- Feng, S.; Hao, Z.; Zhang, X.; Wu, L.; Zhang, Y.; Hao, F. Climate change impacts on concurrences of hydrological droughts and high temperature extremes in a semi-arid river basin of China. J. Arid. Environ. 2022, 202, 104768. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.; Trenberth, K.E. Climate Change and Drought: A Perspective on Drought Indices. Curr. Clim. Chang. Rep. 2018, 4, 145–163. [Google Scholar] [CrossRef]
- Flores, J.; Pérez-Sánchez, R.M.; Jurado, E. The combined effect of water stress and temperature on seed germination of Chihuahuan Desert species. J. Arid. Environ. 2017, 146, 95–98. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of emerging implications of climate change on food production systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef]
- IPCC. Framing and Context. In Global Warming of 1.5 °C; Cambridge University Press: Cambridge, UK, 2022; pp. 49–92. [Google Scholar] [CrossRef]
- Hussain, S.; Huang, J.; Huang, J.; Ahmad, S.; Nanda, S.; Anwar, S.; Shakoor, A.; Zhu, C.; Zhu, L.; Cao, X.; et al. Rice Production Under Climate Change: Adaptations and Mitigating Strategies. In Environment, Climate, Plant and Vegetation Growth; Springer: Cham, Switzerland, 2020; pp. 659–686. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Fahad, S.; Adnan, M.; Hassan, S.; Saud, S.; Hussain, S.; Wu, C.; Wang, D.; Hakeem, K.R.; Alharby, H.F.; Turan, V.; et al. Rice Responses and Tolerance to High Temperature. Adv. Rice Res. Abiotic Stress Toler. 2019, 201–224. [Google Scholar] [CrossRef]
- Aryan, S.; Gulab, G.; Habibi, N.; Kakar, K.; Sadat, M.I.; Zahid, T.; Rashid, R.A. Phenological and physiological responses of hybrid rice under different high-temperature at seedling stage. Bull. Natl. Res. Cent. 2022, 46, 45. [Google Scholar] [CrossRef]
- Aryan, S.; Gulab, G.; Kakar, K.; Habibi, N.; Amin, M.W.; Sadat, M.I.; Zahid, T.; Durani, A.; Baber, B.M.; Safi, Z.; et al. Auxin Application at the Flowering Stage of Rice Alleviates the Negative Impact of Heat Stress on Spikelet Fertility and Yield Attributes. Agriculture 2023, 13, 866. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhou, J.; Hu, S.; Chen, H.; Xiang, J.; Zhang, Y.; Zeng, Y.; Shi, Q.; Zhu, D.; et al. Research Progress on Heat Stress of Rice at Flowering Stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Chidambaranathan, P.; Balasubramaniasai, C.; Behura, N.; Purty, M.; Samantaray, S.; Subudhi, H.; Ngangkham, U.; Devanna, B.N.; Katara, J.L.; Kumar, A.; et al. Effects of high temperature on spikelet sterility in rice (Oryza sativa L.): Association between molecular markers and allelic phenotypic effect in field condition. Genet. Resour. Crop Evol. 2021, 68, 1923–1935. [Google Scholar] [CrossRef]
- Park, J.R.; Kim, E.G.; Jang, Y.H.; Kim, K.M. Screening and identification of genes affecting grain quality and spikelet fertility during high-temperature treatment in grain filling stage of rice. BMC Plant Biol. 2021, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, W.; Lu, Q.; Huang, J.; Peng, S.; Cui, K. Abnormal anther development leads to lower spikelet fertility in rice (Oryza sativa L.) under high temperature during the panicle initiation stage. BMC Plant Biol. 2021, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cui, K.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Nie, L.; Huang, J.; Peng, S.; Ding, Y. Intensified pollination and fertilization ameliorate heat injury in rice (Oryza sativa L.) during the flowering stage. Field Crops Res. 2020, 252, 107795. [Google Scholar] [CrossRef]
- Ye, T.; Li, Y.; Zhang, J.; Hou, W.; Zhou, W.; Lu, J.; Xing, Y.; Li, X. Nitrogen, phosphorus, and potassium fertilization affects the flowering time of rice (Oryza sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Abbas, S.; Mayo, Z.A. Impact of temperature and rainfall on rice production in Punjab, Pakistan. Environ. Dev. Sustain. 2021, 23, 1706–1728. [Google Scholar] [CrossRef]
