Impacts of Nitrogen Fertilizer Application Timing and Rate on Sweet Corn Production Under Subtropical Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
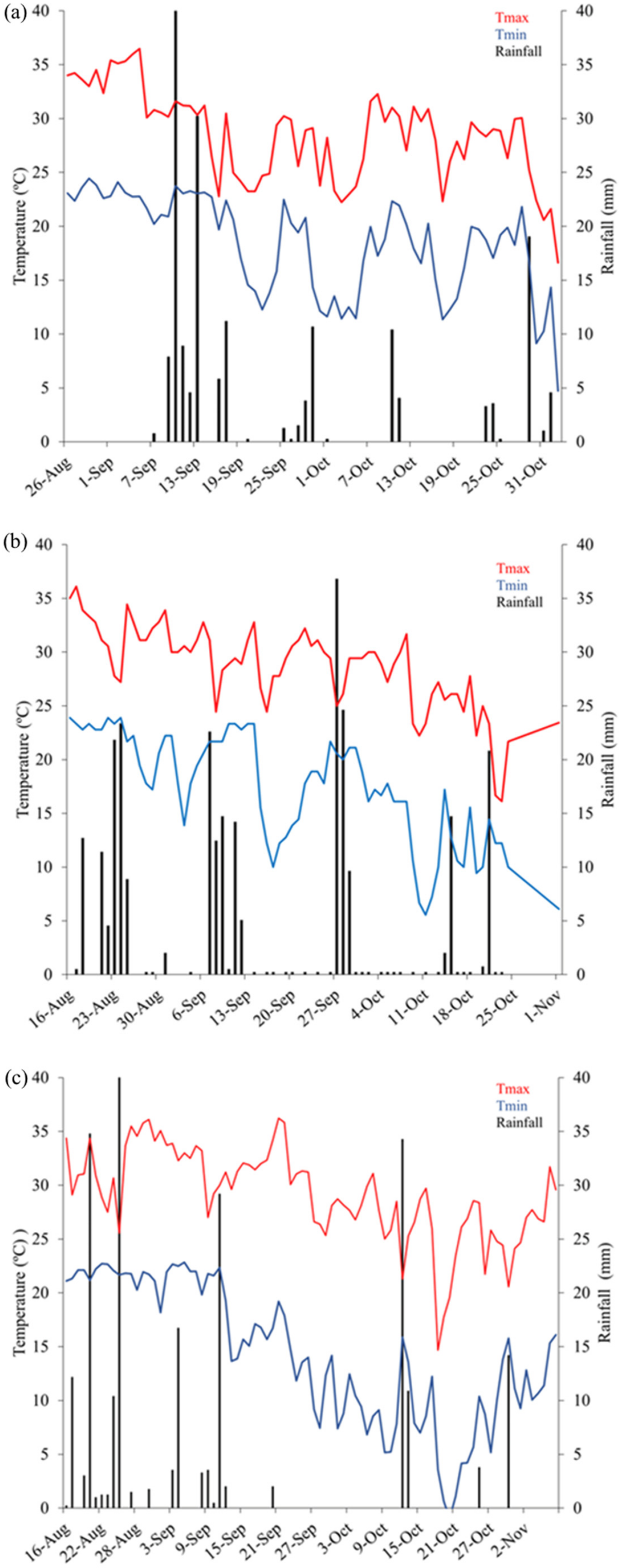
2.2. Weather Conditions
2.3. Soil Total Nitrogen
2.4. Biomass Accumulation and Total Nitrogen
2.5. Yield and Ear Structure
2.6. Statistical Analysis
3. Results
3.1. Weather Data and Growing Degree Days
3.2. Leaf Area Index, Biomass Accumulation, Total Nitrogen, and Soil Total Nitrogen
3.3. Effect of Neme on Biomass Accumulation, Total Nitrogen, and Soil Total Nitrogen Across Growth Stages
3.4. Effect of Nsd on Leaf Area Index and Soil Total Nitrogen Across Growth Stages
