Integrating Agronomic and Molecular Advancements to Enhance Nitrogen Use Efficiency (NUE) and Promote Sustainable Rice Production
Abstract
1. Introduction
2. Concepts of NUE
- Recovery efficiency of N (REN) = (UNN − UN0)/(FN − FN0), kg N uptake per kg N applied;
- Agronomic efficiency of N (AEN) = (GYN − GY0)/(FN − FN0), kg grain yield increase per kg N applied;
- Partial factor productivity of N (PFPN) = GYN/FN, kg grain yield per kg N applied;
- Physiological efficiency of applied N (PEN) = (GYN − GY0)/(UNN − UN0), kg grain yield increase per kg N uptake;
- Internal efficiency of N (IEN) = GYN/UNN, kg grain per kg N uptake;
- Utilization efficiency (UEN) = Physiological efficiency × Apparent N recovery, kg kg−1, where GYN is the total grain yield when N is applied, FN is total N fertilizer applied, GY0 is total grain yield without N application, UNN is total N uptake with N treatment, and UN0 is total N uptake without nitrogen.
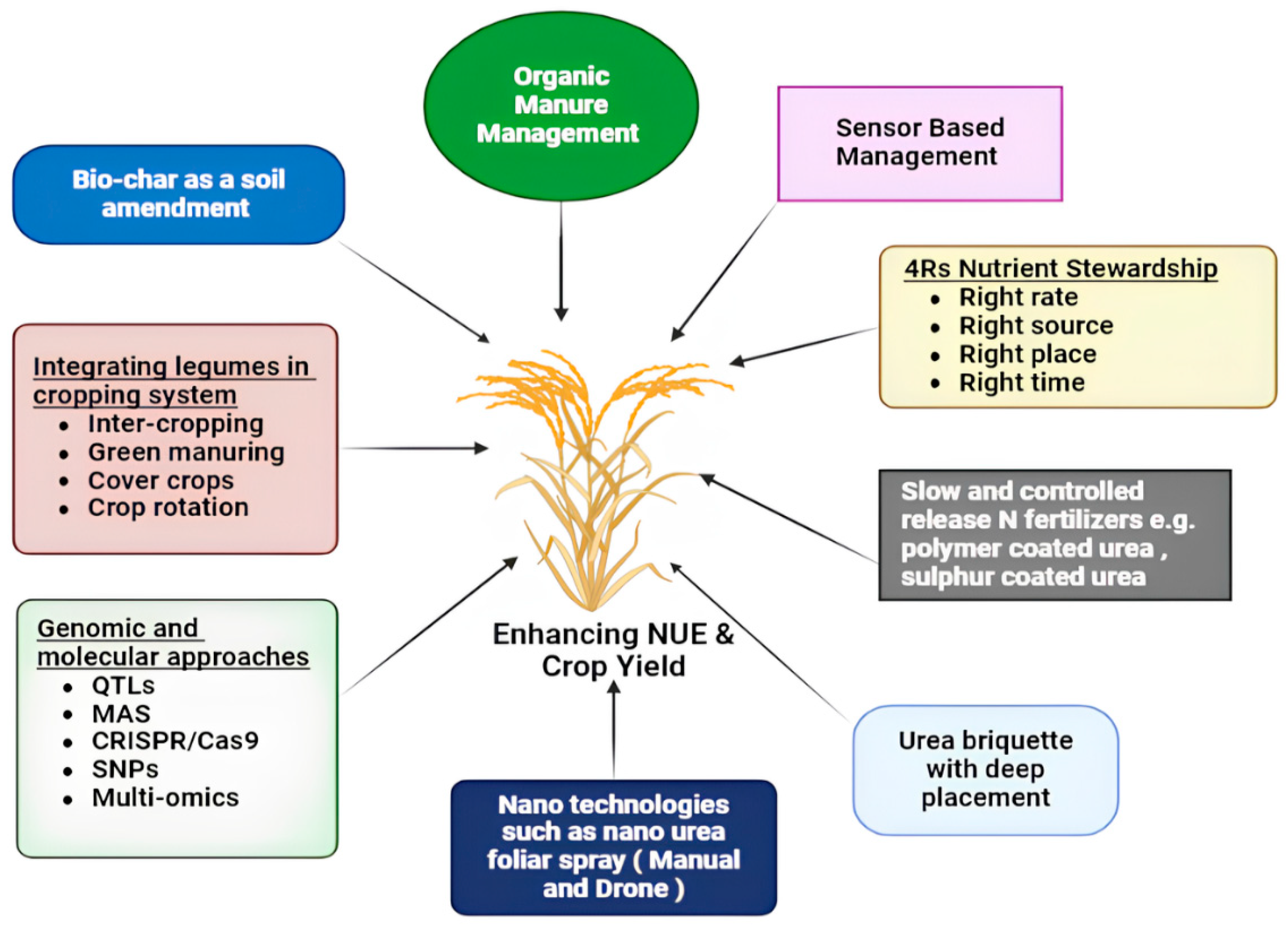
3. Efficient Fertilizer Management in Farmlands
3.1. The 4Rs Nutrient Stewardship Framework
3.2. Enhanced Efficiency Nitrogen Fertilizers (EENFs)
3.3. Nanotechnology
3.4. Digital and Sensor-Based Technologies for Enhancing Fertilizer Use Efficiency
3.5. Conjoint Application of Biochar and Nitrogenous Fertilizers
3.6. Biological N Fixation
| S.N. | Technologies | Country | Crop | NUE Increment | Grain Yield Increment | Economic Benefits | Reduction in Emissions | References |
|---|---|---|---|---|---|---|---|---|
| 1. | 4Rs Technology (right timing- three spilt doses) | Pakistan | Rice | 42 (N uptake), 146 (AFN), | 44% | Reduction in cost of production (31–43%) | [39] | |
| 2. | 4Rs Technology (right timing) | Ethiopia | Rice | 31.4 (PFPN) | 5.6% | Reduction in N fertilizer use by 31.4% | [40] | |
| 3. | 4Rs | Nepal | Maize | 35% | 40% | [35] | ||
| 4. | Polymer-coated urea | China | Rice | 5.2 to 5.9 kg/kg | 1.0 to 1.3 t ha−1 | [52] | ||
| 5. | Urea briquette (UB) | Nepal | Rice | 25% | [23] | |||
| 6. | Deep placement of urea super granules (USGs) | Bangladesh | Rice | NUE −13%, agronomic NUE- 20%, recovery N use efficiency −19% | 5.22% | [103] | ||
| 7. | Slow-release fertilizer | China | Rice | N recovery efficiency by 62.50–91.57% and 24.38–64.24% (two rice cultivars Yongxian15 and Yongyou1540, respectively) | 6.30–11.64% and 6.23–13.11% (two rice cultivars Yongxian15 and Yongyou1540, respectively) | CH4 emissions by 25.34% and N20 emissions by 48.97% | [57] | |
| 8. | Controlled release urea + conventional urea | China | Rice | 24.4% | 25.3% | [104] | ||
| 9. | Polymer-coated urea | China | Wheat | 3–34% | Reduce N volatilization loss (23–62%), ammonia emissions (51.3–91.3%) | [105] | ||
| 10. | Controlled release urea | China | Rice | 0.65–43.96% | 5.21–11.44% | [58] | ||
| 11. | Controlled release urea with deep placement | China | Rice | 41.47% | 76.43% | CH4 accumulation by 25.34% | [59] | |
| 12. | Joint use of PCU and urea briquette | Nepal | Maize and rice | 46% | 23% | [49] | ||
| 13. | Nano-urea | India | Maize, wheat | 80–90% | [67] | |||
| 14. | Application of nano-urea in flooding-irrigated conditions | Bangladesh | Rice | 95.38% | 4.91% | [106] | ||
| 15. | Application of N 150 kg/ha along with nano-urea (4%) in wheat | India | Wheat | 7.8% | Gross return of $2542 and cost–benefit of 2.01 | [107] | ||
| 16. | Co-joint application of biochar (8 t/ha) | India | Peas rabi season and maize kharif season | Benefit–cost ratio of 1.47 | One ton of biochar mitigates 6.22 tonnes of CO2 | [91] | ||
| 17. | NFC combined with 50% chemical fertilizers | China | Rice | 18.62% | 43.83% | NH3 volatilization losses by 41.63–45.34% | [94] |
4. Molecular Mechanisms of Nitrogen Uptake and Assimilation
4.1. Root System Architecture (RSA) and Genes Associated with Improving RSA
4.2. Hormonal Regulation for Enhancing NUE
4.3. Mechanisms of Nitrogen Uptake and Assimilation Through Transporters
4.4. Nitrogen Assimilation and Utilization Pathways
4.5. Key Genes and Transcription Factors (TFs) Associated with Enhanced NUE in Rice
| Category | Gene | Descriptions | Function | Crops | References |
|---|---|---|---|---|---|
| Root System Architecture | OsAMT1;1 | Ammonium transporter 1 member 1 | Increase root length and surface area and encode ammonium transporters, enhancing nitrogen accumulation. | Rice | [114] |
| OsAMT1;2 | Ammonium transporter 1 member 2 | ||||
| RNR10 | Ribonucleoside-diphosphate reductase | Nitrogen-responsive RSA. | [109] | ||
| Nitrate (NO3−) Transporters | AtNRT1.1 | Nitrate transporter 1.1 | Uptake of nitrate NO3− in both low and high nitrogen availability. | Arabidopsis | [134,136,137] |
| NPF6.3 | Nitrate/chlorate transporter | ||||
| AtNRT2.1 | High-affinity nitrate transporter 2.1 | Uptake of nitrate (NO3−) when nitrate is low in soil. | |||
| NRT2.4 | High-affinity nitrate transporter 3.1 | Influx of NO3− into root. | [138,139] | ||
| NRT2.5 | High-affinity nitrate transporter 2.5 | ||||
| OsNRT2.3 | High-affinity nitrate transporter 2.3 | Transportation of nitrate from the root to the stem. | Rice | [140,141] | |
| Ammonia (NH4+) Transporters | AtAMT1;1 | Ammonium transporter 1 member 1 | Decreased uptake of ammonia (NH4+), impedes root and shoot growth. | Arabidopsis | [144,147,148] |
| AtAMT1;2 | Ammonium transporter 1 member 2 | Uptake of ammonia (NH4+), found in root and shoot (leaves). | |||
| AtAMT1;3 | Ammonium transporter 1 member 3 | Uptake of ammonia (NH4+), found in root in low nitrogen availability. | |||
| Nitrogen Assimilation and Utilization | GLN1-1 | Glutamine synthetase cytosolic isozyme 1-1 | Catalyzes synthesis of glutamine from ammonium and glutamate to glutamine synthesis in roots. Ammonium assimilation. | Arabidopsis and Rice | [168,169] |
| NADH-GOGAT 1 | Glutamate synthase 1 [NADH], chloroplast | Glutamate biosynthesis and ammonium ions assimilation in roots. Reutilization of glutamine in developing organs. Development of tillers. | Rice | [170] | |
| GLU2 | Ferredoxin-dependent glutamate synthase 2, chloroplast | Nitrogen assimilation in roots. | Arabidopsis | [171] | |
| Transcription Factors | OsNLP4 | Transcription factor NLP4 | Nitrogen uptake, assimilation, signaling, and utilization in rice. | Rice | [159,160] |
| OsMYB305 | MYB transcription factor | Enhance nitrogen uptake and assimilation, shoot dry weight, and tiller number significantly under low N conditions in rice. | Rice | [165] | |
| MYB61 | Transcription factor MYB61 | Increase biomass and grain yield, under limited nitrogen. | Rice | [10] | |
| OsDOF18 | Dof family transcription factor | Ammonium uptake by regulating the expression of ammonium transporter genes and influencing NUE. | [167] |
5. Genomics and Recent Innovations for Improving NUE in Rice
5.1. Multi-Omics Approaches for Improving NUE in Crops
5.2. MAS and QTLs Mediated NUE Enhancements in Crops
5.3. Genome-Wide Association Study (GWAS) for Improving NUE
5.4. Genetic Modifications and Genome Editing for Improving NUE in Crops
5.5. Metagenomics and Recent Advancements for Enhancing NUE in Crops
6. Conclusions
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandumula, N. Rice Production in Asia: Key to Global Food Security. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1323–1328. [Google Scholar] [CrossRef]
- Muthayya, S.; Hall, J.; Bagriansky, J.; Sugimoto, J.; Gundry, D.; Matthias, D.; Prigge, S.; Hindle, P.; Moench-Pfanner, R.; Maberly, G. Rice Fortification: An Emerging Opportunity to Contribute to the Elimination of Vitamin and Mineral Deficiency Worldwide. Food Nutr. Bull. 2012, 33, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An Overview of Global Rice Production, Supply, Trade, and Consumption. Ann. NY Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Current Status, Challenges, and Opportunities in Rice Production. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–32. ISBN 978-3-319-47514-1. [Google Scholar]
