Abstract
The development of critical levels for sap nitrate and chlorophyll meter reading (SPAD test) in the case of various crops is of great importance for growers in characterizing a plant’s N status. A field experiment with spring oat (Avena sativa L.) was carried out on loamy soil in Debrecen, Hungary, using a small-plot design. Ammonium nitrate was broadcast at rates of 0, 30, 60, and 90 kg N/ha in three replicates. The total N content of the plant, sap nitrate content, and SPAD values were measured at jointing when the first node appeared above the soil surface (Feekes 6) and at boot stage (Feekes 10). Regression analysis between total N content and sap nitrate showed cubic and linear relationships with r2 = 0.7982 (Feekes 6, whole plant) and 0.9625 (Feekes 10, upper developed leaves), respectively. Optimal grain yield was obtained when sap nitrate exceeded 650 mg/L and 540 mg/L at Feekes 6 and Feekes 10, respectively. There were linear and logarithmic relationships between total N content and SPAD values with r2 = 0.8058 and 0.6258 at Feekes 6 and 10. Optimal grain yield occurred over SPAD values of 43 and 48 at Feekes 6 and 10, respectively. Optimal N rate was 60 kg N/ha on the experimental site.
1. Introduction
Optimal N doses can be estimated most accurately by considering the plant N status during the growing season [1]. Performing traditional laboratory plant tests is time consuming and costly. The rapid test of plant sap nitrate and the measurement of SPAD values are two simple and inexpensive methods by which to characterize the N supply of the plant.
The plant sap nitrate test provides information on the actual inorganic N content of the plant, which is not yet assimilated. Some researchers define sap as the liquid from xylem, phloem, and other plant fluids, but there is no agreement on this definition [2,3]. Some consider sap as fluid from all conductive tissues, and others mainly focus on xylem fluids [4]. Regardless of the concept, the nutrients in sap are easily accessible for plant growth [5].
The measurement can be performed semi-quantitatively using test strips, which indicate the nitrate ion concentration of the sample with a color change (Merkoquant test strip). The color of the reactive zones on the test strip can be visually compared with a color scale provided on the packaging. The measurement can be made more accurate (quantitative) by using a handheld reflectometer (RQflex®, Merck, Darmstadt, Germany) to perform the comparison [6]. Since the method only measures the nitrate in the 0–500 mg/L range, and the nitrate content of the plant sap can be much higher than this, the method is difficult to use. Recently, plant sap nitrate content has been measured using a nitrate ion-selective electrode (Cardy meter; Horiba and Spectrum Technologies), and the instrument can measure 3–4 drops of fluid in 0–2000 mg nitrate/L range. The drawback of ion-selective electrodes is that the presence of other ions (chloride, nitrite, bicarbonate) may also affect the measurement [7].
Unfortunately, some other factors also affect the results of sap nitrate test, such as the time of day, the part of the plant, and also the weather conditions [8]. It is not recommended to perform the test after rain or in the case of a prolonged drought.
In recent decades, sap nitrate tests have become widespread. Critical values for the petiole sap nitrate can be found in the international literature on potatoes [9], peppers [10], and many other varieties of vegetables [11,12]. In the case of cereals, sap is pressed from the lower part of the leaf stalk or from the whole plant. Hoel [13] and Liu et al. [14] have provided sap nitrate guidelines for wheat, and Thompson et al. [15] for barley. Hoel [13] found that the critical sap nitrate values varied for different wheat hybrids; moreover, the production site also had an influencing effect on the results. In wheat and rye, Delgado and Follett have shown that a sap test can be used for the determination of additional nitrogen fertilizer requirements [16].
The chlorophyll content of the leaf is proportional to its nutrient supply, and this is one of the indicators of N supply [17]. Because of its simple use, a SPAD-502 (Minolta Corporation, Ramsey, NJ, USA) meter is very commonly applied in fertilization experiments [18,19,20]. The SPAD meter measures light absorption at 650 and 940 nm to estimate chlorophyll content. Since chlorophyll plays a key role in photosynthesis; its levels are used as an indicator of plant health in both agricultural fields and plant physiology research [21]. The disadvantage of this method is that the result may be affected not only by N supply but also by the lack of other nutrients and several other environmental factors [22,23]. The effectiveness of SPAD meters can also be influenced by other factors, including leaf thickness, plant species, growing season, growth stage, and the measurement point on the leaf [24].
The developmental stages were also found to be an important factor that significantly influences the relative chlorophyll content of the leaves. This means that the relative chlorophyll content increases with crop development [25].