3.5. Effect of the Interaction Between Location and Neme on Total Nitrogen and Soil Total Nitrogen Across Growth Stages
3.6. Effect of the Interaction Between Location and Nsd on Leaf Area Index, Biomass Accumulation, and Total Nitrogen Across Growth Stages
3.7. Effect of Location on Sweet Corn Yield and Ear Structure Parameters
3.8. Nitrogen Use Efficiency
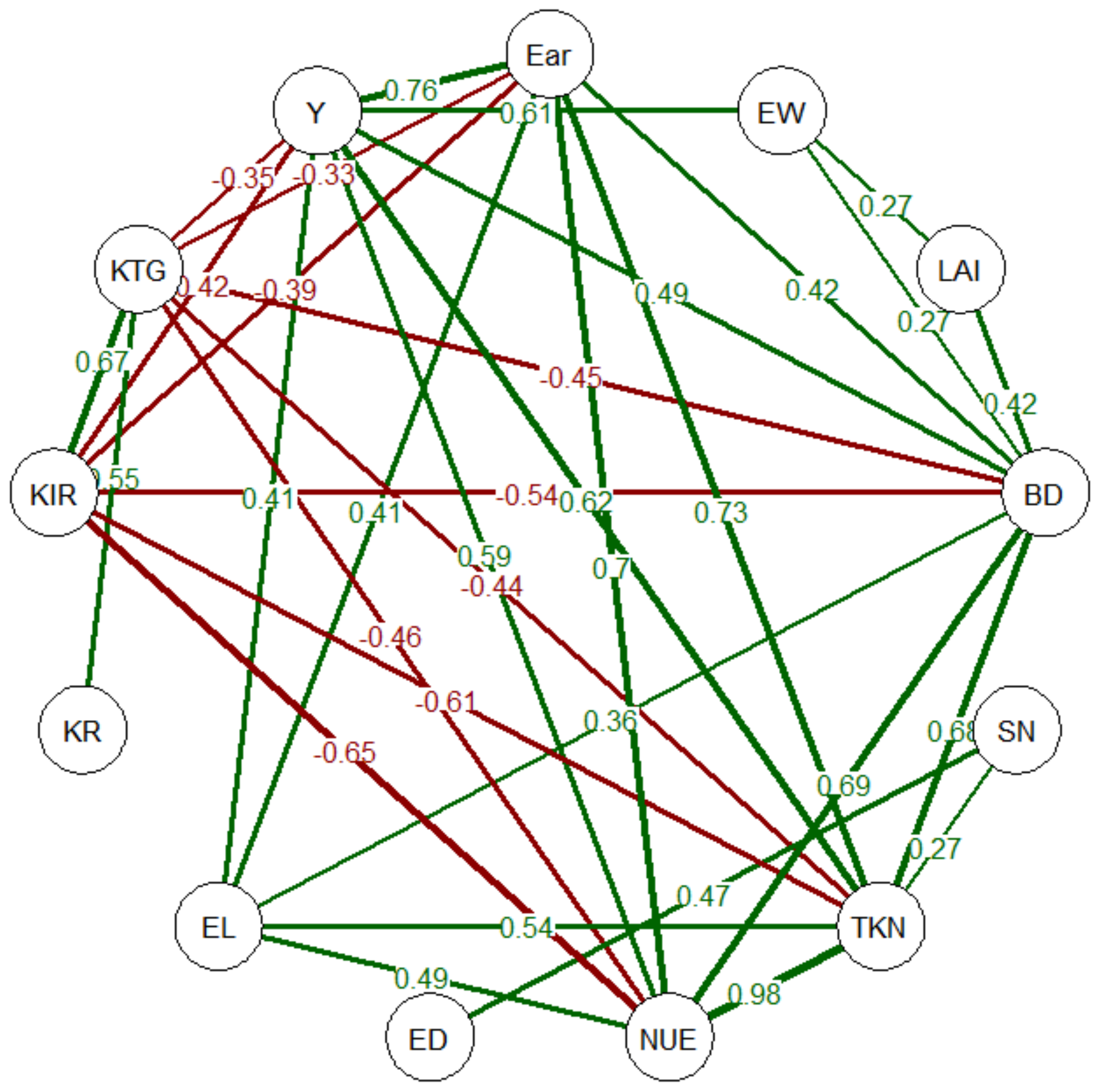
3.9. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. USDA National Agricultural Statistics Service—Vegetables 2021 Summary. Available online: https://quickstats.nass.usda.gov/ (accessed on 20 January 2025).