- Wu, H.; Xiang, J.; Zhang, Y.; Zhang, Y.; Peng, S.; Chen, H.; Zhu, D. Effects of Post-Anthesis Nitrogen Uptake and Translocation on Photosynthetic Production and Rice Yield. Sci. Rep. 2018, 8, 12891. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, Y.K.; Singh, U.; Islam, S.M.M.; Huda, A.; Islam, M.R.; Satter, M.A.; Sanabria, J.; Islam, M.R.; Shah, A.L. Impacts of Urea Deep Placement on Nitrous Oxide and Nitric Oxide Emissions from Rice Fields in Bangladesh. Geoderma 2015, 259–260, 370–379. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of Greenhouse Gas Emissions from Crop Production Systems and Fertilizer Management Effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V. Production Potential and Nitrogen Fractionation of Sugarcane-Based Cropping System as Influenced by Planting Materials and Nitrogen Nutrition. Sugar Tech 2020, 22, 622–629. [Google Scholar] [CrossRef]
- Omara, P.; Aula, L.; Oyebiyi, F.; Raun, W.R. World Cereal Nitrogen Use Efficiency Trends: Review and Current Knowledge. Agrosystems Geosci. Environ. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Chen, J.; Liu, Y.; Chu, C. Genetic Improvement toward Nitrogen-Use Efficiency in Rice: Lessons and Perspectives. Mol. Plant 2023, 16, 64–74. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Kishor, K.; Singh, A.K. Optimizing Nutrient Application and Nitrogen Transformation to Maximize the Growth and Productivity of Bud Chip Transplanted Sugarcane. J. Plant Nutr. 2024, 47, 2583–2596. [Google Scholar] [CrossRef]
- Kunwar, U.B.; Wen, J.; Subedi, R.; Bist, N.S.; Pandit, N.R. Adaptations of Rice Seed Germination to Drought and Hypoxic Conditions: Molecular and Physiological Insights. Seeds 2024, 3, 656–676. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs, P.D. World Population Prospects 2024: Summary of Results; UN DESA/POP/2024/TR/NO. 9; United Nations: New York City, NY, USA, 2024. [Google Scholar]
- Yulong, Y.; Qingfeng, M.; Hao, Y.; Qingsong, Z.; Ye, L.; Cui, Z. Climate Change Increases Nitrogen Concentration in Rice with Low Nitrogen Use Efficiency. Earth Future 2021, 9, e2020EF001878. [Google Scholar] [CrossRef]
- Han, M.; Okamoto, M.; Beatty, P.H.; Rothstein, S.J.; Good, A.G. The Genetics of Nitrogen Use Efficiency in Crop Plants. Annu. Rev. Genet. 2015, 49, 269–289. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced Nitrogen Deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Sapkota, T.B.; Bijay-Singh; Takele, R. Improving Nitrogen Use Efficiency and Reducing Nitrogen Surplus through Best Fertilizer Nitrogen Management in Cereal Production: The Case of India and China. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2023; Volume 178, pp. 233–294. ISBN 978-0-443-19260-9. [Google Scholar]
- Kumar, N.; Rana, L.; Singh, A.K.; Pramanick, B.; Gaber, A.; Alsuhaibani, A.M.; Skalicky, M.; Hossain, A. Precise Macronutrient Application Can Improve Cane Yield and Nutrient Uptake in Widely Spaced Plant-Ratoon Cycles in the Indo-Gangetic Plains of India. Front. Sustain. Food Syst. 2023, 7, 1223881. [Google Scholar] [CrossRef]
- Hou, M.; Yu, M.; Li, Z.; Ai, Z.; Chen, J. Molecular Regulatory Networks for Improving Nitrogen Use Efficiency in Rice. Int. J. Mol. Sci. 2021, 22, 9040. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.A.A.; Kambale, R.; Gnanapanditha Mohan, S.V.; Premnath, A.; Fathy Yousef, A.; Moursy, A.R.A.; Abdelsalam, N.R.; Abd El Moneim, D.; Muthurajan, R.; Manikanda Boopathi, N. Empowering Rice Breeding with NextGen Genomics Tools for Rapid Enhancement Nitrogen Use Efficiency. Gene 2024, 927, 148715. [Google Scholar] [CrossRef] [PubMed]
- Chivenge, P.; Sharma, S.; Bunquin, M.A.; Hellin, J. Improving Nitrogen Use Efficiency—A Key for Sustainable Rice Production Systems. Front. Sustain. Food Syst. 2021, 5, 737412. [Google Scholar] [CrossRef]
- De Datta, S.K. Improving Nitrogen Fertilizer Efficiency in Lowland Rice in Tropical Asia. In Nitrogen Economy of Flooded Rice Soils; De Datta, S.K., Patrick, W.H., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 171–186. ISBN 978-94-010-8471-0. [Google Scholar]
- Baral, B.R.; Pande, K.R.; Gaihre, Y.K.; Baral, K.R.; Sah, S.K.; Thapa, Y.B.; Singh, U. Increasing Nitrogen Use Efficiency in Rice through Fertilizer Application Method Under Rainfed Drought Conditions in Nepal. Nutr. Cycl. Agroecosyst. 2020, 118, 103–114. [Google Scholar] [CrossRef]
- Dobermann, A.R. Nitrogen Use Efficiency—State of the Art; International Fertilizer Industry Association: Paris, France, 2005; p. 31. [Google Scholar]
- Ohnishi, M.; Horie, T.; Homma, K.; Supapoj, N.; Takano, H.; Yamamoto, S. Nitrogen Management and Cultivar Effects on Rice Yield and Nitrogen Use Efficiency in Northeast Thailand. Field Crops Res. 1999, 64, 109–120. [Google Scholar] [CrossRef]
- Zhang, F.S.; Wang, J.Q.; Zhang, W.F.; Cui, Z.L.; Ma, W.Q.; Chen, X.P.; Jiang, R.F. Nutrient Use Efficiencies of Major Cereal Crops in China and Measures for Improvement. Acta Pedol. Sin. 2008, 45, 915–924. [Google Scholar]
- Huda, A.; Gaihre, Y.; Islam, M.; Singh, U.; Islam, M.; Sanabria, J.; Satter, M.; Afroz, H.; Halder, A.; Jahiruddin, M. Floodwater Ammonium, Nitrogen Use Efficiency and Rice Yields with Fertilizer Deep Placement and Alternate Wetting and Drying Under Triple Rice Cropping Systems. Nutr. Cycl. Agroecosyst. 2016, 104, 53–66. [Google Scholar] [CrossRef]
- Liang, X.Q.; Li, H.; Wang, S.X.; Ye, Y.S.; Ji, Y.J.; Tian, G.M.; van Kessel, C.; Linquist, B.A. Nitrogen Management to Reduce Yield-Scaled Global Warming Potential in Rice. Field Crops Res. 2013, 146, 66–74. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Yang, J. Improving Nitrogen Use Efficiency of Rice Crop through an Optimized Root System and Agronomic Practices. Crop Environ. 2023, 2, 192–201. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Morales, A.; Cruz, R.; Thiyagarajan, T.; Nagarajan, R.; Babu, M.; Abdulrachman, S.; Hai, L. Adaptation of the Chlorophyll Meter (SPAD) Technology for Real-Time N Management in Rice: A Review. Int. Rice Res. Notes 2000, 25, 4–8. [Google Scholar]
- Sairam, M. Hand-Held Optical Sensors for Optimizing Nitrogen Application and Improving Nutrient Use Efficiency. Int. J. Bioresour. Sci. 2023, 10, 9–18. [Google Scholar] [CrossRef]
- Argento, F.; Anken, T.; Abt, F.; Vogelsanger, E.; Walter, A.; Liebisch, F. Site-Specific Nitrogen Management in Winter Wheat Supported by Low-Altitude Remote Sensing and Soil Data. Precis. Agric. 2021, 22, 364–386. [Google Scholar] [CrossRef]
- Dobermann, A.; Witt, C.; Dawe, D.; Abdulrachman, S.; Gines, H.C.; Nagarajan, R.; Satawathananont, S.; Son, T.T.; Tan, P.S.; Wang, G.H.; et al. Site-Specific Nutrient Management for Intensive Rice Cropping Systems in Asia. Field Crops Res. 2002, 74, 37–66. [Google Scholar] [CrossRef]
- Muschietti-Piana, M.D.P.; Cipriotti, P.A.; Urricariet, S.; Peralta, N.R.; Niborski, M. Using Site-Specific Nitrogen Management in Rainfed Corn to Reduce the Risk of Nitrate Leaching. Agric. Water Manag. 2018, 199, 61–70. [Google Scholar] [CrossRef]
- Pandit, N.R.; Adhikari, S.; Vista, S.P.; Choudhary, D. Nitrogen Management Utilizing 4R Nutrient Stewardship: A Sustainable Strategy for Enhancing NUE, Reducing Maize Yield Gap and Increasing Farm Profitability. Nitrogen 2025, 6, 7. [Google Scholar] [CrossRef]
- Fixen, P.E. A Brief Account of the Genesis of 4R Nutrient Stewardship. Agron. J. 2020, 112, 4511–4518. [Google Scholar] [CrossRef]
- Jat, M.L.; Satyanarayana, T.; Majumdar, K.; Parihar, C.M.; Jat, S.L.; Tetarwal, J.P.; Jat, R.K.; Saharawat, Y.S. Fertiliser Best Management Practices for Maize Systems. Indian J. Fertil. 2013, 9, 80–94. [Google Scholar]
- Pandit, N.R.; Gaihre, Y.K.; Gautam, S.; Maharjan, S.; Vista, S.P.; Choudhary, D. Enhanced-Efficiency Nitrogen Fertilizer Boosts Cauliflower Productivity and Farmers’ Income: Multi-Location and Multi-Year Field Trials across Nepal. Exp. Agric. 2022, 58, e14. [Google Scholar] [CrossRef]
- Ishfaq, M.; Akbar, N.; Zulfiqar, U.; Ali, N.; Jabran, K.; Nawaz, M.; Farooq, M. Influence of Nitrogen Fertilization Pattern on Productivity, Nitrogen Use Efficiencies, and Profitability in Different Rice Production Systems. J. Soil Sci. Plant Nutr. 2021, 21, 145–161. [Google Scholar] [CrossRef]