Sap nitrate testing and SPAD measurements can be essential tools for managing the crop N levels. Sap nitrate testing allows farmers to improve fertilization by providing real-time data on inorganic N availability [26], while SPAD tests estimate chlorophyll content to indicate N status without destructive sampling [27]. These methods help to improve the nitrogen use efficiency (NUE) by preventing the over or under application of fertilizers, reducing environmental impact and lowering costs. They provide a faster, more cost-effective alternative to traditional laboratory nitrogen analysis and allow for frequent monitoring throughout the growing season [28]. By determining the critical thresholds values for different growth stages, farmers can ensure that crops receive optimum N at key stages of development. They also help to minimize the nitrate leaching to groundwater, thus enhancing environmental sustainability while maintaining high yields [29]. Overall, the sap nitrate and SPAD tests can support agriculture by improving both economic and environmental sustainability. To the best of our knowledge, the critical sap nitrate values at different growth stages for oat have not yet been established in the literature. While this test has been widely used for other crops, oat-specific values are lacking.
We hypothesized that there is a strong correlation between oat sap nitrate content and yield, as well as between SPAD values and yield. Therefore, our research aims to determine the lower limit of critical sap nitrate and the SPAD value in the case of the spring oat variety “Lota”.
2. Materials and Methods
2.1. Soil Characteristics and Experimental Setup
A small plot-field experiment was set up with spring oat (Avena Sativa L.), variety “Lota”, at the University of Debrecen, Hungary (GPS coordinates: 47°33′2.82″ N, 21°35′55.58″ E) in 2021. The size of the plots was 10.5 m2. The pre-harvest crop was winter wheat with uniform nitrogen fertilizer application rates in the former year.
Two weeks before the experiment was set up, 20 composite soil samples from the 0–20 cm layer were collected from the studied area and analyzed. For soil sampling, we used a soil sampling probe. Soil organic matter (SOM) and the mineral N (Nmin) content were 3.6% and 10.07 mg/kg in the upper layer, respectively. The mineral nitrogen content of soil samples (NO3− and NH4+ fractions) were measured by 0.01 M CaCl2 extractant [30] with a SKALAR continuous flow analyzer. The soil mineral N content was calculated as the sum of NO3− and NH4+ fractions. The thickness of the humus layer was 80 cm. According to the Hungarian Fertilizer Advisory System, the N status of the soil was good, and the phosphorus and potassium status were excessive (ammonium lactate soluble P and K content were AL − P2O5 = 1671 mg/kg and AL − K2O = 659 mg/kg). The soil was of a loamy texture, with a CaCO3 content of 8.1% and a slightly alkaline pH(H2O) = 8.3.
In the experiment, four treatments were set up as follows: control (no N fertilizer was applied) and three treatments with increasing nitrogen doses (30, 60, and 90 kg N/ha). As the P and K supply of the soil was excessive, no PK fertilizers were applied to the experimental area. Nitrogen was added in the form of ammonium nitrate on 6 March 2021. Oat seeds were sown a few days later, with the number of seeds released being 180 kg/ha (200 g/plot).
Precipitation data were measured during the oat growing season, and they were as follows: 19 mm in March; 32 mm in April; 64 mm in May; 6 mm in June; and 7 mm in July, i.e., a total of 128 mm in 4.5 months. The summer of 2021 was very dry; the total rainfall was just 13 mm in June and July, whereas the 30-year average rainfall for June and July was 131 mm. The temperature was also very high; in June and July, it was 4.5 °C higher than the 30-year average. Due to the drought, ripening occurred earlier than average; hence, harvesting was conducted on 14 July 2021.
2.2. Plant and Soil Sampling, Measurement of Plant Sap Nitrate, Total N, SPAD Values, and Soil Mineral N Content
Plant samples for the sap nitrate test were collected at Feekes 6 and 10 on 11 May and 8 June, respectively. Ten plants were collected in each plot between 10 and 11 a.m. in sunny weather. The samples were stored on ice in a cooler bag and processed within a few hours. At Feekes 6, the sap was pressed from the entire above ground part of the plant, while at Feekes 10, the upper two fully developed leaves and the lower 5 cm of the stems of 10 plants were squeezed out. Sap was extracted by a horizontal twin gear juicer (Angel Juicer 7500, Angel Co., Busan, Republic of Korea), and the nitrate measurement was conducted with the nitrate-ion selective electrode (Cardy meter, Horiba Laquatwin NO3−-11).