- Qutbudin, I.; Shiru, M.S.; Sharafati, A.; Ahmed, K.; Al-Ansari, N.; Yaseen, Z.M.; Shahid, S.; Wang, X. Seasonal drought pattern changes due to climate variability: Case study in Afghanistan. Water 2019, 11, 1096. [Google Scholar] [CrossRef]
- Ishfaq, M.; Akbar, N.; Zulfiqar, U.; Ali, N.; Jabran, K.; Nawaz, M.; Farooq, M. Influence of Nitrogen Fertilization Pattern on Productivity, Nitrogen Use Efficiencies, and Profitability in Different Rice Production Systems. J. Soil Sci. Plant Nutr. 2021, 21, 145–161. [Google Scholar] [CrossRef]
- Yousaf, M.; Li, J.; Lu, J.; Ren, T.; Cong, R.; Fahad, S.; Li, X. Effects of fertilization on crop production and nutrient-supplying capacity under rice-oilseed rape rotation system. Sci. Rep. 2017, 7, 1270. [Google Scholar] [CrossRef]
- Ferdous, J.; Mahjabin, F.; al Asif, M.A.; Riza, I.J.; Jahangir, M.M.R. Gaseous Losses of Nitrogen from Rice Field: Insights into Balancing Climate Change and Sustainable Rice Production. In Sustainable Rice Production-Challenges, Strategies and Opportunities; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Gu, B.; van Grinsven, H.J.; Lam, S.K.; Oenema, O.; Sutton, M.A.; Mosier, A.; Chen, D. A Credit System to Solve Agricultural Nitrogen Pollution. Innovation 2021, 2, 100079. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Huang, S.; Zhao, Y. Synthesis composite hydrogels from inorganic-organic hybrids based on leftover rice for environment-friendly controlled-release urea fertilizers. Sci. Total Environ. 2018, 615, 422–430. [Google Scholar] [CrossRef]
- Hou, W.; Shen, J.; Xu, W.; Khan, M.R.; Wang, Y.; Zhou, X.; Gao, Q.; Murtaza, B.; Zhang, Z. Recommended nitrogen rates and the verification of effects based on leaf SPAD readings of rice. PeerJ 2021, 9, e12107. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yang, J.; Wang, H.; Zou, J.; He, J. Effects of Nitrogen Application Rate and Leaf Age on the Distribution Pattern of Leaf SPAD Readings in the Rice Canopy. PLoS ONE 2014, 9, e88421. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Tränkner, M.; Lu, J.; Yan, J.; Huang, S.; Ren, T.; Cong, R.; Li, X. Diagnosis of Nitrogen Nutrition in Rice Leaves Influenced by Potassium Levels. Front. Plant Sci. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of nitrogen application rate on the photosynthetic pigment, leaf fluorescence characteristics, and yield of indica hybrid rice and their interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef] [PubMed]
- Caddell, D.; Langenfeld, N.J.; Eckels, M.J.; Zhen, S.; Klaras, R.; Mishra, L.; Bugbee, B.; Coleman-Derr, D. Photosynthesis in rice is increased by CRISPR/Cas9-mediated transformation of two truncated light-harvesting antenna. Front. Plant Sci. 2023, 14, 1050483. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Gu, J.F.; Zhou, Z.X.; Li, Z.K.; Dai, Q.X.; Kong, X.S.; Wang, Z.Q.; Yang, J.C. Effects of the mutant with low chlorophyll content on photosynthesis and yield in rice. Acta Agron. Sin. 2016, 42, 551–560. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, T.; Yang, Z.; Wang, T.; Fu, Y.; Chen, Y.; Hu, B.; Ren, W. Morphophysiological mechanism of rice yield increase in response to optimized nitrogen management. Sci. Rep. 2017, 7, 17226. [Google Scholar] [CrossRef]
- Fu, P.; Wang, J.; Zhang, T.; Huang, J.; Peng, S. High nitrogen input causes poor grain filling of spikelets at the panicle base of super hybrid rice. Field Crops Res. 2019, 244, 107635. [Google Scholar] [CrossRef]
- MAFF (Ministry of Agriculture, Forestry and Fisheries). 2023. Available online: https://www.maff.go.jp/e/ (accessed on 21 May 2025).