- Redda, A.; Redae, W.; Hailegebriel, K.; Weldegerima, G.; Tsegay, G.; Yirgalem, T.; Eyasu, A.; Hussien, S. Nitrogen Fertilizer Management Factors Affecting Nitrogen Use Efficiency and Yield of Rice (Oryza sativa L.): A REVIEW. Asian J. Plant Soil. Sci. 2022, 7, 1–22. [Google Scholar] [CrossRef]
- Pandit, N.R.; Choudhary, D.; Maharjan, S.; Dhakal, K.; Vista, S.P.; Gaihre, Y.K. Optimum Rate and Deep Placement of Nitrogen Fertilizer Improves Nitrogen Use Efficiency and Tomato Yield in Nepal. Soil Syst. 2022, 6, 72. [Google Scholar] [CrossRef]
- Thapa, G.; Choudhary, D.; Pandit, N.R.; Dongol, P. Fertilizer Demonstration, Agricultural Performance, and Food Security of Smallholder Farmers: Empirical Evidence from Nepal. World Dev. Sustain. 2025, 6, 100196. [Google Scholar] [CrossRef]
- Bruulsema, T. Managing Nutrients to Mitigate Soil Pollution. Environ. Pollut. 2018, 243, 1602–1605. [Google Scholar] [CrossRef]
- Feng, J.; Li, F.; Deng, A.; Feng, X.; Fang, F.; Zhang, W. Integrated Assessment of the Impact of Enhanced-Efficiency Nitrogen Fertilizer on N2O Emission and Crop Yield. Agric. Ecosyst. Environ. 2016, 231, 218–228. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Snyder, C.S.; Blaylock, A.D.; Del Grosso, S.J. Enhanced-Efficiency Nitrogen Fertilizers: Potential Role in Nitrous Oxide Emission Mitigation. Agron. J. 2014, 106, 715–722. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Ma, J.; Zou, P.; Jiang, L. Impact of Controlled-Release Urea on Rice Yield, Nitrogen Use Efficiency and Soil Fertility in a Single Rice Cropping System. Sci. Rep. 2020, 10, 10432. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of Fertilizers for Enhanced Nitrogen Use Efficiency—Trends and Perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Linquist, B.A.; Liu, L.; Van Kessel, C.; Van Groenigen, K.J. Enhanced Efficiency Nitrogen Fertilizers for Rice Systems: Meta-Analysis of Yield and Nitrogen Uptake. Field Crops Res. 2013, 154, 246–254. [Google Scholar] [CrossRef]
- Pandit, N.R.; Gaihre, Y.K.; Choudhary, D.; Subedi, R.; Thapa, S.B.; Maharjan, S.; Khadka, D.; Vista, S.P.; Rusinamhodzi, L. Slow but Sure: The Potential of Slow-Release Nitrogen Fertilizers to Increase Crop Productivity and Farm Profit in Nepal. J. Plant Nutr. 2022, 45, 2986–3003. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, 2nd ed.; International Fertilizer Industry Association (IFA): Paris, France, 2010; ISBN 978-2-9523139-7-1. [Google Scholar]
- Zheng, W.; Zhang, M.; Liu, Z.; Zhou, H.; Lu, H.; Zhang, W.; Yang, Y.; Li, C.; Chen, B. Combining Controlled-Release Urea and Normal Urea to Improve the Nitrogen Use Efficiency and Yield under Wheat-Maize Double Cropping System. Field Crops Res. 2016, 197, 52–62. [Google Scholar] [CrossRef]
- Xu, R.; Chen, S.; Xu, C.M.; Liu, Y.H.; Zhang, X.F.; Wang, D.Y.; Chu, G. Polymer-Coated Urea Application Can Increase Both Grain Yield and Nitrogen Use Efficiency in Japonica-Indica Hybrid Rice. J. Agric. Sci. 2023, 161, 51–59. [Google Scholar] [CrossRef]
- Tang, C.; Han, M.; Yang, X.; Shen, T.; Gao, Y.; Wang, Y.; Zhang, S.; Chen, D.; He, D.; Li, Y.C. Gene Expression, Enzyme Activity, Nitrogen Use Efficiency, and Yield of Rice Affected by Controlled-Release Nitrogen. ACS Omega 2023, 8, 23772–23781. [Google Scholar] [CrossRef]
- Agyin-Birikorang, S.; Winings, J.H.; Yin, X.; Singh, U.; Sanabria, J. Field Evaluation of Agronomic Effectiveness of Multi-Nutrient Fertilizer Briquettes for Upland Crop Production. Nutr. Cycl. Agroecosyst. 2018, 110, 395–406. [Google Scholar] [CrossRef]
- Dhakal, K.; Baral, B.R.; Pokhrel, K.R.; Pandit, N.R.; Thapa, S.B.; Gaihre, Y.K.; Vista, S.P. Deep Placement of Briquette Urea Increases Agronomic and Economic Efficiency of Maize in Sandy Loam Soil. Agrivita J. Agric. Sci. 2020, 42, 499–508. [Google Scholar] [CrossRef]
- Eric Derrick, B.; Etienne, I.; Mathusalem, K. Comparative Study of Urea in Prilled and Briquette Forms on Rice Production in Marshlands of Rwanda. J. Fertil. Pestic. 2017, 8, 178. [Google Scholar] [CrossRef]
- Zhu, C.; Xiang, J.; Zhang, Y.; Zhang, Y.; Zhu, D.; Chen, H. Mechanized Transplanting with Side Deep Fertilization Increases Yield and Nitrogen Use Efficiency of Rice in Eastern China. Sci. Rep. 2019, 9, 5653. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Yang, X.; Pan, H.; Zhang, X.; Cao, H.; Ulgiati, S.; Wu, J.; Zhang, Y.; Wang, G.; Xiao, Y. Impact of Fertilization Schemes with Different Ratios of Urea to Controlled Release Nitrogen Fertilizer on Environmental Sustainability, Nitrogen Use Efficiency and Economic Benefit of Rice Production: A Study Case from Southwest China. J. Clean. Prod. 2021, 293, 126198. [Google Scholar] [CrossRef]
- Lan, C.; Zou, J.; Li, J.; Xu, H.; Lin, W.; Weng, P.; Fang, C.; Zhang, Z.; Chen, H.; Lin, W. Slow-Release Fertilizer Deep Placement Increased Rice Yield and Reduced the Ecological and Environmental Impact in Southeast China: A Life-Cycle Perspective. Field Crops Res. 2024, 306, 109224. [Google Scholar] [CrossRef]
- Iqbal, M.A. Nano-Fertilizers for Sustainable Crop Production Under Changing Climate: A Global Perspective; IntechOpen: London, UK, 2019; ISBN 978-1-78985-317-9. [Google Scholar]
- Rana, L.; Kumar, M.; Rajput, J.; Kumar, N.; Sow, S.; Kumar, S.; Kumar, A.; Singh, S.N.; Jha, C.K.; Singh, A.K.; et al. Nexus between Nanotechnology and Agricultural Production Systems: Challenges and Future Prospects. Discov. Appl. Sci. 2024, 6, 555. [Google Scholar] [CrossRef]
- Kumar, A.; Sheoran, P.; Kumar, N.; Devi, S.; Kumar, A.; Malik, K.; Rani, M.; Bhardwaj, A.K.; Mann, A. Elucidating Morphogenic and Physiological Traits of Rice with Nitrogen Substitution through Nano-Nitrogen under Salt Stress Conditions. BMC Plant Biol. 2024, 24, 908. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Romanovski, V.; Thirumalaisamy, S.K.; Palanimuthu, V.; Sampath, M.P.; Anilkumar, A.; Sivaraj, D.K.; Ahamed, N.A.N.; Murugesan, S.; Chandrasekar, D.; et al. Innovations in Modern Nanotechnology for the Sustainable Production of Agriculture. ChemEngineering 2023, 7, 61. [Google Scholar] [CrossRef]
- Rahale, C.S. Nutrient Release Pattern of Nanofertilizer Formulation. Ph.D. Thesis, Tamilnadu Agricultural University, Coimbatore, Tamil Nadu, India, 2011. [Google Scholar]
- Upadhyay, P.K.; Dey, A.; Singh, V.K.; Dwivedi, B.S.; Singh, T.; Rajanna, G.A.; Babu, S.; Rathore, S.S.; Singh, R.K.; Shekhawat, K.; et al. Conjoint Application of Nano-Urea with Conventional Fertilizers: An Energy Efficient and Environmentally Robust Approach for Sustainable Crop Production. PLoS ONE 2023, 18, e0284009. [Google Scholar] [CrossRef]
- Cowling, S.A.; Field, C.B. Environmental Control of Leaf Area Production: Implications for Vegetation and Land-surface Modeling. Glob. Biogeochem. Cycles 2003, 17, 7-1–7-14. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, T.; Raliya, R.; Tiwari, K. Nano Fertilizers for Sustainable Crop Production, Higher Nutrient Use Efficiency and Enhanced Profitability. Indian J. Fertil. 2021, 17, 1206–1214. [Google Scholar]
- Rai, M.; Ingle, A.; Pandit, R. Nanotechnology in Agro-Food: From Field to Plate. Food Nutr. J. 2015, 6, 40–47. [Google Scholar]
- Singh, B.V.; Rana, N.S.; Sharma, K.; Verma, A.; Rai, A.K.; Singh, N.K.; Pandey, S.K. Impact of Nano-Fertilizers on Productivity and Profitability of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2024, 25, 69–76. [Google Scholar] [CrossRef]
- Bashir, S.D.; Bhat, T.A.; Jamsheed, B.; Nazir, A.; Jan, B.; Kanth, R.H.; Saxena, A.; Dar, K.A.; Khan, I.M.; Wani, F.J.; et al. Effect of Nano-Urea Based Nitrogen Application on the Growth, Phenology and Yield of Direct Seeded Rice (Oryza sativa L.). Arch. Curr. Res. Int. 2024, 24, 385–395. [Google Scholar] [CrossRef]
- Prem, B. Nano Urea the Philosophy of Future. Res. Gate 2021. [Google Scholar] [CrossRef]
- Kumar, Y.; Tiwari, K.N.; Singh, T.; Sain, N.K.; Laxmi, S.; Verma, R.; Sharma, G.C.; Raliya, R. Nanofertilizers for Enhancing Nutrient Use Efficiency, Crop Productivity and Economic Returns in Winter Season Crops of Rajasthan. Res. Gate 2020. [Google Scholar] [CrossRef]