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Morton, L.W.; Cooley, D.; Clements, J.; Gleason, M. Climate, Weather and Apples; Department of Sociology, Iowa State University: Ames, IN, USA, 2017; p. 16. [Google Scholar]
- Revilla, P.; Anibas, C.M.; Tracy, W.F. Sweet corn research around the world 2015–2020. Agronomy 2021, 11, 534. [Google Scholar] [CrossRef]
- Khan, A.A.; Hussain, A.; Ganai, M.A.; Sofi, N.; Hussain, S.T. Yield, nutrient uptake and quality of sweet corn as influenced by transplanting dates and nitrogen levels. J. Pharmacogn. Phytochem. 2018, 7, 3567–3571. [Google Scholar]
- Sugiyama, T.; Sakakibara, H. Regulation of carbon and nitrogen assimilation through gene expression. In Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism; Springer: Dordrecht, The Netherlands, 2002; pp. 227–238. [Google Scholar]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [PubMed]
- Calabi-Floody, M.; Medina, J.; Rumpel, C.; Condron, L.M.; Hernandez, M.; Dumont, M.; de La Luz Mora, M. Smart fertilizers as a strategy for sustainable agriculture. Adv. Agron. 2018, 147, 119–157. [Google Scholar]
- Oktem, A.; Oktem, A.; Emeklier, H. Effect of nitrogen on yield and some quality parameters of sweet corn. Commun. Soil Sci. Plant Anal. 2010, 41, 832–847. [Google Scholar]
- Stephens, J.M.; Liu, G. Soil Preparation and Liming for Vegetable Gardens. Available online: https://edis.ifas.ufl.edu/publication/VH024 (accessed on 12 June 2024).
- Camberato, J.; Nielsen, R.; Joern, B.; Nitrogen Management Guidelines for Corn in Indiana. Purdue Nitrogen Management Update. 2017. Available online: https://www.agry.purdue.edu/ext/corn/news/timeless/nitrogenmgmt.pdf (accessed on 14 December 2016).
- Panison, F.; Sangoi, L.; Durli, M.M.; Leolato, L.S.; Coelho, A.E.; Kuneski, H.F.; Liz, V.O.d. Timing and splitting of nitrogen side-dress fertilization of early corn hybrids for high grain yield. Rev. Bras. Ciência Solo 2019, 43, e0170338. [Google Scholar]
- Gao, L.; Li, Y.-L.; Li, W.; Yu, T.; Li, G.-K.; Li, C.-Y.; Hu, J.-G. Effects of nitrogen application on yields and nitrogen use efficiencies of sweet corn in south China. J. Plant Nutr. Fertil. 2017, 23, 1215–1224. [Google Scholar]
- Malik, W.; Isla, R.; Dechmi, F. DSSAT-CERES-maize modelling to improve irrigation and nitrogen management practices under Mediterranean conditions. Agric. Water Manag. 2019, 213, 298–308. [Google Scholar] [CrossRef]
- Kalvová, J.; Halenka, T.; Bezpalcová, K.; Nemešová, I. Köppen climate types in observed and simulated climates. Stud. Geophys. Geod. 2003, 47, 185–202. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Kemble, J.M.; Bertucci, T.R.; Jennings, K.M.; Meadows, I.M.; Rodrigues, C.; Walgenbach, J.F.; Wszelaki, A.L. Southeastern U.S. 2022 Vegetable Crop Handbook; Great American Media Services: Sparta, MI, USA, 2022; Volume 2023, p. 365. [Google Scholar]