- Van, R.L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Centre (ISRIC), Food and Agriculture Organization of the United Nations: Wageningen, The Netherlands, 2002; Available online: https://www.isric.org/documents/document-type/technical-paper-09-procedures-soil-analysis-6th-edition (accessed on 14 January 2024).
- Aryan, S.; Gulab, G.; Hashemi, T.; Habibi, S.; Kakar, K.; Habibi, N.; Amin, M.W.; Sadat, M.I.; Zahid, T.; Zerak, A. Pre-spike emergence nitrogen fertilizer application as a strategy to improve floret fertility and production efficiency in wheat. Field Crops Res. 2024, 319, 109623. [Google Scholar] [CrossRef]
- Sanwong, P.; Sanitchon, J.; Dongsansuk, A.; Jothityangkoon, D. High Temperature Alters Phenology, Seed Development and Yield in Three Rice Varieties. Plants 2023, 12, 666. [Google Scholar] [CrossRef]
- Weather Meta-Meteoblue. Available online: https://www.meteoblue.com/en/weather/week/meta_italy_3173524 (accessed on 3 April 2023).
- Shrestha, S.; Mahat, J.; Shrestha, J.; Madhav, K.C.; Paudel, K. Influence of high-temperature stress on rice growth and development. A review. Heliyon 2022, 8, e12651. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cui, K.; Fahad, S. Heat Stress Decreases Rice Grain Weight: Evidence and Physiological Mechanisms of Heat Effects Prior to Flowering. Int. J. Mol. Sci. 2022, 23, 10922. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Bao, J.; Gao, Z.; Sun, D.; Zheng, S.; Bai, J. How rice adapts to high temperatures. Front. Plant Sci. 2023, 14, 1137923. [Google Scholar] [CrossRef]
- Wu, C.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Ding, Y. Roles of phytohormone changes in the grain yield of rice plants exposed to heat: A review. PeerJ 2019, 7, e7792. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef]
- Gu, J.; Yang, J. Nitrogen (N) transformation in paddy rice field: Its effect on N uptake and relation to improved N management. Crop Environ. 2022, 1, 7–14. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, G.; Guo, S.; Li, X.; Yuan, J.; Hu, A. Improving Nitrogen Use Efficiency in Rice for Sustainable Agriculture: Strategies and Future Perspectives. Life 2022, 12, 1653. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Ishimaru, T.; Hlaing, K.T.; Oo, Y.M.; Lwin, T.M.; Sasaki, K.; Lumanglas, P.D.; Simon, E.-V.M.; Myint, T.T.; Hairmansis, A.; Susanto, U.; et al. An early-morning flowering trait in rice can enhance grain yield under heat stress field conditions at flowering stage. Field Crops Res. 2022, 277, 108400. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sathishraj, R.; Manoharan, M.; Sumanth, H.; Muthurajan, R.; Ishimaru, T.; Krishna, J.S. Is early morning flowering an effective trait to minimize heat stress damage during flowering in rice? Field Crops Res. 2017, 203, 238–242. [Google Scholar] [CrossRef]
- Mboyerwa, P.A.; Kibret, K.; Mtakwa, P.; Aschalew, A. Rice Yield and Nitrogen Use Efficiency With System of Rice Intensification and Conventional Management Practices in Mkindo Irrigation Scheme, Tanzania. Front. Sustain. Food Syst. 2022, 6, 802267. [Google Scholar] [CrossRef]