- Ravikumar, S.; Vellingiri, G.; Sellaperumal, P.; Pandian, K.; Sivasankar, A.; Sangchul, H. Real-Time Nitrogen Monitoring and Management to Augment N Use Efficiency and Ecosystem Sustainability—A Review. J. Hazard. Mater. Adv. 2024, 16, 100466. [Google Scholar] [CrossRef]
- Gautam, S.; Tiwari, U.; Sapkota, B.; Sharma, B.; Parajuli, S.; Pandit, N.R.; Gaihre, Y.K.; Dhakal, K. Field Evaluation of Slow-Release Nitrogen Fertilizers and Real-Time Nitrogen Management Tools to Improve Grain Yield and Nitrogen Use Efficiency of Spring Maize in Nepal. Heliyon 2022, 8, e09566. [Google Scholar] [CrossRef]
- Gorai, T.; Yadav, P.K.; Choudhary, G.L.; Kumar, A. Site-Specific Crop Nutrient Management for Precision Agriculture—A Review. Curr. J. Appl. Sci. Technol. 2021, 37–52. [Google Scholar] [CrossRef]
- Takoutsing, B. Digital Soil Mapping Using Uncertain Soil Observations to Support Agricultural Intensification in West and Central Africa. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2024. [Google Scholar]
- Mohan, S.S. GPS and Sensor Based Technologies in Variable Rate Fertilizer Application. Int. J. Agric. Environ. Biotechnol. 2021, 14, 21–27. [Google Scholar] [CrossRef]
- Soussi, A.; Zero, E.; Sacile, R.; Trinchero, D.; Fossa, M. Smart Sensors and Smart Data for Precision Agriculture: A Review. Sensors 2024, 24, 2647. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strat. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Kang, S.-W.; Cheong, Y.H.; Yun, J.-J.; Park, J.-H.; Park, J.-H.; Seo, D.-C.; Cho, J.-S. Effect of Biochar Application on Nitrogen Use Efficiency for Sustainable and Productive Agriculture under Different Field Crops. J. Plant Nutr. 2021, 44, 2849–2862. [Google Scholar] [CrossRef]
- Kimani, S.M.; Bimantara, P.O.; Kautsar, V.; Tawaraya, K.; Cheng, W. Poultry Litter Biochar Application in Combination with Chemical Fertilizer and Azolla Green Manure Improves Rice Grain Yield and Nitrogen Use Efficiency in Paddy Soil. Biochar 2021, 3, 591–602. [Google Scholar] [CrossRef]
- Oladele, S.; Adeyemo, A.; Awodun, M.; Ajayi, A.; Fasina, A. Effects of Biochar and Nitrogen Fertilizer on Soil Physicochemical Properties, Nitrogen Use Efficiency and Upland Rice (Oryza sativa) Yield Grown on an Alfisol in Southwestern Nigeria. Int. J. Recycl. Org. Waste Agric. 2019, 8, 295–308. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Li, Y.; Liu, S.; Ji, J.; Tong, Y. Effects of Mixed Controlled Release Nitrogen Fertilizer with Rice Straw Biochar on Rice Yield and Nitrogen Balance in Northeast China. Sci. Rep. 2020, 10, 9452. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, C.; Zhao, X.; Wang, Y.; Lv, X.; Zhu, X.; Song, X. Beneficial Effects of Biochar Application with Nitrogen Fertilizer on Soil Nitrogen Retention, Absorption and Utilization in Maize Production. Agronomy 2022, 13, 113. [Google Scholar] [CrossRef]
- Selvarajh, G.; Ch’ng, H.Y. Enhancing Soil Nitrogen Availability and Rice Growth by Using Urea Fertilizer Amended with Rice Straw Biochar. Agronomy 2021, 11, 1352. [Google Scholar] [CrossRef]
- Pandit, N.R.; Sipkhan, P.; Sharma, S.S.; Dawadi, D.; Vista, S.P.; Raut, P. Cattle-Urine-Enriched Biochar Enhances Soil Fertility, Nutrient Uptake, and Yield of Maize in a Low-Productive Soil. Nitrogen 2024, 5, 16–27. [Google Scholar] [CrossRef]
- Schmidt, H.; Pandit, B.; Martinsen, V.; Cornelissen, G.; Conte, P.; Kammann, C. Fourfold Increase in Pumpkin Yield in Response to Low-Dosage Root Zone Application of Urine-Enhanced Biochar to a Fertile Tropical Soil. Agriculture 2015, 5, 723–741. [Google Scholar] [CrossRef]
- Selvarajh, G.; Ch’ng, H.Y.; Md Zain, N.; Seong Wei, L.; Liew, J.Y.; Mohammad Azmin, S.N.H.; Naher, L.; Abdullah, P.S.; Ahmed, O.H.; Jalloh, M.B.; et al. Enriched Rice Husk Biochar Superior to Commercial Biochar in Ameliorating Ammonia Loss from Urea Fertilizer and Improving Plant Uptake. Heliyon 2024, 10, e32080. [Google Scholar] [CrossRef]
- Utomo, W.; Islami, T.; Wisnubroto, E.; Soelistyari, H.T. Biochar as a Carrier for Nitrogen Plant Nutrition: 3. Effect of Enriched Biochar on Rice (Oryza sativa L.) Yield and Soil Qualities. Int. J. Appl. Eng. Res. 2017, 12, 10426–10432. [Google Scholar]
- Hagemann, N.; Joseph, S.; Conte, P.; Albu, M.; Obst, M.; Borch, T.; Orsetti, S.; Subdiaga, E.; Behrens, S.; Kappler, A. Composting-Derived Organic Coating on Biochar Enhances Its Affinity to Nitrate. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 23–28 April 2017; p. 10775. [Google Scholar]
- Patel, M.R.; Panwar, N.L. Evaluating the Agronomic and Economic Viability of Biochar in Sustainable Crop Production. Biomass Bioenergy 2024, 188, 107328. [Google Scholar] [CrossRef]
- Collino, D.J.; Salvagiotti, F.; Perticari, A.; Piccinetti, C.; Ovando, G.; Urquiaga, S.; Racca, R.W. Biological Nitrogen Fixation in Soybean in Argentina: Relationships with Crop, Soil, and Meteorological Factors. Plant Soil 2015, 392, 239–252. [Google Scholar] [CrossRef]
- Zhang, Z.; Masuda, Y.; Xu, Z.; Shiratori, Y.; Ohba, H.; Senoo, K. Active Nitrogen Fixation by Iron-Reducing Bacteria in Rice Paddy Soil and Its Further Enhancement by Iron Application. Appl. Sci. 2023, 13, 8156. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Li, D.; Peng, C. Nitrogen-Fixing Cyanobacteria Have the Potential to Improve Nitrogen Use Efficiency through the Reduction of Ammonia Volatilization in Red Soil Paddy Fields. Soil Tillage Res. 2022, 217, 105274. [Google Scholar] [CrossRef]
- Choudhury, A.T.M.A.; Kennedy, I.R. Prospects and Potentials for Systems of Biological Nitrogen Fixation in Sustainable Rice Production. Biol. Fertil. Soils 2004, 39, 219–227. [Google Scholar] [CrossRef]
- Das, K.; Biswakarma, N.; Zhiipao, R.; Kumar, A.; Ghasal, P.C.; Pooniya, V. Significance and Management of Green Manures. In Soil Health; Giri, B., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 59, pp. 197–217. ISBN 978-3-030-44363-4. [Google Scholar]
- Meelu, O.P.; Morris, R.A. Green Manure Management in Rice Based Cropping Systems. In Sustainable Agriculture: Green Manure in Rice Farming; International Rice Research Institute: Manila, Philippines, 1988; pp. 209–222. [Google Scholar]
- Qaswar, M.; Huang, J.; Ahmed, W.; Liu, S.; Li, D.; Zhang, L.; Liu, L.; Xu, Y.; Han, T.; Du, J.; et al. Substitution of Inorganic Nitrogen Fertilizer with Green Manure (GM) Increased Yield Stability by Improving C Input and Nitrogen Recovery Efficiency in Rice Based Cropping System. Agronomy 2019, 9, 609. [Google Scholar] [CrossRef]
- Gebru, H. A Review on the Comparative Advantages of Intercropping to Mono-Cropping System. J. Biol. Agric. Healthc. 2015, 5, 1–13. [Google Scholar]
- Rusinamhodzi, L.; Corbeels, M.; Nyamangara, J.; Giller, K.E. Maize–Grain Legume Intercropping Is an Attractive Option for Ecological Intensification That Reduces Climatic Risk for Smallholder Farmers in Central Mozambique. Field Crops Res. 2012, 136, 12–22. [Google Scholar] [CrossRef]
- Hei, Z.; Xiang, H.; Zhang, J.; Liang, K.; Zhong, J.; Li, M.; Lu, Y. Rice Intercropping with Water Mimosa (Neptunia oleracea Lour.) Can Facilitate Soil N Utilization and Alleviate Apparent N Loss. Agric. Ecosyst. Environ. 2021, 313, 107378. [Google Scholar] [CrossRef]
- Peoples, M.B.; Herridge, D.F.; Ladha, J.K. Biological Nitrogen Fixation: An Efficient Source of Nitrogen for Sustainable Agricultural Production? Plant Soil 1995, 174, 3–28. [Google Scholar] [CrossRef]
- Kamuruzzaman, M.; Rees, R.M.; Islam, M.T.; Drewer, J.; Sutton, M.; Bhatia, A.; Bealey, W.J.; Hasan, M.M. Improving Nitrogen Fertilizer Management for Yield and N Use Efficiency in Wetland Rice Cultivation in Bangladesh. Agronomy 2024, 14, 2758. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Y.; Wu, G.; Wang, X.; Yuan, M.; Wang, J.; He, W.; Chen, F.; LeCocq, K.; Wang, L.; et al. Amendment with Controlled Release Urea Increases Leaf Morpho-physiological Traits, Grain Yield and NUE in a Double-cropping Rice System in Southern China. J. Sci. Food Agric. 2023, 103, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Guo, X.; Zhou, D.; Zhang, Q.; Ren, X.; Wang, R.; Wang, X.; Chen, X.; Li, J. Quantify the Effect of Manure Fertilizer Addition and Optimal Nitrogen Input on Rainfed Wheat Yield and Nitrogen Requirement Using Nitrogen Nutrition Index. Agric. Ecosyst. Environ. 2023, 345, 108319. [Google Scholar] [CrossRef]