For measuring the total N content of plant, an additional 10 plants per plot were collected at Feekes 6 and 10. The plant samples were dried at 50 °C. The nitrogen content for the whole plant was determined at Feekes 6 and for the upper two fully developed leaves at Feekes 10. The total N content of the samples was measured via the Kjeldahl method using a Velp Scientifica UDK 129 distillation unit.
At Feekes 6 and 10, the photosynthetic activity (SPAD values) of the upper fully developed leaves was measured with a portable chlorophyll meter (SPAD-502, Minolta Corporation, Ramsey, NJ, USA). Five plants per plot were measured, and the average of 5 measurements per leaf were taken.
After harvest, soil samples were collected at the following depths: 0–20 cm; 20–40 cm; 40–60 cm. Each soil sample was a mixture of ten sub-samples. The soil mineral N content (NO3− and NH4+ fractions) was measured by 0.01 M CaCl2 extractant [30].
2.3. Measurements of Yield Reference Values
The yield from each plot was harvested using the Wintersteiger 125 plot machine. The thousand kernel weight and the moisture content of the grains were determined from sub-samples of the grain yield per plot. The moisture content of grain was measured by drying the sample at 105 °C. Before harvest, 10 plant samples per plot were collected and stem lengths (height to top of panicle) were measured. The harvest index was determined using the following formula [31]:
For the calculation of the relative yield, the maximum yield was considered to be 100%.
2.4. Statistical Evaluation and Estimation of Critical Nutrient Supply Values
Statistical analysis of the results was performed with SPSS 20.0. The effects of the N rates on the grain yield, stem length, thousand kernel weight, harvest index, sap nitrate concentration, N content of leaves, SPAD values, and soil nitrate content were analyzed via one-way ANOVA and Tukey’s post hoc test. Before conducting the one-way ANOVA, we tested the assumption of homogeneity of variances using Levene’s test. If the assumption of homogeneity is violated, the Welch test, followed by the Games–Howell post hoc test, was performed, and these cases are denoted by * in the tables and figures. Significantly different values at p ≤ 0.05 probability level are indicated by different letters. The data in the figures and tables are presented as the mean ± 2 SEM (standard error of the means). To estimate the optimal N rate for maximum yield and maximum profit, quadratic functions were fitted for grain yield response to N rate and yield value response to N rate. For the calculation, we used oat yield and fertilizer prices related to 2021.
Regression analysis was also performed to determine the relationship between the plant total N content and sap nitrate content and the N content and SPAD values.
Critical plant sap nitrate concentration and SPAD values were determined by the graphical method of Cate and Nelson [32]. Sap nitrate and SPAD values belonging to the 90% relative yield were considered as critical values.
3. Results and Discussion
Table 1 shows the effect of N fertilization on oat yield and some reference values. The average moisture content of the grain yield was 7.2%; the data in the table refer to wet grain weight. As a result of N fertilization, the yield increased compared to the control, and the highest yield was observed at 60 kg N/ha. Maximum thousand kernel weight was also at 60 kg N/ha dose, but the differences between treatments could not be verified statistically at the p ≤ 0.05 probability level. The stem length increased gradually with N doses. However, no significant difference between the 60 kg N/ha and 90 kg N/ha doses was found. The increase in height of the plant with N doses was expected as N has a crucial role in vegetative growth. Excess N application rates are often cited in the literature as contributing factors to lodging as they increase plant length while decreasing its strength [33]. In our experiment, the 90 kg N/ha dose did not cause lodging, suggesting that under the experimental circumstances, the N excess was not severe. Although N is generally the primary biomass limiting factor in agroecosystems [34], the effect of N fertilizers may be moderate in soils with high organic matter content [35] or sensitive to acidification [36]. The highest harvest index was observed at 0 kg; here, the limited N availability may have restricted vegetative growth, leading to a relatively higher proportion of the harvestable product. The lowest harvest index occurred at 90 kg N/ha, which promoted excessive biomass, leading to a lower proportion of economic yield.

Table 1.
Effect of N fertilization on spring oat yield and its reference values.
The relationship between yield and N rates could be well fitted using a quadratic function with an R2 value of 0.9987 (Figure 1a). The N rate for maximum yield is the point where the slope (dy/dx) of the yield response curve is zero [37]. Therefore, the derivative of the yield response equation to zero was set and solved for x.
dy/dx = −0.0004x + 0.0208
0 = −0.0004x + 0.0208
x = 52 kgN/ha
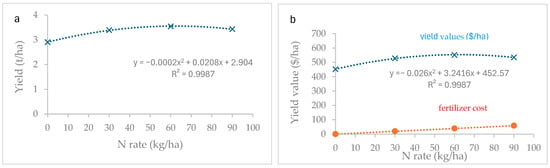
Figure 1.