- Julia, C.; Dingkuhn, M. Predicting temperature induced sterility of rice spikelets requires simulation of crop-generated microclimate. Eur. J. Agron. 2013, 49, 50–60. [Google Scholar] [CrossRef]
- Zhang, C.X.; Fu, G.F.; Yang, X.Q.; Yang, Y.J.; Zhao, X.; Chen, T.T.; Zhang, X.F.; Jin, Q.Y.; Tao, L.X. Heat Stress Effects are Stronger on Spikelets Than on Flag Leaves in Rice Due to Differences in Dissipation Capacity. J. Agron. Crop Sci. 2016, 202, 394–408. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef]
- Fu, G.; Feng, B.; Zhang, C.; Yang, Y.; Yang, X.; Chen, T.; Zhao, X.; Zhang, X.; Jin, Q.; Tao, L. Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures. Front. Plant Sci. 2016, 7, 1637. [Google Scholar] [CrossRef]
- Hakata, M.; Wada, H.; Kubo, C.M.; Tanaka, R.; Sato, H.; Morita, S. Development of a new heat tolerance assay system for rice spikelet sterility. Plant Methods 2017, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Kamiji, Y.; Yoshida, H.; Palta, J.A.; Sakuratani, T.; Shiraiwa, T. N applications that increase plant N during panicle development are highly effective in increasing spikelet number in rice. Field Crops Res. 2011, 122, 242–247. [Google Scholar] [CrossRef]
- Pereira, E.G.; Bucher, C.P.C.; Santos, L.A.; Lerin, J.; Catarina, C.S.; Fernandes, M.S. The amino acid transporter OsAAP1 regulates the fertility of spikelets and the efficient use of N in rice. Plant Soil 2022, 480, 507–521. [Google Scholar] [CrossRef]
- Chuma, B.A.; Cotter, M.; Kalisa, A.; Rajaona, A.; Senthilkumar, K.; Stuerz, S.; Vincent, I.; Asch, F. Altitude, temperature, and N Management effects on yield and yield components of contrasting lowland rice cultivars. J. Agron. Crop Sci. 2020, 206, 456–465. [Google Scholar] [CrossRef]
- Sonjaroon, W.; Jutamanee, K.; Khamsuk, O.; Thussagunpanit, J.; Kaveeta, L.; Suksamrarn, A. Impact of brassinosteroid mimic on photosynthesis, carbohydrate content and rice seed set at reproductive stage under heat stress. Agric. Nat. Resour. 2018, 52, 234–240. [Google Scholar] [CrossRef]
- Oh-E, I.; Saitoh, K.; Kuroda, T. Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Prod. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Pinter, P.J., Jr.; Kimball, B.; Adamsen, F.; LaMorte, R.; Wall, G.; Hunsaker, D.; Adam, N.; Brooks, T.; Garcia, R.; et al. Leaf nitrogen concentration of wheat subjected to elevated [CO2] and either water or N deficits. Agric. Ecosyst. Environ. 2000, 79, 53–60. [Google Scholar] [CrossRef]
- Ookawa, T.; Naruoka, Y.; Yamazaki, T.; Suga, J.; Hirasawa, T. A comparison of the accumulation and partitioning of nitrogen in plants between two rice cultivars, Akenohoshi and Nipponbare, at the ripening stage. Plant Prod. Sci. 2003, 6, 172–178. [Google Scholar] [CrossRef]
- Pantoja-Benavides, A.D.; Garces-Varon, G.; Restrepo-Díaz, H. Foliar Growth Regulator Sprays Induced Tolerance to Combined Heat Stress by Enhancing Physiological and Biochemical Responses in Rice. Front. Plant Sci. 2021, 12, 702892. [Google Scholar] [CrossRef] [PubMed]