- Asif, M.A.A.; Mahjabin, F.; Singha, S.K.; Rahman Jahangir, M.M.; Hoque, S.M. Application of Nano-Urea in Conventional Flood-Irrigated Boro Rice in Bangladesh and Nitrogen Losses Investigation. Heliyon 2024, 10, e37150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Tripathi, S.C.; Yadav, D.B.; Samota, S.R.; Venkatesh, K.; Sareen, S.; Singh, G. Boosting Wheat Yield, Profitability and NUE with Prilled and Nano Urea in Conservation Tillage. Sci. Rep. 2023, 13, 18073. [Google Scholar] [CrossRef]
- Adams, A.; Gore, J.; Musser, F.; Cook, D.; Catchot, A.; Walker, T.; Awuni, G.A. Impact of Water Management on Efficacy of Insecticide Seed Treatments Against Rice Water Weevil (Coleoptera: Curculionidae) in Mississippi Rice. J. Econ. Entomol. 2015, 108, 1079–1085. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.; Ji, Z.; Tao, Y.; Wei, S.; Jiao, W.; Fang, Y.; Jian, P.; Shen, C.; Qin, Y.; Zhang, S.; et al. Improving Rice Nitrogen-Use Efficiency by Modulating a Novel Monouniquitination Machinery for Optimal Root Plasticity Response to Nitrogen. Nat. Plants 2023, 9, 1902–1914. [Google Scholar] [CrossRef]
- Mandal, V.K.; Sharma, N.; Raghuram, N. Molecular Targets for Improvement of Crop Nitrogen Use Efficiency: Current and Emerging Options. In Engineering Nitrogen Utilization in Crop Plants; Shrawat, A., Zayed, A., Lightfoot, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 77–93. ISBN 978-3-319-92957-6. [Google Scholar]
- Yan, J.; Wu, Q.; Qi, D.; Zhu, J. Rice Yield, Water Productivity, and Nitrogen Use Efficiency Responses to Nitrogen Management Strategies under Supplementary Irrigation for Rain-Fed Rice Cultivation. Agric. Water Manag. 2022, 263, 107486. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and Defixation of Ammonium in Soils: A Review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Hawkesford, M.J.; Barraclough, P.B.; Holdsworth, M.J.; Kerr, S.; Kightley, S.; Shewry, P.R. Identifying Traits to Improve the Nitrogen Economy of Wheat: Recent Advances and Future Prospects. Field Crops Res. 2009, 114, 329–342. [Google Scholar] [CrossRef]
- Liu, L.; Cui, K.; Qi, X.; Wu, Y.; Huang, J.; Peng, S. Varietal Responses of Root Characteristics to Low Nitrogen Application Explain the Differing Nitrogen Uptake and Grain Yield in Two Rice Varieties. Front. Plant Sci. 2023, 14, 1244281. [Google Scholar] [CrossRef] [PubMed]
- Anis, G.B.; Zhang, Y.; Islam, A.; Zhang, Y.; Cao, Y.; Wu, W.; Cao, L.; Cheng, S. RDWN6XB, a Major Quantitative Trait Locus Positively Enhances Root System Architecture under Nitrogen Deficiency in Rice. BMC Plant Biol. 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liang, Z.; Chen, S.; Sun, H.; Fan, X.; Wang, C.; Xu, G.; Zhang, Y. A Transcription Factor, OsMADS57, Regulates Long-Distance Nitrate Transport and Root Elongation. Plant Physiol. 2019, 180, 882–895. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Xu, J.; Zhu, C.; Hu, Q.; Xu, K. Nitrogen Uptake and Carbon-Nitrogen Synergistic Translocation Improve Yield and Nitrogen Use Efficiency in the Dep1 Rice Line. J. Integr. Agric. 2025. [Google Scholar] [CrossRef]
- Gao, J.; Ge, S.; Wang, H.; Fang, Y.; Sun, L.; He, T.; Cheng, X.; Wang, D.; Zhou, X.; Cai, H.; et al. Biochar-Extracted Liquor Stimulates Nitrogen Related Gene Expression on Improving Nitrogen Utilization in Rice Seedling. Front. Plant Sci. 2023, 14, 1131937. [Google Scholar] [CrossRef] [PubMed]
- Al Aasmi, A.; Li, J.; Hamoud, Y.A.; Lan, Y.; Alordzinu, K.E.; Appiah, S.A.; Shaghaleh, H.; Sheteiwy, M.; Wang, H.; Qiao, S.; et al. Impacts of Slow-Release Nitrogen Fertilizer Rates on the Morpho-Physiological Traits, Yield, and Nitrogen Use Efficiency of Rice under Different Water Regimes. Agriculture 2022, 12, 86. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, W.; Wu, B.; Fang, Z.; Wang, X. The Amino Acid Transporter AAP1 Mediates Growth and Grain Yield by Regulating Neutral Amino Acid Uptake and Reallocation in Oryza sativa. J. Exp. Bot. 2020, 71, 4763–4777. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Shen, C.; Ji, Z.; Zhang, H.; Zhang, T.; Li, Y.; Yu, J.; Yang, N.; He, Y.; et al. Natural Allelic Variation in a Modulator of Auxin Homeostasis Improves Grain Yield and Nitrogen Use Efficiency in Rice. Plant Cell 2021, 33, 566–580. [Google Scholar] [CrossRef]
- Jiang, N.; Zou, T.; Huang, H.; Li, C.; Xia, Y.; Yang, L. Auxin Synthesis Promotes N Metabolism and Optimizes Root Structure Enhancing N Acquirement in Maize (Zea mays L.). Planta 2024, 259, 46. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Y.; Liu, X.; Zhang, B.; Si, J.; Xia, H.; Fan, S.; Kong, L. Nitrate Starvation Induces Lateral Root Organogenesis in Triticum aestivum via Auxin Signaling. Int. J. Mol. Sci. 2024, 25, 9566. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Shabir, P.A.; Hakeem, K.R. Advances in Plant Nitrogen Metabolism, 1st ed.; CRC Press: New York City, NY, USA, 2022; ISBN 978-1-00-324836-1. [Google Scholar]
- Li, Z.; Chen, H.; Guan, Q.; Li, L.; Xuan, Y.H. Gibberellic Acid Signaling Promotes Resistance to Saline-Alkaline Stress by Increasing the Uptake of Ammonium in Rice. Plant Physiol. Biochem. 2024, 207, 108424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Q.; Zhang, Y.; Zhang, Y.; Xing, J.; Yang, B.; Mi, G.; Li, Z.; Zhang, M. The Role of Gibberellins in Regulation of Nitrogen Uptake and Physiological Traits in Maize Responding to Nitrogen Availability. Int. J. Mol. Sci. 2020, 21, 1824. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal Control of Nitrogen Acquisition: Roles of Auxin, Abscisic Acid, and Cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, L.; Duan, X.; Lv, Y.; Ye, C.; Ding, C.; Zhang, Y.; Qi, W.; Motte, H.; Beeckman, T.; et al. A Nitrogen-responsive Cytokinin Oxidase/Dehydrogenase Regulates Root Response to High Ammonium in Rice. New Phytol. 2024, 244, 1391–1407. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Mao, Y.; Struik, P.C.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J. Roles of Nitrogen and Cytokinin Signals in Root and Shoot Communications in Maximizing of Plant Productivity and Their Agronomic Applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, G.; Gao, Y.; Lu, G.; Habben, J.E.; Mao, G.; Chen, G.; Wang, J.; Yang, F.; Zhao, X.; et al. A Cytokinin-Activation Enzyme-like Gene Improves Grain Yield under Various Field Conditions in Rice. Plant Mol. Biol. 2020, 102, 373–388. [Google Scholar] [CrossRef]
- Xing, J.; Wang, Y.; Yao, Q.; Zhang, Y.; Zhang, M.; Li, Z. Brassinosteroids Modulate Nitrogen Physiological Response and Promote Nitrogen Uptake in Maize (Zea mays L.). Crop J. 2022, 10, 166–176. [Google Scholar] [CrossRef]
- Yang, W.; Wan, G.-F.; Zhou, J.-Q.; Song, G.-C.; Zhao, J.; Huang, F.-L.; Meng, S. The Effects of Brassinosteroids on Nitrogen Utilization in Rice. Agronomy 2024, 14, 604. [Google Scholar] [CrossRef]
- Hang, J.; Wu, B.; Qiu, D.; Yang, G.; Fang, Z.; Zhang, M. OsNPF3.1, a Nitrate, Abscisic Acid and Gibberellin Transporter Gene, Is Essential for Rice Tillering and Nitrogen Utilization Efficiency. J. Integr. Agric. 2024, 23, 1087–1104. [Google Scholar] [CrossRef]
- Aluko, O.O.; Kant, S.; Adedire, O.M.; Li, C.; Yuan, G.; Liu, H.; Wang, Q. Unlocking the Potentials of Nitrate Transporters at Improving Plant Nitrogen Use Efficiency. Front. Plant Sci. 2023, 14, 1074839. [Google Scholar] [CrossRef]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant Nitrate Transporters: From Gene Function to Application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Tsay, Y.-F. Switching between the Two Action Modes of the Dual-Affinity Nitrate Transporter CHL1 by Phosphorylation. EMBO J. 2003, 22, 1005–1013. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate Transport and Signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Von Wittgenstein, N.J.; Le, C.H.; Hawkins, B.J.; Ehlting, J. Evolutionary Classification of Ammonium, Nitrate, and Peptide Transporters in Land Plants. BMC Evol. Biol. 2014, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fan, X.; Li, Q.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Knockdown of a Rice Stelar Nitrate Transporter Alters Long-Distance Translocation But Not Root Influx. Plant Physiol. 2012, 160, 2052–2063. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Gao, N.; Liu, M.; Zhang, C.; Luo, J.; Sun, Y.; Feng, Y. Nitrate Transporters and Mechanisms of Nitrate Signal Transduction in Arabidopsis and Rice. Physiol. Plant. 2024, 176, e14486. [Google Scholar] [CrossRef]