Graphical representation of the N rate (a) for the maximum rate (b) and the optimal N rate for the maximum profit.
Similarly, the calculation with which to obtain the maximum profit was also performed using the quadratic fit to yield value. However, in this case, the first derivative was equal to the fertilizer price per kilogram of nitrogen active ingredient (i.e., USD 0.65/kgN) (Figure 1b).
dy/dx = −0.052x + 3.2416
0.65 = −0.052x + 3.2416
x = 49.8 kgN/ha
In our experiment, the N rate for the maximum yield was 52 kg/ha, which was very close to the rate for maximum profit (49.8 kg N/ha).
The mineral N content of the soil after harvest (Table 2) reflects the environmental impact of the different N rates. These data show that the 60 kg/ha N dose increased the soil mineral N concentration compared to control, which increased further at 90 kg N/ha rate. We can conclude that unsplit N doses at 90 kg/ha N rates caused N leaching.

Table 2.
Effect of N fertilization the mineral N content of the soil after harvest.
Sap nitrate content (Figure 2 and Figure 3a) measured at Feekes GS 6 increased more drastically with increasing N doses than the total N content of the plant. The sap nitrate values measured at the 90 kg N/ha dose were more than five times greater than that measured in the control plot. Here, the assumption of homogeneity of variances was violated; therefore, we conducted the Welch test, which showed significant differences. In the later growth stage (Feekes 10), both the plant’s nitrate and total nitrogen concentration decreased compared to Feekes 6 values. Sap nitrate values at Feekes 10 were different in the lower part of the stem and in the upper leaves. Typically, higher nitrate values were obtained in the leaf than in the stem. In contrast, in the case of barley and wheat, Thompson [15] found that the nitrate concentration was higher in the lower part of the stem. In his experiment, only the stem was collected on the field, and later, the nitrate concentration was measured from dry matter. In contrast, we collected the entire plant, placed it in a cooler, and conducted the measurements within 2–3 h. We separated the stem and leaves from the plant just before the nitrate measurements. The difference in the results may be due to the different sampling methods.
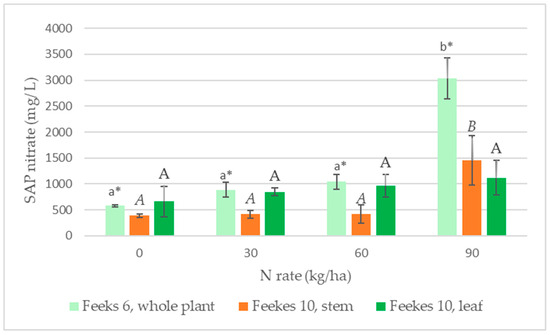
Figure 2.
Effect of N fertilization on the plant sap nitrate content at different growth stages. Note: data marked with the same letter are not significantly different in columns of the same color at the significant level of p ≤ 0.05; * denotes the case where the Welch test was performed instead of one-way ANOVA as homogeneity of variances was not fulfilled. Small letters indicate the difference between the values measured for Feekes 6, italic capital letters for Feekes 10 stem and capital letters for Feekes 10 leaf.
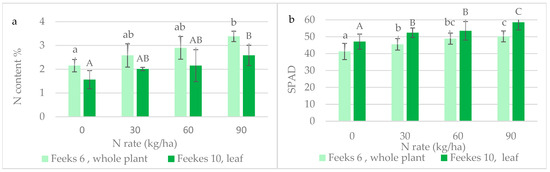
Figure 3.
Effect of N fertilization on (a) the total N content of the plant and (b) SPAD values at different growth stages. Note: data marked with the same letter are not significantly different at the significant level of p ≤ 0.05.
The total nitrogen (N) content of the plant (Figure 3a) increased with the increasing N doses. At 90 kg N/ha, the N content was approximately 1.5 times higher than that of the control at both the Feekes 6 and Feekes 10 growth stages. The increase in the SPAD values of the leaves (Figure 3b) was less steep with the increasing N doses at Feekes 6. The SPAD value at the highest N dose was 1.2 times higher than that of the control.