- Lestari, A.P.; Suwarno; Trikoesoemaningtyas; Sopandie, D.; Aswidinnoor, H. Panicle Length and Weight Performance of F3 Population from Local and Introduction Hybridization of Rice Varieties. HAYATI J. Biosci. 2015, 22, 87–92. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, W.; Chen, N.; Wang, Z.; Li, G.; Ding, Y.; Ninomiya, S.; Mu, Y. Analyzing Nitrogen Effects on Rice Panicle Development by Panicle Detection and Time-Series Tracking. Plant Phenomics 2023, 5, 0048. [Google Scholar] [CrossRef]
- Mboyerwa, P.A.; Kibret, K.; Mtakwa, P.; Aschalew, A. Lowering nitrogen rates under the system of rice intensification enhanced rice productivity and nitrogen use efficiency in irrigated lowland rice. Heliyon 2022, 8, e09140. [Google Scholar] [CrossRef]
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of Organic and Inorganic Fertilizer Application on Growth, Yield, and Grain Quality of Rice. Agriculture 2020, 10, 544. [Google Scholar] [CrossRef]
- Ding, C.; You, J.; Chen, L.; Wang, S.; Ding, Y. Nitrogen fertilizer increases spikelet number per panicle by enhancing cytokinin synthesis in rice. Plant Cell Rep. 2014, 33, 363–371. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Mean Daytime Temperature (°C) | Mean Nighttime Temperature (°C) | ||
|---|---|---|---|---|
| NPB | IR64 | NPB | IR64 | |
| Ambient | 29.6 | 25.6 | 25.7 | 22.8 |
| HS | 34.5 | 31.2 | 27.0 | 24.9 |
| Variety | Treatment | Photosynthetic Rate (μmol CO2 m−2 s−1) | Stomatal Conductance (mol CO2 m−2 s−1) | Transpiration Rate (μmol m−2 s−1) | Leaf Temperature (°C) |
|---|---|---|---|---|---|
| NPB | Control | 7.5 ± 0.65 a | 0.17 ± 0.02 a | 4.5 ± 1.18 a | 40.1 ± 1.53 ab |
| Control + TD | 8.1 ± 1.01 a | 0.12 ± 0.01 a | 4.8 ± 1.04 a | 40.9 ± 1.82 ab | |
| Heat stress | 1.8 ± 0.70 b | 0.09 ± 0.03 ab | 5.6 ± 1.54 a | 43.2 ± 1.45 a | |
| Heat stress + TD | 3.8 ± 1.28 b | 0.11 ± 0.04 a | 6.3 ± 1.78 a | 43.0 ± 1.34 a | |
| Significance | *** | * | ns | * | |
| IR64 | Control | 7.5 ± 0.78 a | 0.20 ± 0.02 a | 2.4 ± 0.86 b | 36.7 ± 1.78 b |
| Control + TD | 9.4 ± 1.87 a | 0.21 ± 0.04 a | 4.1 ± 1.41 ab | 36.3 ± 1.75 b | |
| Heat stress | 5.5 ± 1.40 ab | 0.05 ± 0.04 b | 8.6 ± 1.15 a | 43.1 ± 2.13 a | |
| Heat stress + TD | 6.6 ± 1.14 a | 0.11 ± 0.03 ab | 8.8 ± 1.87 a | 42.8 ± 2.13 a | |
| Significance | * | ** | * | * |
| Variety | Treatment | NPP | PL (cm) | PW (g) | NPBP | NSBP | FGP | UGP | TSP | 1000-GW (g) | YP (g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPB | Experiment I | Control | 6.4 ± 0.8 b | 20.2 ± 0.6 a | 1.88 ± 0.10 a | 8.2 ± 0.3 a | 15.7 ± 1.2 a | 63.3 ± 3.6 a | 7.1 ± 1.9 b | 69.0 ± 3.1 a | 54.8 ± 0.5 a | 21.7 ± 2.1 b |
| Control + TD | 12.8 ± 2.0 a | 20.5 ± 0.5 a | 1.78 ± 0.10 a | 8.6 ± 0.2 a | 16.1 ± 1.0 a | 65.6 ± 2.9 a | 4.2 ± 2.1 b | 69.8 ± 1.8 a | 51.0 ± 2.3 a | 43.7 ± 8.3 a | ||
| Heat stress | 5.6 ± 0.51b | 17.3 ± 0.4 b | 0.70 ± 0.05 b | 7.4 ± 0.4 a | 9.1 ± 0.7 b | 21.3 ± 1.4 b | 29.7 ± 2.4 a | 51.0 ± 2.5 b | 48.7 ± 5.5 a | 5.7 ± 1.7 c | ||