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and Genetic Approaches Improving Nitrogen Use Efficiency in Cereal Crops: A Review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in Plants: From Nutrition to the Modulation of Abiotic Stress Adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Yuan, L.; Loqué, D.; Kojima, S.; Rauch, S.; Ishiyama, K.; Inoue, E.; Takahashi, H.; Von Wirén, N. The Organization of High-Affinity Ammonium Uptake in Arabidopsis Roots Depends on the Spatial Arrangement and Biochemical Properties of AMT1-Type Transporters. Plant Cell 2007, 19, 2636–2652. [Google Scholar] [CrossRef]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.B.; Von Wirén, N. Three Functional Transporters for Constitutive, Diurnally Regulated, and Starvation-Induced Uptake of Ammonium into Arabidopsis Roots. Plant Cell 1999, 11, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, B.; Chu, C. Nitrogen Use Efficiency in Crops: Lessons from Arabidopsis and Rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Li, C.; Tang, Z.; Wei, J.; Qu, H.; Xie, Y.; Xu, G. The OsAMT1.1 Gene Functions in Ammonium Uptake and Ammonium–Potassium Homeostasis over Low and High Ammonium Concentration Ranges. J. Genet. Genom. 2016, 43, 639–649. [Google Scholar] [CrossRef]
- Sonoda, Y.; Ikeda, A.; Saiki, S.; Yamaya, T.; Yamaguchi, J. Feedback Regulation of the Ammonium Transporter Gene Family AMT1 by Glutamine in Rice. Plant Cell Physiol. 2003, 44, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.C.; Eriksson, D.; Møller, I.S.; Schjoerring, J.K. Cytosolic Glutamine Synthetase: A Target for Improvement of Crop Nitrogen Use Efficiency? Trends Plant Sci. 2014, 19, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Schauser, L.; Wieloch, W.; Stougaard, J. Evolution of NIN-Like Proteins in Arabidopsis, Rice, and Lotus japonicus. J. Mol. Evol. 2005, 60, 229–237. [Google Scholar] [CrossRef]
- Boisson, M.; Mondon, K.; Torney, V.; Nicot, N.; Laine, A.-L.; Bahrman, N.; Gouy, A.; Daniel-Vedele, F.; Hirel, B.; Sourdille, P.; et al. Partial Sequences of Nitrogen Metabolism Genes in Hexaploid Wheat. Theor. Appl. Genet. 2005, 110, 932–940. [Google Scholar] [CrossRef]
- Campbell, W.H. Molecular Control of Nitrate Reductase and Other Enzymes Involved in Nitrate Assimilation. In Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism; Foyer, C.H., Noctor, G., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2002; Volume 12, pp. 35–48. ISBN 978-0-7923-6336-1. [Google Scholar]
- Sétif, P.; Hirasawa, M.; Cassan, N.; Lagoutte, B.; Tripathy, J.N.; Knaff, D.B. New Insights into the Catalytic Cycle of Plant Nitrite Reductase. Electron Transfer Kinetics and Charge Storage. Biochemistry 2009, 48, 2828–2838. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; De Pinto, M.C. The Role of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) in the Improvement of Nitrogen Use Efficiency in Cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Brauer, E.K.; Rochon, A.; Bi, Y.; Bozzo, G.G.; Rothstein, S.J.; Shelp, B.J. Reappraisal of Nitrogen Use Efficiency in Rice Overexpressing Glutamine synthetase1. Physiol. Plant. 2011, 141, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Chichkova, S.; Arellano, J.; Vance, C.P.; Hernández, G. Transgenic Tobacco Plants That Overexpress Alfalfa NADH-Glutamate Synthase Have Higher Carbon and Nitrogen Content. J. Exp. Bot. 2001, 52, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Xu, Z.; Li, C.; Rasheed, A.; Pan, X.; Shi, Q.; Wu, Z. The Removal of Nitrate Reductase Phosphorylation Enhances Tolerance to Ammonium Nitrogen Deficiency in Rice. J. Integr. Agric. 2022, 21, 631–643. [Google Scholar] [CrossRef]
- Wang, M.; Hasegawa, T.; Beier, M.; Hayashi, M.; Ohmori, Y.; Yano, K.; Teramoto, S.; Kamiya, T.; Fujiwara, T. Growth and Nitrate Reductase Activity Are Impaired in Rice Osnlp4 Mutants Supplied with Nitrate. Plant Cell Physiol. 2021, 62, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.; Xia, J.; Alfatih, A.; Song, Y.; Huang, Y.; Wan, G.; Sun, L.; Tang, H.; Liu, Y.; et al. Rice NIN-LIKE PROTEIN 4 Plays a Pivotal Role in Nitrogen Use Efficiency. Plant Biotechnol. J. 2021, 19, 448–461. [Google Scholar] [CrossRef]
- Sun, H.; Li, J.; Song, W.; Tao, J.; Huang, S.; Chen, S.; Hou, M.; Xu, G.; Zhang, Y. Nitric Oxide Generated by Nitrate Reductase Increases Nitrogen Uptake Capacity by Inducing Lateral Root Formation and Inorganic Nitrogen Uptake Under Partial Nitrate Nutrition in Rice. J. Exp. Bot. 2015, 66, 2449–2459. [Google Scholar] [CrossRef]
- Yang, X.; Nong, B.; Chen, C.; Wang, J.; Xia, X.; Zhang, Z.; Wei, Y.; Zeng, Y.; Feng, R.; Wu, Y.; et al. OsNPF3.1, a Member of the NRT1/PTR Family, Increases Nitrogen Use Efficiency and Biomass Production in Rice. Crop J. 2023, 11, 108–118. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, C. Nitrogen-Use Divergence Between Indica and Japonica Rice: Variation at Nitrate Assimilation. Mol. Plant 2020, 13, 6–7. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, Y.; Ying, S.; Tang, Y.; Niu, J.; Wang, T.; Huang, R.; Xie, H.; Wang, W.; Peng, X. OsCBL1 Modulates Rice Nitrogen Use Efficiency via Negative Regulation of OsNRT2.2 by OsCCA1. BMC Plant Biol. 2023, 23, 502. [Google Scholar] [CrossRef]
- Wang, D.; Xu, T.; Yin, Z.; Wu, W.; Geng, H.; Li, L.; Yang, M.; Cai, H.; Lian, X. Overexpression of OsMYB305 in Rice Enhances the Nitrogen Uptake Under Low-Nitrogen Condition. Front. Plant Sci. 2020, 11, 369. [Google Scholar] [CrossRef]
- Araus, V.; Vidal, E.A.; Puelma, T.; Alamos, S.; Mieulet, D.; Guiderdoni, E.; Gutiérrez, R.A. Members of BTB Gene Family Regulate Negatively Nitrate Uptake and Nitrogen Use Efficiency in Arabidopsis thaliana and Oryza sativa. Plant Physiol. 2016, 171, 1523–1532. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Wei, J.; Yoon, H.; An, G. Transcription Factor OsDOF18 Controls Ammonium Uptake by Inducing Ammonium Transporters in Rice Roots. Mol. Cells 2017, 40, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Inoue, E.; Watanabe-Takahashi, A.; Obara, M.; Yamaya, T.; Takahashi, H. Kinetic Properties and Ammonium-Dependent Regulation of Cytosolic Isoenzymes of Glutamine Synthetase in Arabidopsis. J. Biol. Chem. 2004, 279, 16598–16605. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Fukushima, A.; Tabuchi-Kobayashi, M.; Funayama, K.; Kojima, S.; Maruyama, K.; Yamamoto, Y.Y.; Nishizawa, T.; Kobayashi, M.; Wakazaki, M.; et al. Cytosolic GLUTAMINE SYNTHETASE1;1 Modulates Metabolism and Chloroplast Development in Roots. Plant Physiol. 2020, 182, 1894–1909. [Google Scholar] [CrossRef] [PubMed]
- Tamura, W.; Hidaka, Y.; Tabuchi, M.; Kojima, S.; Hayakawa, T.; Sato, T.; Obara, M.; Kojima, M.; Sakakibara, H.; Yamaya, T. Reverse Genetics Approach to Characterize a Function of NADH-Glutamate Synthase1 in Rice Plants. Amino Acids 2010, 39, 1003–1012. [Google Scholar] [CrossRef]
- Potel, F.; Valadier, M.; Ferrario-Méry, S.; Grandjean, O.; Morin, H.; Gaufichon, L.; Boutet-Mercey, S.; Lothier, J.; Rothstein, S.J.; Hirose, N.; et al. Assimilation of Excess Ammonium into Amino Acids and Nitrogen Translocation in Arabidopsis thaliana—Roles of Glutamate Synthases and Carbamoylphosphate Synthetase in Leaves. FEBS J. 2009, 276, 4061–4076. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Qiao, H.; Zheng, Y.; Hou, X.; Shi, L. An Integrated Transcriptome and Physiological Analysis of Nitrogen Use Efficiency in Rice (Oryza sativa L. Ssp. Indica) under Drought Stress. Front. Genet. 2024, 15, 1483113. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Qian, Y.; Miao, S.; Wang, W.; Xu, J.; Fu, B.; Zhang, F.; Zhao, X. Multi-Omics Analysis Reveals the Regulatory and Metabolic Mechanisms Underlying Low-Nitrogen Tolerance at the Flowering Stage in Rice. Agronomy 2023, 13, 578. [Google Scholar] [CrossRef]