For SPAD values, there were significant differences between almost all N treatments, while for sap nitrate, the significant difference can only be proved between the highest N rate and other treatments. This is due to the fact that the data of SPAD were less dispersed than the data of sap nitrate and the total N content of the plant.
For the SPAD measurement, five data per plot were available, each averaging five measurements. Thus, a treatment effect was characterized by 5 × 5 × 3 = 75 data, as the measurement could be performed quickly. In the case of sap nitrate and total N content, only three data characterized the treatment, as these tests were more time consuming.
The relationship between the total N content and sap nitrate content at Feekes 6 can best be described with a polynomial equation (Figure 4a). This equation is valid for the studied range (N% > 1.8). At excess N supply, the nitrate content increased drastically. It shows that the rate of nitrate (N) uptake is larger than the N assimilation at large N rates. Similarly, Mahal et al. also found that at the early developmental stage (V7–V9) of maize, the stalk sap nitrate concentration increased much more intensely than the total N content of the plant [38]. At Feekes 10, however, the relationship between total N content and sap nitrate can be characterized with a linear equation (Figure 4b). Presumably, the increased biomass causes greater assimilation.
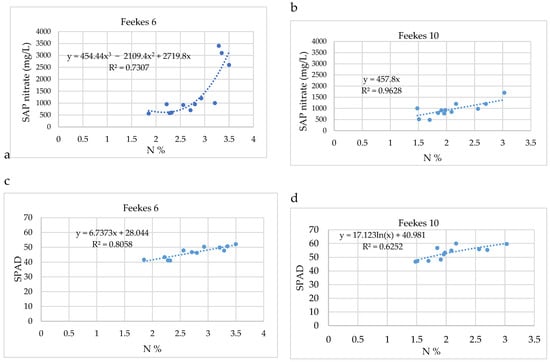
Figure 4.
The relationship between sap nitrate and total N content (%) (a) at Feekes 6 (b) at Feekes 10 and the relationship between SPAD values and total N content (c) at Feekes 6 (d) and at Feekes 10 growth stages.
At Feekes 6 of the crop, the change in the SPAD values versus the total N content could best be characterized by a linear fit (Figure 4c). The steady increase in SPAD values with the increasing N dose means that the plants continuously produced chlorophyll, which was less sensitive to rapid changes in nitrogen levels compared to nitrate accumulation. The SPAD values did not indicate the oversupply of nitrate. At Feekes 10, the SPAD values increased in a plateau, and the relationship can best be described with s logarithmic equation, which shows that excess N does not cause a further increase in the SPAD values (Figure 4d).
To determine the critical sap nitrate and SPAD values, the relative yield was plotted as a function of the sap nitrate and SPAD values (Figure 5).
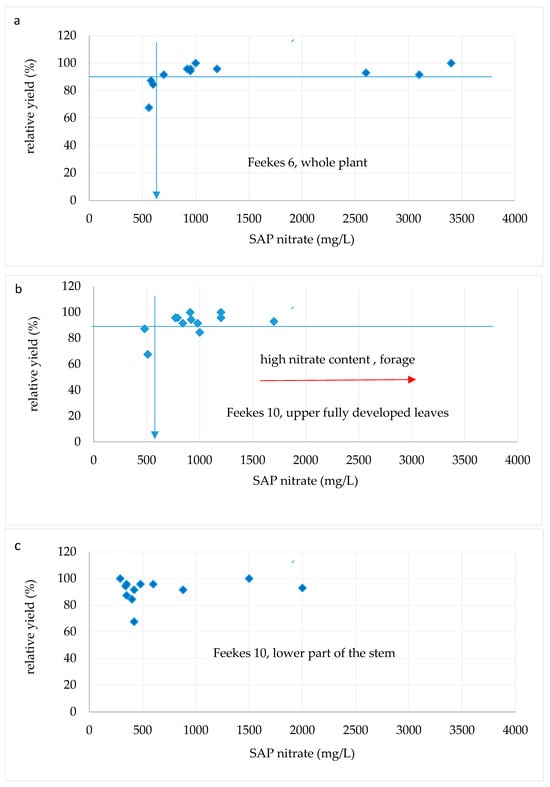
Figure 5.
Relationship between relative yield and sap nitrate concentration at different growth stages: (a) Feekes 6, whole plant; (b) Feekes 10, upper leaves; (c) Feekes 10, lower part of stem.