- McMaster, G.S.; Wilhelm, W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Eck, M.A.; Murray, A.R.; Ward, A.R.; Konrad, C.E. Influence of growing season temperature and precipitation anomalies on crop yield in the southeastern United States. Agric. For. Meteorol. 2020, 291, 108053. [Google Scholar]
- Zebarth, B.; Leclerc, Y.; Moreau, G. Rate and timing of nitrogen fertilization of Russet Burbank potato: Nitrogen use efficiency. Can. J. plant Sci. 2004, 84, 845–854. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Khan, Z.H.; Khalil, S.K.; Iqbal, A.; Ullah, I.; Ali, M.; Shah, T.; Wu, W.; Shah, F. Nitrogen doses and plant density affect phenology and yield of sweet corn. Fresenius Environ. Bull. 2017, 26, 3809–3815. [Google Scholar]
- Kar, P.; Barik, K.; Mahapatra, P.; Garnayak, L.; Rath, B.; Bastia, D.; Khanda, C. Effect of plantinggeometry and nitrogen on yield, economics and nitrogen uptake of sweet corn (Zea mays). Indian J. Agron. 2006, 51, 43–45. [Google Scholar] [CrossRef]
- Abewoy, D. Review on impacts of climate change on vegetable production and its management practices. Adv. Crop Sci. Technol. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Marañón, E.; Lorenzo, M.P.; Cermeño, P.; Mouriño-Carballido, B. Nutrient limitation suppresses the temperature dependence of phytoplankton metabolic rates. ISME J. 2018, 12, 1836–1845. [Google Scholar] [CrossRef]
- Jones, C.; Brown, B.D.; Engel, R.; Horneck, D.; Olson-Rutz, K. Nitrogen Fertilizer Volatilization; Montana State University Extension: Bozeman, MT, USA, 2013. [Google Scholar]
- Cazetta, J.O.; Seebauer, J.R.; Below, F.E. Sucrose and nitrogen supplies regulate growth of maize kernels. Ann. Bot. 1999, 84, 747–754. [Google Scholar] [CrossRef]
- Guo, R.; Li, X.; Christie, P.; Chen, Q.; Zhang, F. Seasonal temperatures have more influence than nitrogen fertilizer rates on cucumber yield and nitrogen uptake in a double cropping system. Environ. Pollut. 2008, 151, 443–451. [Google Scholar] [CrossRef][Green Version]
- Subedi, K.; Ma, B. Corn Crop Production: Growth, Fertilization and Yield; Nova Science Publisher: New York, NY, USA, 2009. [Google Scholar]
- Liliane, T.N.; Charles, M.S. Factors affecting yield of crops. In Agronomy-Climate Change & Food Security; IntechOpen: London, UK, 2020; p. 9. [Google Scholar]
- Dhaliwal, D.S.; Williams, M.M. Evidence of sweet corn yield losses from rising temperatures. Sci. Rep. 2022, 12, 18218. [Google Scholar]
- Ben-Asher, J.; Garcia y Garcia, A.; Hoogenboom, G. Effect of high temperature on photosynthesis and transpiration of sweet corn (Zea mays L. var. rugosa). Photosynthetica 2008, 46, 595–603. [Google Scholar]