| Heat stress + TD | 8.2 ± 1.2 ab | 18.8 ± 0.2 ab | 0.76 ± 0.06 b | 7.6 ± 0.2 a | 11.9 ± 1.1 ab | 24.3 ± 4.1 b | 27.3 ± 2.2 a | 51.6 ± 1.3 b | 44.1 ± 2.3 a | 8.0 ±1.9 c | ||
| Significance | ** | ** | *** | ns | *** | *** | *** | *** | ns | *** | ||
| Experiment II | Control | 27.8 ± 9.3 ns | 26.0 ± 1.0 ns | 2.5 ± 0.3 ns | 11.3 ± 1.1 * | 19.1 ± 4.0 ns | 97.9 ± 10.7 * | 19.3 ± 8.4 * | 116.5 ± 15.4 ns | 34.1 ± 4.9 * | 93.1 ± 16.8 * | |
| Control TD | 28.1 ± 12.9 | 26.8 ± 1.3 | 2.9 ± 0.3 | 12.1 ± 1.1 | 19.4 ± 3.7 | 103.3 ± 13.1 | 13.1 ± 4.9 | 117.2 ± 12.7 | 37.4 ± 6.0 | 115.2 ± 30.5 | ||
| IR64 | Experiment I | Control | 10.9 ± 0.9 b | 22.7 ± 0.4 b | 1.99 ± 0.13 b | 9.5 ± 0.2 a | 25.0 ± 1.8 b | 75.4 ± 4.6 b | 31.5 ± 7.7 ab | 106.9 ± 8.6 a | 46.2 ± 0.7 a | 77.3 ± 5.8 a |
| Control + TD | 22.0 ± 1.4 a | 24.7 ± 0.3 a | 2.54 ± 0.12 a | 9.6 ± 0.1 a | 29.3 ± 1.0 a | 97.2 ± 5.5 a | 22.3 ± 2.7 b | 119.5 ± 4.3 a | 46.4 ± 0.5 a | 92.0 ± 7.6 a | ||
| Heat stress | 10.0 ± 1.0 b | 22.4 ± 0.5 b | 1.42 ± 0.04 c | 9.3 ± 0.3 a | 24.8 ± 1.9 b | 46.2 ± 2.5 c | 53.5 ± 6.8 a | 99.7 ± 5.0 ab | 46.0 ± 4.3 a | 22.9 ± 3.0 b | ||
| Heat stress + TD | 20.6 ± 1.7 a | 24.6 ± 0.5 a | 1.95 ± 0.10 b | 9.5 ± 0.1 a | 25.9 ± 2.2 b | 69.2 ± 4.3 b | 44.6 ± 4.4 ab | 113.8 ± 4.5 a | 52.1 ± 5.3 a | 35.6 ± 6.1 b | ||
| Significance | *** | *** | *** | ns | * | *** | ** | * | ns | *** | ||
| Experiment II | Control | 34.8 ± 5.5 ns | 19.9 ± 1.6 ns | 2.3 ± 0.5 ns | 8.7 ± 0.5 ns | 22.2 ± 3.9 ns | 92.1 ± 9.6 * | 27.8 ± 8.2 *** | 119.9 ± 11.3 ns | 40.9 ± 6.1 ns | 126.9 ± 23.6 * | |
| Control + TD | 34.2 ± 10.7 | 20.6 ± 1.1 | 2.6 ± 0.2 | 9.1 ± 0.8 | 22.4 ± 2.3 | 100.6 ± 12.1 | 19.2 ± 4.3 | 120.1 ± 10.9 | 42.9 ± 3.5 | 148.5 ± 29.8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aryan, S.; Gulab, G.; Habibi, S.; Zahid, T.; Safi, Z.; Habibi, N.; Mahmoodzada, A.B.; Amin, M.W.; Samsor, I.A.; Erie, K. Optimizing Rice Yield and Heat Stress Resilience Through Nitrogen Top Dressing Before Panicle Emergence. Nitrogen 2025, 6, 40. https://doi.org/10.3390/nitrogen6020040
Aryan S, Gulab G, Habibi S, Zahid T, Safi Z, Habibi N, Mahmoodzada AB, Amin MW, Samsor IA, Erie K. Optimizing Rice Yield and Heat Stress Resilience Through Nitrogen Top Dressing Before Panicle Emergence. Nitrogen. 2025; 6(2):40. https://doi.org/10.3390/nitrogen6020040
Chicago/Turabian StyleAryan, Shafiqullah, Gulbuddin Gulab, Safiullah Habibi, Tayebullah Zahid, Zabihullah Safi, Nasratullah Habibi, Abdul Basir Mahmoodzada, Mohammad Wasif Amin, Ijaz Ahmad Samsor, and Kenji Erie. 2025. "Optimizing Rice Yield and Heat Stress Resilience Through Nitrogen Top Dressing Before Panicle Emergence" Nitrogen 6, no. 2: 40. https://doi.org/10.3390/nitrogen6020040
APA StyleAryan, S., Gulab, G., Habibi, S., Zahid, T., Safi, Z., Habibi, N., Mahmoodzada, A. B., Amin, M. W., Samsor, I. A., & Erie, K. (2025). Optimizing Rice Yield and Heat Stress Resilience Through Nitrogen Top Dressing Before Panicle Emergence. Nitrogen, 6(2), 40. https://doi.org/10.3390/nitrogen6020040