At Feekes 6, the critical value for sap nitrate was 650 mg/L (Figure 5a). This is lower than that of Liu et al. [14] for winter wheat stem (<1000 mg/L) and that of Thomson et al. [15] for malting barley stem at Feekes 7 (<797 mg/L). At Feekes 10, the critical SAP or sap nitrate value was 540 mg/L, related to the upper fully developed leaves (Figure 5b), which was very similar to the result of Thompson et al. for malting barley stem at Feekes 10 (<531 mg/L). At the same time, Hoel [13] suggested that 4000 mg/L of stem nitrate at Feekes 6 and 2000 mg/L of nitrate at Feekes 10 provides sufficient grain protein content in winter wheat when a single nitrogen application was undertaken in spring. However, he mentioned that due to the high variability of sap nitrate values, the sap nitrate method is not precise.
The critical nitrate in the lower part of the stem could not be evaluated as the shape of the curve did not allow for the use of the Cate–Nelson method (Figure 5c). Westcott et al. [39] found that oat used as fodder is generally safe when sap nitrate is below 1540 mg/L. This critical upper limit is denoted in Figure 4b.
The SPAD critical value at Feekes 6 was 43 (Figure 6a), which is similar to that found by Liu et al. [14], who considered winter wheat to N to be deficient under SPAD = 44. At Feekes 10, the SPAD critical value was 48 (Figure 6b).
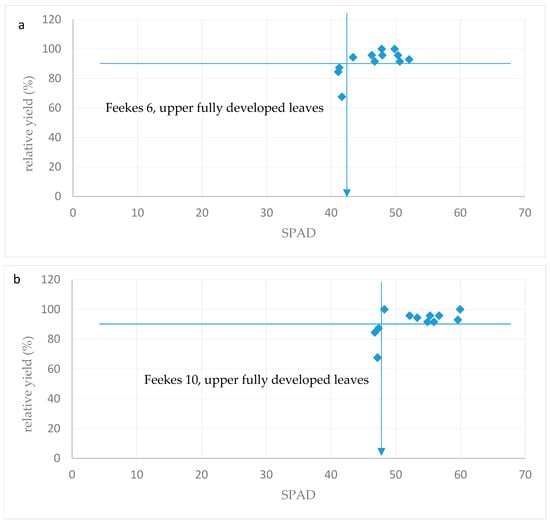
Figure 6.
Relationship between relative yield and SPAD values across various growth stages: (a) Feekes 6, upper leaves; (b) Feekes 10, upper leaves.
In our experiment, the sap nitrate values of the whole plant at Feekes 6 and that of the upper two leaves at Feekes 10 were appropriate to determine the critical nitrate level with the Cate and Nelson method [32]. The sap nitrate values varied widely, and they were good indicators of the plant N supply. Sap nitrate values related to the lower stem, however, were not consistent at Feekes 10. Presumably more than three replications are needed for reliable data. Our experience showed the measured sample variance by the sap nitrate meter was very sensitive to the temperature and the clarity of the electrodes. These effects could also result in deviations in the data.
4. Conclusions
SPAD values varied in a narrow range versus N fertilizer doses, but they were good indicators of the N deficiency of spring oat. Of the two methods, SPAD tests are much simpler and faster to perform, so we mostly recommend this method to track the N supply. In case the SPAD value below 43 at Feekes 6, additional split nitrogen application is required for the spring oat variety “Lota”. However, if the aim is to check the excessive nitrate content of the fodder, the SPAD test is not appropriate; the sap nitrate test provides this information. The relative chlorophyll content may also decrease due to the lack of other nutrients. In case of its suspicion, the sap nitrate test is recommended to confirm the existence of a N deficiency. For sap nitrate, we obtained lower threshold values of 650 mg/L (whole plant) at Feekes 6 and 540 mg/L (upper fully developed leaves) at Feekes 10, respectively. The critical values identified in this study are valid only under the conditions studied and for this particular oat variety. The data could be further strengthened with additional research across more soil types, years, and oat varieties.