- Ciampitti, I.A.; Vyn, T.J. A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res. 2011, 121, 2–18. [Google Scholar]
- Cheng, Q.; Xu, H.; Fei, S.; Li, Z.; Chen, Z. Estimation of maize LAI using ensemble learning and UAV multispectral imagery under different water and fertilizer treatments. Agriculture 2022, 12, 1267. [Google Scholar] [CrossRef]
- Li, G.; Long, H.; Zhang, R.; Xu, A.; Niu, L. Photosynthetic traits, water use and the yield of maize are influenced by soil water stability. BMC Plant Biol. 2024, 24, 1235. [Google Scholar]
- Yan, Y.; Duan, F.; Li, X.; Zhao, R.; Hou, P.; Zhao, M.; Li, S.; Wang, Y.; Dai, T.; Zhou, W. Photosynthetic capacity and assimilate transport of the lower canopy influence maize yield under high planting density. Plant Physiol. 2024, 195, 2652–2667. [Google Scholar]
- Sangoi, L.; Picoli Junior, G.J.; Vargas, V.P.; Vieira, J.; Schmitt, A.; Zoldan, S.R.; Siega, E.; Carniel, G. Nitrogen side-dress as a strategy to reduce defoliation damages at different growth stages of maize. Semin. Ciências Agrárias 2014, 35, 671–682. [Google Scholar]
- Yang, T.; Zhao, J.; Fu, Q. Quantitative Relationship of Plant Height and Leaf Area Index of Spring Maize under Different Water and Nitrogen Treatments Based on Effective Accumulated Temperature. Agronomy 2024, 14, 1018. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2005; Volume 88, pp. 97–185. [Google Scholar]
- Ren, Y.; Sun, Z.; Hu, X.; Liu, Q.; Xu, Q.; Qin, D.; Wang, X.; Liu, S.; Ma, C.; Wei, X. Effects of Different Nitrogen Allocation Ratios and Period on Cotton Yield and Nitrogen Utilization. Water 2023, 15, 3011. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; GK, S.; Vadivel, R.; TK, D. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar]
- Xu, A.; Li, L.; Xie, J.; Wang, X.; Coulter, J.A.; Liu, C.; Wang, L. Effect of long-term nitrogen addition on wheat yield, nitrogen use efficiency, and residual soil nitrate in a semiarid area of the loess plateau of China. Sustainability 2020, 12, 1735. [Google Scholar] [CrossRef]
- Flynn, N.E.; Comas, L.H.; Stewart, C.E.; Fonte, S.J. High N availability decreases N uptake and yield under limited water availability in maize. Sci. Rep. 2023, 13, 14269. [Google Scholar]
- Zhou, S.; Xia, P.; Chen, J.; Xiong, Q.; Li, G.; Tian, J.; Wu, B.; Zhou, F. Optimizing nitrogen application position to change root distribution in soil and regulate maize growth and yield formation in a wide–narrow row cropping system: Pot and field experiments. Front. Plant Sci. 2024, 15, 1298249. [Google Scholar]
- Yadete, E.; Gurmu, S.; Biya, M. Effects of Time and Rate of Nitrogen Fertilizer Application on Phenology, Growth and Yield of Maize at Jimma, Southwestern Ethiopia. World J. Agric. Sci. Technol. 2024, 2, 185–197. [Google Scholar]