- Liang, T.; Yuan, Z.; Fu, L.; Zhu, M.; Luo, X.; Xu, W.; Yuan, H.; Zhu, R.; Hu, Z.; Wu, X. Integrative Transcriptomic and Proteomic Analysis Reveals an Alternative Molecular Network of Glutamine Synthetase 2 Corresponding to Nitrogen Deficiency in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2021, 22, 7674. [Google Scholar] [CrossRef]
- Nan, Y.; Xie, Y.; He, H.; Wu, H.; Gao, L.; Atif, A.; Zhang, Y.; Tian, H.; Hui, J.; Gao, Y. Integrated BSA-Seq and RNA-Seq Analysis to Identify Candidate Genes Associated with Nitrogen Utilization Efficiency (NUtE) in Rapeseed (Brassica napus L.). Int. J. Biol. Macromol. 2024, 254, 127771. [Google Scholar] [CrossRef]
- Guo, Y.; Kong, F.; Xu, Y.; Zhao, Y.; Liang, X.; Wang, Y.; An, D.; Li, S. QTL Mapping for Seedling Traits in Wheat Grown Under Varying Concentrations of N, P and K Nutrients. Theor. Appl. Genet. 2012, 124, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Kapoor, N. Molecular Breeding Strategies for Genetic Improvement in Rice (Oryza sativa L.). In Advances in Plant Breeding Strategies: Cereals; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 317–341. ISBN 978-3-030-23107-1. [Google Scholar]
- Kumar, R.; Vinod, K.K.; Krishnan, S.G.; Kumar, D.; Mehrotra, S.; Sathee, L.; Ellur, R.K.; Singh, A.K.; Bhowmick, P.K.; Bollinedi, H.; et al. Meta-QTLs Linked to Nitrogen Use Efficiency Are Randomly Distributed in Indian Rice Germplasm. Indian J. Genet. Plant Breed. 2022, 82, 7–15. [Google Scholar] [CrossRef]
- Ogawa, S.; Valencia, M.O.; Lorieux, M.; Arbelaez, J.D.; McCouch, S.; Ishitani, M.; Selvaraj, M.G. Identification of QTLs Associated with Agronomic Performance under Nitrogen-Deficient Conditions Using Chromosome Segment Substitution Lines of a Wild Rice Relative, Oryza Rufipogon. Acta Physiol. Plant 2016, 38, 103. [Google Scholar] [CrossRef]
- Neeraja, C.N.; Subramanyam, D.; Surekha, K.; Rao, P.R.; Rao, L.V.S.; Babu, M.B.B.P.; Voleti, S.R. Advances in Genetic Basis of Nitrogen Use Efficiency of Rice. Indian J. Plant Physiol. 2016, 21, 504–513. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Sun, Y.; Zhang, J.; Du, W.; Guo, X.; Li, S.; Zhao, Y.; Xia, L. Efficient Allelic Replacement in Rice by Gene Editing: A Case Study of the NRT1.1B Gene. J. Integr. Plant Biol. 2018, 60, 536–540. [Google Scholar] [CrossRef]
- Yadav, M.R.; Kumar, S.; Lal, M.K.; Kumar, D.; Kumar, R.; Yadav, R.K.; Kumar, S.; Nanda, G.; Singh, J.; Udawat, P.; et al. Mechanistic Understanding of Leakage and Consequences and Recent Technological Advances in Improving Nitrogen Use Efficiency in Cereals. Agronomy 2023, 13, 527. [Google Scholar] [CrossRef]
- Fiaz, S.; Wang, X.; Khan, S.A.; Ahmar, S.; Noor, M.A.; Riaz, A.; Ali, K.; Abbas, F.; Mora-Poblete, F.; Figueroa, C.R.; et al. Novel Plant Breeding Techniques to Advance Nitrogen Use Efficiency in Rice: A Review. GM Crops Food 2021, 12, 627–646. [Google Scholar] [CrossRef]
- Panahabadi, R.; Ahmadikhah, A.; Farrokhi, N.; Bagheri, N. Genome-Wide Association Study (GWAS) of Germination and Post-Germination Related Seedling Traits in Rice. Euphytica 2022, 218, 112. [Google Scholar] [CrossRef]
- Zhu, C.; Gore, M.; Buckler, E.S.; Yu, J. Status and Prospects of Association Mapping in Plants. Plant Genome 2008, 1, 5–20. [Google Scholar] [CrossRef]
- Mallikarjuna, B.P.; Shettigar, N.; Radhika, D.H.; Devi, E.L.; Bhat, J.S.; Patil, B.S.; Lohithaswa, H.C.; Mallikarjuna, M.G. Genome-Wide Association Studies and Genomic Selection for Nutrient Use Efficiency in Cereals. In Next-Generation Plant Breeding Approaches for Stress Resilience in Cereal Crops; Gowdra Mallikarjuna, M., Nayaka, S.C., Kaul, T., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 161–197. ISBN 978-981-19144-4-7. [Google Scholar]
- Liao, Z.; Xia, X.; Zhang, Z.; Nong, B.; Guo, H.; Feng, R.; Chen, C.; Xiong, F.; Qiu, Y.; Li, D.; et al. Genome-Wide Association Study Using Specific-Locus Amplified Fragment Sequencing Identifies New Genes Influencing Nitrogen Use Efficiency in Rice Landraces. Front. Plant Sci. 2023, 14, 1126254. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, J.; Wang, Y.; Wang, Q.; Lu, X.; Hu, H.; Qian, Q.; Guo, L.; Shang, L. Loci and Natural Alleles for Low-Nitrogen-Induced Growth Response Revealed by the Genome-Wide Association Study Analysis in Rice (Oryza sativa L.). Front. Plant Sci. 2021, 12, 770736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, C.; Xu, W.; Li, Y.; Qi, X.; Zhao, M. Genome-Wide Association Study of Agronomic Traits Related to Nitrogen Use Efficiency in Henan Wheat. BMC Genom. 2024, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, S.D.; Han, Y.; Zhang, X.-Q.; Zhou, G.; Hill, C.B.; Chen, K.; Angessa, T.; Li, C. Genome-Wide Association Study and Identification of Candidate Genes for Nitrogen Use Efficiency in Barley (Hordeum vulgare L.). Front. Plant Sci. 2020, 11, 571912. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Subbaiah, M.; Roy, J.; Phogat, S.; Kaushik, M.; Saini, M.R.; Madhavan, J.; Sevanthi, A.M.; Mandal, P.K. Strategies to Utilize Genome Editing for Increasing Nitrogen Use Efficiency in Crops. Nucleus 2024, 67, 205–225. [Google Scholar] [CrossRef]
- Sathee, L.; Jagadhesan, B.; Pandesha, P.H.; Barman, D.; Adavi, B.S.; Nagar, S.; Krishna, G.K.; Tripathi, S.; Jha, S.K.; Chinnusamy, V. Genome Editing Targets for Improving Nutrient Use Efficiency and Nutrient Stress Adaptation. Front. Genet. 2022, 13, 900897. [Google Scholar] [CrossRef]
- Neeraja, C.N.; Barbadikar, K.M.; Mangrauthia, S.K.; Rao, P.R.; Subrahmanayam, D.; Sundaram, R.M. Genes for NUE in Rice: A Way Forward for Molecular Breeding and Genome Editing. Plant Physiol. Rep. 2021, 26, 587–599. [Google Scholar] [CrossRef]
- Karunarathne, S.D.; Han, Y.; Zhang, X.; Li, C. CRISPR/Cas9 Gene Editing and Natural Variation Analysis Demonstrate the Potential for HvARE1 in Improvement of Nitrogen Use Efficiency in Barley. J. Integr. Plant Biol. 2022, 64, 756–770. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Gao, Z.; Tian, H.; Gao, Y.; Kariman, K. Genetically Modified Crops Are Superior in Their Nitrogen Use Efficiency-A Meta-Analysis of Three Major Cereals. Sci. Rep. 2020, 10, 8568. [Google Scholar] [CrossRef]
- Fraisier, V.; Gojon, A.; Tillard, P.; Daniel-Vedele, F. Constitutive Expression of a Putative High-Affinity Nitrate Transporter in Nicotiana Plumbaginifolia: Evidence for Post-Transcriptional Regulation by a Reduced Nitrogen Source. Plant J. 2000, 23, 489–496. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Babalola, O.O. Metagenomics: A Tool for Exploring Key Microbiome With the Potentials for Improving Sustainable Agriculture. Front. Sustain. Food Syst. 2022, 6, 886987. [Google Scholar] [CrossRef]
- Chandrashekharaiah, P.S.; Kodgire, S.; Sanyal, D.; Dasgupta, S. Battling Climate Change: Improving Crop Productivity and Quality by Increasing Photosynthetic Efficiency, Deploying Microbiome Metagenomics, and Effectively Utilizing Digital Technology. In Climate Change and the Microbiome: Sustenance of the Ecosphere; Choudhary, D.K., Mishra, A., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 637–660. ISBN 978-3-030-76863-8. [Google Scholar]
- Sun, C.; Lu, Y.; Tang, G.; Wang, R.; Wu, H.; Zhang, J.; Cai, S.; Zhu, J.; Xiong, Q. Integrated Metagenomics and 15N Isotope Tracing Reveal the Mechanisms Through Which the Nitrogen-Planting Density Interaction Impacts Rice Root Nitrogen Uptake Efficiency. J. Soil Sci. Plant Nutr. 2024, 24, 2665–2678. [Google Scholar] [CrossRef]
- Xue, S.; Yi, X.; Peng, J.; Bak, F.; Zhang, L.; Duan, G.; Liesack, W.; Zhu, Y. Fulvic Acid Enhances Nitrogen Fixation and Retention in Paddy Soils through Microbial-Coupled Carbon and Nitrogen Cycling. Environ. Sci. Technol. 2024, 58, 18777–18787. [Google Scholar] [CrossRef]
- Wu, W.-F.; Li, X.-Y.; Chen, S.-C.; Jin, B.-J.; Wu, C.-Y.; Li, G.; Sun, C.; Zhu, Y.-G.; Lin, X.-Y. Nitrogen Fertilization Modulates Rice Phyllosphere Functional Genes and Pathogens through Fungal Communities. Sci. Total Environ. 2024, 929, 172622. [Google Scholar] [CrossRef]