Author Contributions
Conceptualization, R.K. and A.B.K.; formal analysis, T.N. and R.K.; investigation, E.K.J., I.K., S.S. and Z.S.; data curation, A.B.K.; writing—original draft, R.K. and A.S.; writing—review and editing, A.S. and Á.B.; project administration, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was supported by the tender project EFOP-3.6.1-16-2016-00022 entitled “Debrecen Venture Catapult” and supported by the University of Debrecen Program for Scientific Publication.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Preparation of the paper was supported by the University of Debrecen Program for Scientific Publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Thompson, R.B.; Tremblay, N.; Fink, M.; Gallardo, M.; Padilla, F.M. Tools and strategies for sustainable nitrogen fertilisation of vegetable crops. In Advances in Research on Fertilization Management of Vegetable Crops; Springer: Cham, Switzerland, 2017; pp. 11–63. [Google Scholar] [CrossRef]
- Farneselli, M.; Tei, F.; Simonne, E. Reliability of petiole sap test for N nutritional status assessing in processing tomato. J. Plant Nutr. 2014, 37, 270–278. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquinto, G.; Tei, F.; Thompson, R.B. Monitoring nitrogen status of vegetable crops and soils for optimal nitrogen management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Llanderal, A.; García-Caparrós, P.; Segura, M.L.; Contreras, J.I.; Lao, M.T. Nutritional changes in petiole sap over space and time in a tomato crop greenhouse. J. Plant Nutr. 2019, 42, 1205–1217. [Google Scholar] [CrossRef]
- Esteves, E.; Locatelli, G.; Bou, N.A.; Ferrarezi, R.S. Sap analysis: A powerful tool for monitoring plant nutrition. Horticulturae 2021, 7, 426. [Google Scholar] [CrossRef]
- Parks, S.E.; Irving, D.E.; Milham, P.J. A critical evaluation of on-farm rapid tests for measuring nitrate in leafy vegetables. Sci. Hortic. 2012, 134, 1–6. [Google Scholar] [CrossRef]
- Anderson, K.A.; Case, T.E. Evaluation of plant nitrate extraction techniques and effect of commonly used analytical methods of detection. Commun. Soil Sci. Plant Anal. 1999, 30, 1479–1495. [Google Scholar] [CrossRef]
- Zhang, H.; Smeal, D.; Arnold, R.N.; Gregory, E.J. Potato nitrogen management by monitoring petiole nitrate level. J. Plant Nutr. 1996, 19, 1405–1412. [Google Scholar] [CrossRef]
- Williams, C.M.J.; Maier, N.A. Determination of the nitrogen status of irrigated potato crop. I. Critical nutrient ranges for nitrate–nitrogen in petioles. J. Plant Nutr. 1990, 13, 971–984. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peña-Fleitas, M.T.; Padilla, F.M.; Gallardo, M.; Thompson, R.B. Petiole sap nitrate concentration to assess crop nitrogen status of greenhouse sweet pepper. Sci. Hortic. 2021, 110, 157–285. [Google Scholar] [CrossRef]
- Hochmuth, G.; Maynard, D.; Vavrina, C.; Hanlon, E.; Simonne, E. Plant tissue analysis and interpretation for vegetable crops in Florida. In Nutrient Management of Vegetable and Row Crops Handbook; University of Florida Press: Gainesville, FL, USA, 2012; pp. 45–92. [Google Scholar] [CrossRef]
- Hochmuth, G.J. Plant Petiole Sap Testing: Guide for Vegetable Crops; University of Florida Cooperative Extension Service: Gainesville, FL, USA, 1994. [Google Scholar]
- Hoel, B.O. Determination of nitrogen status in winter wheat by measuring basal stem tissue sap nitrate concentration. Acta Agric. Scand. Sect. B-Plant Soil Sci. 1999, 49, 82–91. [Google Scholar] [CrossRef]
- Liu, X.; Ju, X.; Zhang, F.; Chen, X. Nitrogen recommendation for winter wheat using N min test and rapid plant tests in North China Plain. Commun. Soil Sci. Plant Anal. 2003, 34, 2539–2551. [Google Scholar] [CrossRef]
- Thompson, T.L.; Ottman, M.J.; Riley-Saxton, E. Basal stem nitrate tests for irrigated malting barley. Agron. J. 2004, 96, 516–524. [Google Scholar] [CrossRef]
- Delgado, J.A.; Follett, R.F. Sap test to determine nitrate-nitrogen concentrations in aboveground biomass of winter cover crops. Commun. Soil Sci. Plant Anal. 1998, 29, 545–559. [Google Scholar] [CrossRef]