- Nemeskéri, E.; Helyes, L. Physiological responses of selected vegetable crop species to water stress. Agronomy 2019, 9, 447. [Google Scholar] [CrossRef]
- Nielsen, R. N loss mechanisms and nitrogen use efficiency. In Purdue Nitrogen Management Workshops; Purdue University: West Lafayette, IN, USA, 2006; pp. 1–5. [Google Scholar]

| Location | Geographic Coordinates | Year | Season | Soil Type | IRS 1 (cm) | PS 2 (cm) | PD 3 | Harvest | GDD 4 (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Georgia | 32.01814° N, 82.22138° W | 2020 | Fall | Irvington loamy sand | 91.44 | 17.78 | Aug. 26 | Nov. 2 | 928 |
| Alabama | 32.50053° N, 85.89281° W | 2021 | Fall | Kalmia loamy sand | 91.44 | 17.78 | Aug. 16 | Nov. 1 | 921 |
| Alabama | 32.50053° N, 85.89281° W | 2022 | Fall | Kalmia loamy sand | 91.44 | 17.78 | Aug. 17 | Nov. 7 | 980 |
| Treatments | N Rates (kg ha−1) | |||
|---|---|---|---|---|
| Npl 1 | Neme 2 | Nsd 3 | Total N | |
| 1 | 34 | 56 | 134 | 224 |
| 2 | 34 | 56 | 162 | 252 |
| 3 | 34 | 56 | 190 | 280 |
| 4 | 34 | 112 | 134 | 280 |
| 5 | 34 | 112 | 162 | 308 |
| 6 | 34 | 112 | 190 | 336 |
| Stages | DAP 1 | |||||
|---|---|---|---|---|---|---|
| Soil Total N (NO3− + NH4+) | Biomass and Total N | |||||
| 2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |
| PL 2 | 0 | 0 | 0 | - | - | - |
| EME 3 | 19 | 25 | 28 | 19 | 25 | 28 |
| SD 4 | 40 | 44 | 40 | 40 | 44 | 40 |
| Silk | 54 | 66 | 62 | 54 | 66 | 62 |
| Harvest | 68 | 74 | 82 | 68 | 74 | 82 |
| Neme 1 (kg ha−1) | Biomass (kg ha−1) | Total N Uptake (kg ha−1) | Soil Total N (kg ha−1) | |||
|---|---|---|---|---|---|---|
| SD 2 | Silk | SD | Harvest | EME 3 | SD | |
| 56 | 2298 a 4 | 2604 a | 69.5 a | 98.0 a | 12.4 a | 27.3 b |
| 112 | 1992 b | 2797 a | 61.4 b | 108.8 a | 19.3 a | 50.9 a |
| p-value 5 | ** | ns | * | ns | ns | *** |
| Nsd 1 (kg ha−1) | LAI 2 (cm2) | Soil Total N (kg ha−1) |
|---|---|---|
| SD 3 | Silk | |
| 190 | 1870 a 4 | 138.9 a |
| 162 | 2069 a | 93.1 b |
| 134 | 1953 a | 81.4 b |
| p-value 5 | ns | * |
| Neme 1 (kg ha−1) | Georgia (2020) | Alabama (2021) | Alabama (2022) |
|---|---|---|---|
| Harvest | |||
| Total N Uptake (kg ha−1) | |||
| 56 | - | 41.40 aB | 154.59 bA |
| 112 | - | 39.78 aB | 177.77 aA |
| p-value 2 | - | * | * |
| Neme (kg ha−1) | SD 3 | ||
| Soil total N (kg ha−1) | |||
| 56 | 24.36 b 4 B 5 | 3.78 aB | 53.93 aA |
| 112 | 83.54 aA | 2.99 aB | 66.41 aA |
| p-value | ** | ** | ** |
| Nsd 1 (kg ha−1) | Georgia (2020) | Alabama (2021) | Alabama (2022) |
|---|---|---|---|
| EME 2 | |||
| LAI (cm2) 3 | |||
| 134 | 303 a 4 C 5 | 777 bB | 1467 aA |
| 162 | 322 aC | 814 abB | 1335 abA |
| 190 | 344 aC | 965 aB | 1268 bA |
| p-value 6 | * | * | * |
| Nsd (kg ha−1) | SD 7 | ||
| Biomass (kg ha−1) | |||
| 134 | 1179 aC | 2116 aB | 3217 aA |
| 162 | 1573 aB | 1890 aB | 2724 bA |
| 190 | 1475 aC | 2035 aB | 3095 aA |