- Kaviraj, M.; Kumar, U.; Chatterjee, S.; Parija, S.; Padbhushan, R.; Nayak, A.K.; Gupta, V.V.S.R. Dissimilatory Nitrate Reduction to Ammonium (DNRA): A Unique Biogeochemical Cycle to Improve Nitrogen (N) Use Efficiency and Reduce N-Loss in Rice Paddy. Rhizosphere 2024, 30, 100875. [Google Scholar] [CrossRef]
- Babajanpour Bora, A.; Ranjbar, G.; Hashemi-Petroudi, S.H.; Nematzadeh, G.; Najafi Zarini, H. The Growth Promoting Effect of Native Cyanobacterial Strains on Rice Germination, as Well as a In Silico Analysis of Ammonium Transporter (OsAMT) Gene Family. Crop Biotechnol. 2024, 14, 45–65. [Google Scholar] [CrossRef]
- Wang, R.; Tang, G.; Lu, Y.; Zhang, D.; Cai, S.; He, H.; Zhang, H.; Xiong, Q. Root Physiological and Soil Microbial Mechanisms Underlying Responses to Nitrogen Deficiency and Compensation in Indica and Japonica Rice. Physiol. Plant. 2024, 176, e14549. [Google Scholar] [CrossRef]
- Zhu, K.; Fu, J.; Zhang, Y.; Ren, W.; Zhang, W.; Gu, J.; Xu, Y.; Zhang, H.; Wang, Z.; Liu, L.; et al. Root Activity and Rhizospheric Bacteria in Response to Nitrogen Management in Rice (Oryza sativa L.). Eur. J. Agron. 2024, 159, 127294. [Google Scholar] [CrossRef]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and Shoot Traits for Rice Varieties with Higher Grain Yield and Higher Nitrogen Use Efficiency at Lower Nitrogen Rates Application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Singh, U.; Ladha, J.K.; Castillo, E.G.; Punzalan, G.; Tirol-Padre, A.; Duqueza, M. Genotypic Variation in Nitrogen Use Efficiency in Medium- and Long-Duration Rice. Field Crops Res. 1998, 58, 35–53. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Y.; Zhao, G.; Cheng, W.; Guo, S.; Zhang, H.; Shi, W. Do High Nitrogen Use Efficiency Rice Cultivars Reduce Nitrogen Losses from Paddy Fields? Agric. Ecosyst. Environ. 2015, 209, 26–33. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, S.; Huang, L.; Sun, F.; Zhu, G.; Li, G.; Fahad, S.; Peng, S.; Wang, F. Physiological Mechanisms Underlying the High-Grain Yield and High-Nitrogen Use Efficiency of Elite Rice Varieties Under a Low Rate of Nitrogen Application in China. Front. Plant Sci. 2016, 7, 1024. [Google Scholar] [CrossRef]
- Natarajan, S.; Karuppasamy, K.S.; Ramasamy, A.; Chellamuthu, T.; Sadasivam, N.; Kovilpillai, B.; Govindan, S.K.; Veerasamy, R.; Muthurajan, R. Exploring Rice Genotypes for Nitrogen Use Efficiency under Different Nitrogen Regimes. Plant Physiol. Rep. 2024, 30, 175–190. [Google Scholar] [CrossRef]
- Tyagi, A.; Chakraborty, N.; Raghuram, N. Morpho-Physiological Evaluation of Indica Rice Genotypes with Contrasting Crop Duration for Nitrogen Use Efficiency Under Graded Urea Doses. J. Plant Growth Regul. 2024, 43, 4826–4847. [Google Scholar] [CrossRef]
- Srikanth, B.; Subrahmanyam, D.; Sanjeeva Rao, D.; Narender Reddy, S.; Supriya, K.; Raghuveer Rao, P.; Surekha, K.; Sundaram, R.M.; Neeraja, C.N. Promising Physiological Traits Associated with Nitrogen Use Efficiency in Rice under Reduced N Application. Front. Plant Sci. 2023, 14, 1268739. [Google Scholar] [CrossRef]
- Gawdiya, S.; Kumar, D.; Shivay, Y.S.; Bhatia, A.; Mehrotra, S.; Chandra, M.S.; Kumawat, A.; Kumar, R.; Price, A.H.; Raghuram, N.; et al. Field-Based Evaluation of Rice Genotypes for Enhanced Growth, Yield Attributes, Yield and Grain Yield Efficiency Index in Irrigated Lowlands of the Indo-Gangetic Plains. Sustainability 2023, 15, 8793. [Google Scholar] [CrossRef]
- Padhan, B.K.; Sathee, L.; Kumar, S.; Chinnusamy, V.; Kumar, A. Variation in Nitrogen Partitioning and Reproductive Stage Nitrogen Remobilization Determines Nitrogen Grain Production Efficiency (NUEg) in Diverse Rice Genotypes Under Varying Nitrogen Supply. Front. Plant Sci. 2023, 14, 1093581. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiang, H.; Fu, Y.; Zhou, C.; Wang, X.; Yuan, S.; Yu, X.; Peng, S. Optimal Nitrogen Management Increases Nitrogen Use Efficiency of Direct-Seeded Double-Season Rice Using Ultrashort-Duration Cultivars. Field Crops Res. 2024, 316, 109495. [Google Scholar] [CrossRef]
- Hussain, T.; Hussain, N.; Ahmed, M.; Nualsri, C.; Duangpan, S. Impact of Nitrogen Application Rates on Upland Rice Performance, Planted under Varying Sowing Times. Sustainability 2022, 14, 1997. [Google Scholar] [CrossRef]
- Jagadhesan, B.; Meena, H.S.; Jha, S.K.; Krishna, K.G.; Kumar, S.; Elangovan, A.; Chinnusamy, V.; Kumar, A.; Sathee, L. Association of Nitrogen Utilisation Efficiency with Sustenance of Reproductive Stage Nitrogen Assimilation, Transcript Abundance and Sequence Variation of Nitrogen Metabolism Genes in Rice (Oryza sativa L.) Sub-Species. Plant Physiol. Rep. 2024, 29, 931–947. [Google Scholar] [CrossRef]
- Haefele, S.M.; Jabbar, S.M.A.; Siopongco, J.D.L.C.; Tirol-Padre, A.; Amarante, S.T.; Sta Cruz, P.C.; Cosico, W.C. Nitrogen Use Efficiency in Selected Rice (Oryza sativa L.) Genotypes under Different Water Regimes and Nitrogen Levels. Field Crops Res. 2008, 107, 137–146. [Google Scholar] [CrossRef]


| S.N. | Country | Cultivars and Genotypes with High NUE | Characteristics | References |
|---|---|---|---|---|
| 1. | China | Huaidao 5 (HD-5) and Lianjing 7 (LJ-7) | Deeper roots, higher root oxidation, and enhanced photosynthetic NUE at low N conditions | [206] |
| 2. | Philippines | IR54790-B-B-38, BG380-2, BG90-2 (medium duration), IR3932-182-2-3-3-2, IR54853-B-B-318, and IR29723-88-2-3-3 (long duration) | [207] | |
| 3. | China | Wuyunjing 23 (W23) and Zhendao 11 (Z11) | Cultivars W23 and Z11 have 21–27% less NH3 volatilization, 23–26% less N2O emission, 23–33% less N leaching, and 13–24% less N in runoff | [208] |
| 4. | China | HY549 and YY4949 | NUE for grain production (NUEg) 35.2 to 62.0 kg kg−1 | [209] |
| 5. | Nepal | Arize 6444 | Use of Arize 6444 along with fertilization with deep-placed briquette urea increased grain yield by 21–23% | [23] |
| 6. | India | Rice genotypes (IRGC 6386-1, WAS 20, IRGC 116967-1 WAS 207), Variety (IR64) | Higher yield and NUE under low N conditions | [210] |
| 7. | India | Dhala Heera | Higher yield and NUE across soil and various N conditions | [211] |
| 8. | India | Birupa | High NUE and crop yield under low nitrogen | [212] |
| 9. | India | Nidhi and Daya | Higher grain yield, NUE, and grain yield efficiency index GYEI | [213] |
| 10. | India | R-83929-B-B-291-3-1-1, Kalinga-1, APO, Pusa Basmati-1, and Nerica-L-44 | Higher grain yield and NUE under N-deficit conditions | [214] |
| 11. | China | Nconv and Nweek | Higher leaf area index, stem numbers, and N uptake | [215] |
| 12. | Thailand | Dawk Pa–yawm | Showed higher NUE and nitrogen agronomic efficiency (NAE) at application of 90 kg N ha−1 | [216] |
| 13. | India | Apo and Nerica-L-42 | Change in gene expression and amino acid sequence of NLP3, NLP4, and NLP5 make them potential resources for genome editing for enhancing NUE | [217] |
| 14. | Philippines | CT6510-24-1-2, IR55423-01, IR72, and IR57514-PMI5-B-1-2 | Higher NUE under limited N and water supply | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunwar, U.B.; Manzoor, N.; Wen, J.; Pandit, N.R. Integrating Agronomic and Molecular Advancements to Enhance Nitrogen Use Efficiency (NUE) and Promote Sustainable Rice Production. Nitrogen 2025, 6, 34. https://doi.org/10.3390/nitrogen6020034
Kunwar UB, Manzoor N, Wen J, Pandit NR. Integrating Agronomic and Molecular Advancements to Enhance Nitrogen Use Efficiency (NUE) and Promote Sustainable Rice Production. Nitrogen. 2025; 6(2):34. https://doi.org/10.3390/nitrogen6020034
Chicago/Turabian StyleKunwar, Uttam Bahadur, Nazer Manzoor, Jiancheng Wen, and Naba Raj Pandit. 2025. "Integrating Agronomic and Molecular Advancements to Enhance Nitrogen Use Efficiency (NUE) and Promote Sustainable Rice Production" Nitrogen 6, no. 2: 34. https://doi.org/10.3390/nitrogen6020034
APA StyleKunwar, U. B., Manzoor, N., Wen, J., & Pandit, N. R. (2025). Integrating Agronomic and Molecular Advancements to Enhance Nitrogen Use Efficiency (NUE) and Promote Sustainable Rice Production. Nitrogen, 6(2), 34. https://doi.org/10.3390/nitrogen6020034