- Veres, S.; Setzka, D.; Zsombik, L.; Rátonyi, T.; Makleit, P. Changes in Photosynthetic Capacity of Cucumber Seedlings in Response to Different Nitrogen Supply. Int. J. Food Eng. 2018, 4, 165–169. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, X. Assessing nitrogen nutritional status, biomass and yield of cotton with NDVI, SPAD and petiole sap nitrate concentration. Exp. Agric. 2018, 54, 531–548. [Google Scholar] [CrossRef]
- Ali, A.M.; Salem, H.M. Site-Specific Nitrogen Fertilizer Management Using Canopy Reflectance Sensors, Chlorophyll Meters and Leaf Color Charts: A Review. Nitrogen 2024, 5, 828–856. [Google Scholar] [CrossRef]
- Rhezali, A.; Aissaoui, A.E. Feasibility study of using absolute SPAD values for standardized evaluation of corn nitrogen status. Nitrogen 2021, 2, 298–307. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmer, A.M.; Ellsworth, J.W.; Koehler, K.J. Sensitivity of chlorophyll meters for diagnosing nitrogen deficiencies of corn in production agriculture. Agron. J. 2008, 100, 543–550. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Liu, X. Optimal leaf positions for SPAD meter measurement in rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Bytyqi, B.; Kutasy, E. Leaf reflectance characteristics and yield of spring oat varieties as influenced by varietal divergences and nutritional supply. Acta Agrar. Debreceniensis 2023, 1, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, L.; Massa, D.; Pardossi, A. New trends in the fertigation management of irrigated vegetable crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Zhang, L.; Hashimoto, N.; Saito, Y.; Obara, K.; Ishibashi, T.; Ito, R.; Yamamoto, S.; Maki, M.; Homma, K. Validation of relation between SPAD and rice grain protein content in farmer fields in the coastal area of Sendai, Japan. AgriEngineering 2023, 5, 369–379. [Google Scholar] [CrossRef]
- Fiorentini, M.; Zenobi, S.; Giorgini, E.; Basili, D.; Conti, C.; Pro, C.; Monaci, E.; Orsini, R. Nitrogen and chlorophyll status determination in durum wheat as influenced by fertilization and soil management: Preliminary results. PLoS ONE 2019, 14, e0225126. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, D.; Liu, M.; Hafeez, M.B.; Wen, P.; Wang, X.; Wang, R.; Zhang, X.; Li, J. Optimized fertilizer recommendation method for nitrate residue control in a wheat–maize double cropping system in dryland farming. Field Crop. Res. 2021, 271, 108258. [Google Scholar] [CrossRef]
- Houba, V.; Temminghoff, E.; Gaikhorst, G.; Van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Sinclair, T.R. Historical changes in harvest index and crop nitrogen accumulation. Crop Sci. 1998, 38, 638–643. [Google Scholar] [CrossRef]
- Cate, R.B.; Nelson, L.A. Soil Testing Series Technical Bulletin 1; North Carolina State University International North Carolina State University: Raleigh, NC, USA, 1965. [Google Scholar]
- Berry, P.; Sterling, M.; Spink, J.; Baker, C.; Sylvester-Bradley, R.; Mooney, S.; Tams, A.; Ennos, A. Understanding and reducing lodging in cereals. Adv. Agron. 2004, 84, 215–269. [Google Scholar]
- Ragályi, P.; Szabó, A.; Rékási, M.; Csathó, P.; Csontos, P. Effect of Different Macronutrient Supply Levels on the Drought Tolerance of Rainfed Grass Based on Biomass Production, Water Use Efficiency and Macroelement Content. Horticulturae 2023, 9, 1337. [Google Scholar] [CrossRef]
- Mulvaney, R.; Khan, S.; Hoeft, R.; Brown, H. A soil organic nitrogen fraction that reduces the need for nitrogen fertilization. Soil Sci. Soc. Am. J. 2001, 65, 1164–1172. [Google Scholar] [CrossRef]
- Kádár, I.; Ragályi, P.; Murányi, A.; Radimszky, L.; Gajdó, A. Effect of Gérce alginit on the fertility of an acid sandy soil. Agrokémia Talajt. 2015, 64, 437–452. [Google Scholar] [CrossRef]
- Havlin, J.L. Soil: Fertility and nutrient management. In Landscape and Land Capacity; CRC Press: Boca Raton, FL, USA, 2020; pp. 251–265. [Google Scholar]
- Mahal, N.K.; Miguez, F.E.; Sawyer, J.E.; Dong, L.; Schnable, P.S.; Castellano, M.J. Stalk sap nitrate test as a potential tool for nitrogen fertilizer recommendations for maize. Field Crop. Res. 2024, 310, 109330. [Google Scholar] [CrossRef]
- Westcott, M.P.; Cash, S.D.; Jacobsen, J.S.; Carlson, G.R.; Welty, L.E. Sap analysis for diagnosis of nitrate accumulation in cereal forages. Commun. Soil Sci. Plant Anal. 1998, 29, 1355–1363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).