| p-value | * | * | * |
| Nsd (kg ha−1) | EME | ||
| Total N uptale (kg ha−1) | |||
| 134 | - | 13.00 aB | 67.58 aA |
| 162 | - | 15.01 aA | 21.36 bA |
| 190 | - | 17.71 aA | 20.67 bA |
| p-value | - | * | * |
| Nsd (kg ha−1) | SD | ||
| Total N uptake (kg ha−1) | |||
| 134 | 41.91 aB | 57.37 aB | 96.44 aA |
| 162 | 53.83 aB | 50.52 aB | 83.18 bA |
| 190 | 50.54 aB | 52.61 aB | 102.61 aA |
| p-value | * | * | * |
| Location | Yield (kg ha−1) | Ears per Plant | ED 1 (cm) | EL 2 (cm) | KIR 3 | KTG 4 |
|---|---|---|---|---|---|---|
| Georgia (2020) | 15,951 ab 5 | 1 b | 4.17 c | 17 c | 33 a | 472 ab |
| Alabama (2021) | 14,470 b | 1 b | 4.77 a | 18 b | 36 a | 503 ab |
| Alabama (2022) | 17,380 a | 1.13 a | 4.49 b | 19 a | 30 b | 437 b |
| p-value 6 | *** | *** | *** | *** | *** | ** |
| NUEeme 1 (%) | NUEsd 2 (%) | NUEsilk 3 (%) | NUEharv 4 (%) | |
|---|---|---|---|---|
| Location | ||||
| Georgia (2020) | - | 43.0 b | 15.5 b | 21.0 b |
| Alabama (2021) | 23.0 b 5 | 37.5 b | 22.1 b | 13.2 c |
| Alabama (2022) | 28.9 a | 60.6 a | 51.0 a | 51.6 a |
| p-value 6 | * | *** | *** | *** |
| Neme 7 (kg ha−1) | ||||
| 56 | 26.5 a | 58.8 a | 31.7 a | 29.3 a |
| 112 | 25.4 a | 35.2 b | 27.3 b | 27.8 a |
| p-value | ns | *** | * | ns |
| Nsd 8 (kg ha−1) | ||||
| 134 | 25.9 a | 46.3 a | 33.7 a | 29.8 ab |
| 162 | 25.2 a | 45.2 a | 27.6 b | 30.5 a |
| 190 | 26.8 a | 49.5 a | 27.2 b | 25.4 b |
| p-value | ns | ns | * | ** |
| Location*Neme | ns | ns | ns | ns |
| Location*Nsd | ns | ns | ns | ns |
| Neme*Nsd | ns | ns | ns | ns |
| Location*Neme*Nsd | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paranhos, J.; Foshee, W.; Coolong, T.; Torres-Quezada, E.; da Silva, A.L.B.R. Impacts of Nitrogen Fertilizer Application Timing and Rate on Sweet Corn Production Under Subtropical Environmental Conditions. Nitrogen 2025, 6, 20. https://doi.org/10.3390/nitrogen6020020
Paranhos J, Foshee W, Coolong T, Torres-Quezada E, da Silva ALBR. Impacts of Nitrogen Fertilizer Application Timing and Rate on Sweet Corn Production Under Subtropical Environmental Conditions. Nitrogen. 2025; 6(2):20. https://doi.org/10.3390/nitrogen6020020
Chicago/Turabian StyleParanhos, Jessica, Wheeler Foshee, Timothy Coolong, Emmanuel Torres-Quezada, and Andre Luiz Biscaia Ribeiro da Silva. 2025. "Impacts of Nitrogen Fertilizer Application Timing and Rate on Sweet Corn Production Under Subtropical Environmental Conditions" Nitrogen 6, no. 2: 20. https://doi.org/10.3390/nitrogen6020020
APA StyleParanhos, J., Foshee, W., Coolong, T., Torres-Quezada, E., & da Silva, A. L. B. R. (2025). Impacts of Nitrogen Fertilizer Application Timing and Rate on Sweet Corn Production Under Subtropical Environmental Conditions. Nitrogen, 6(2), 20. https://doi.org/10.3390/nitrogen6020020







