Enhancing Sustainability in Potato Crop Production: Mitigating Greenhouse Gas Emissions and Nitrate Accumulation in Potato Tubers through Optimized Nitrogen Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Experimental Design and Treatments
2.3. Experimental Setup
2.4. Characteristics Evaluated
2.5. Statistical Analysis
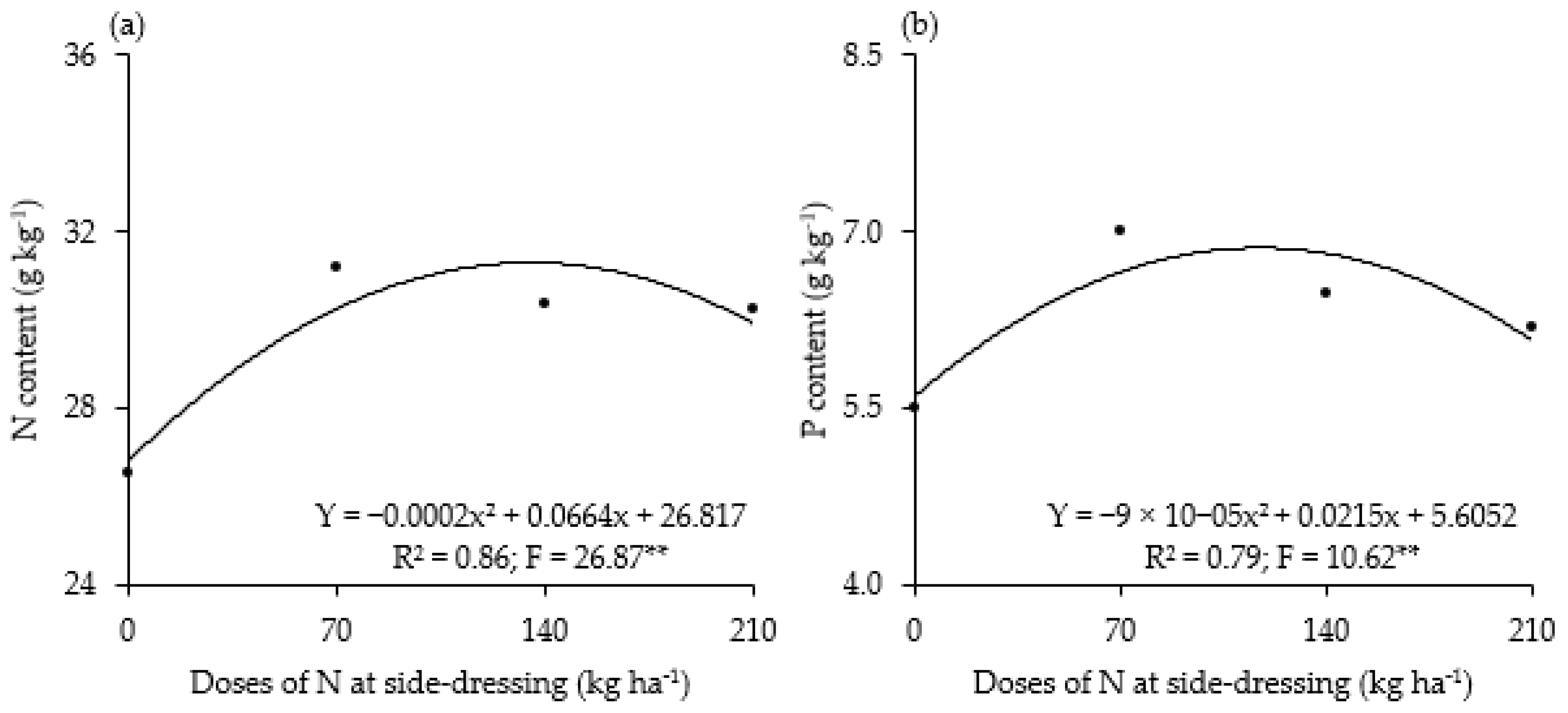
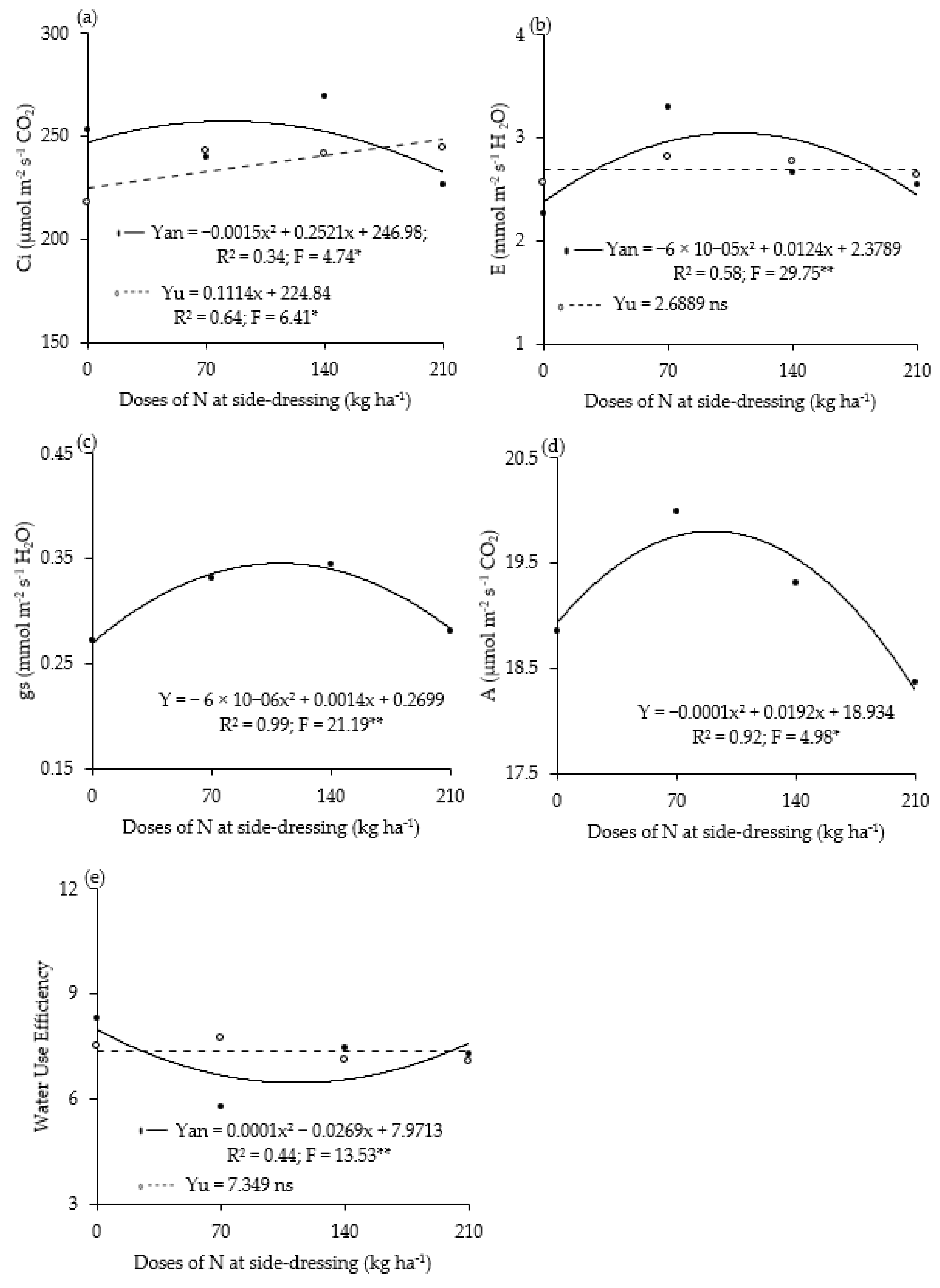
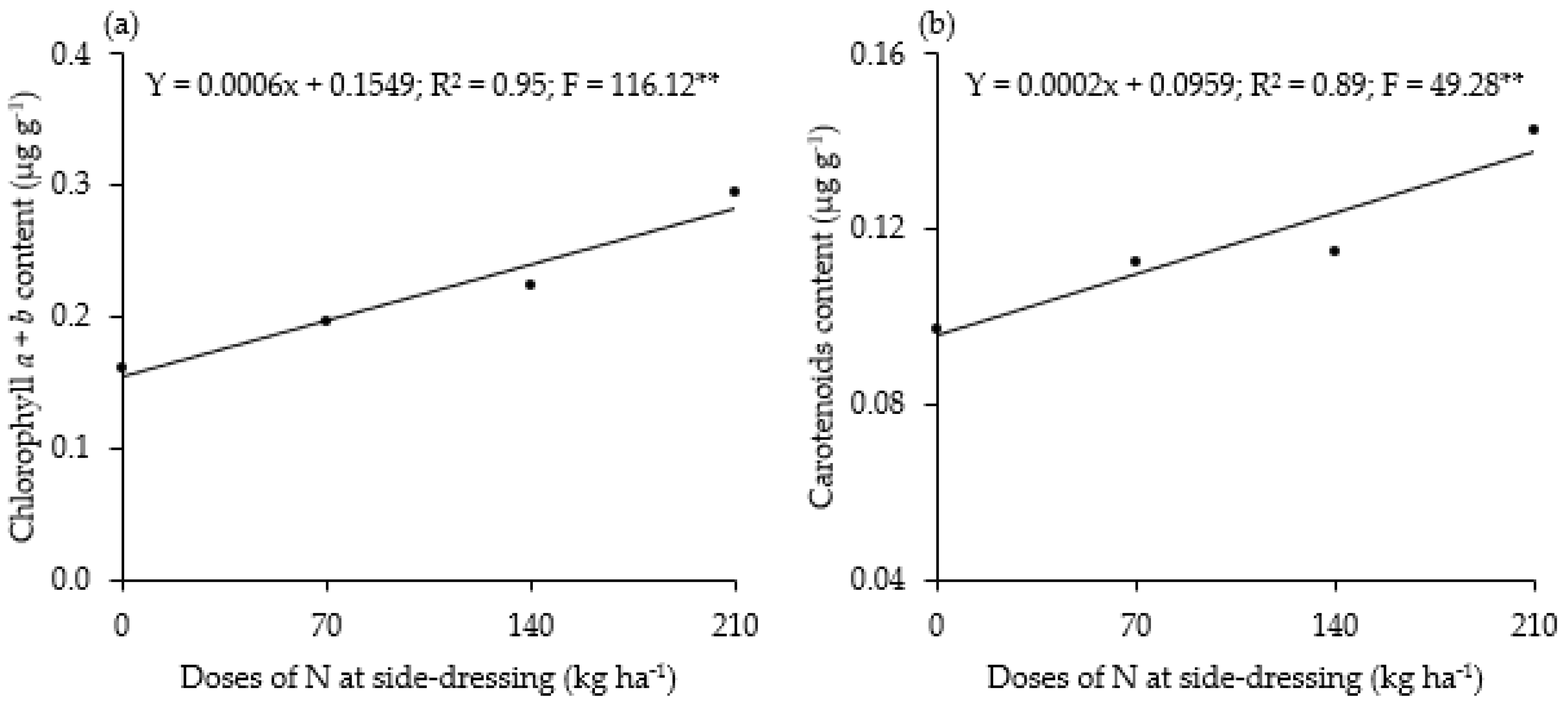
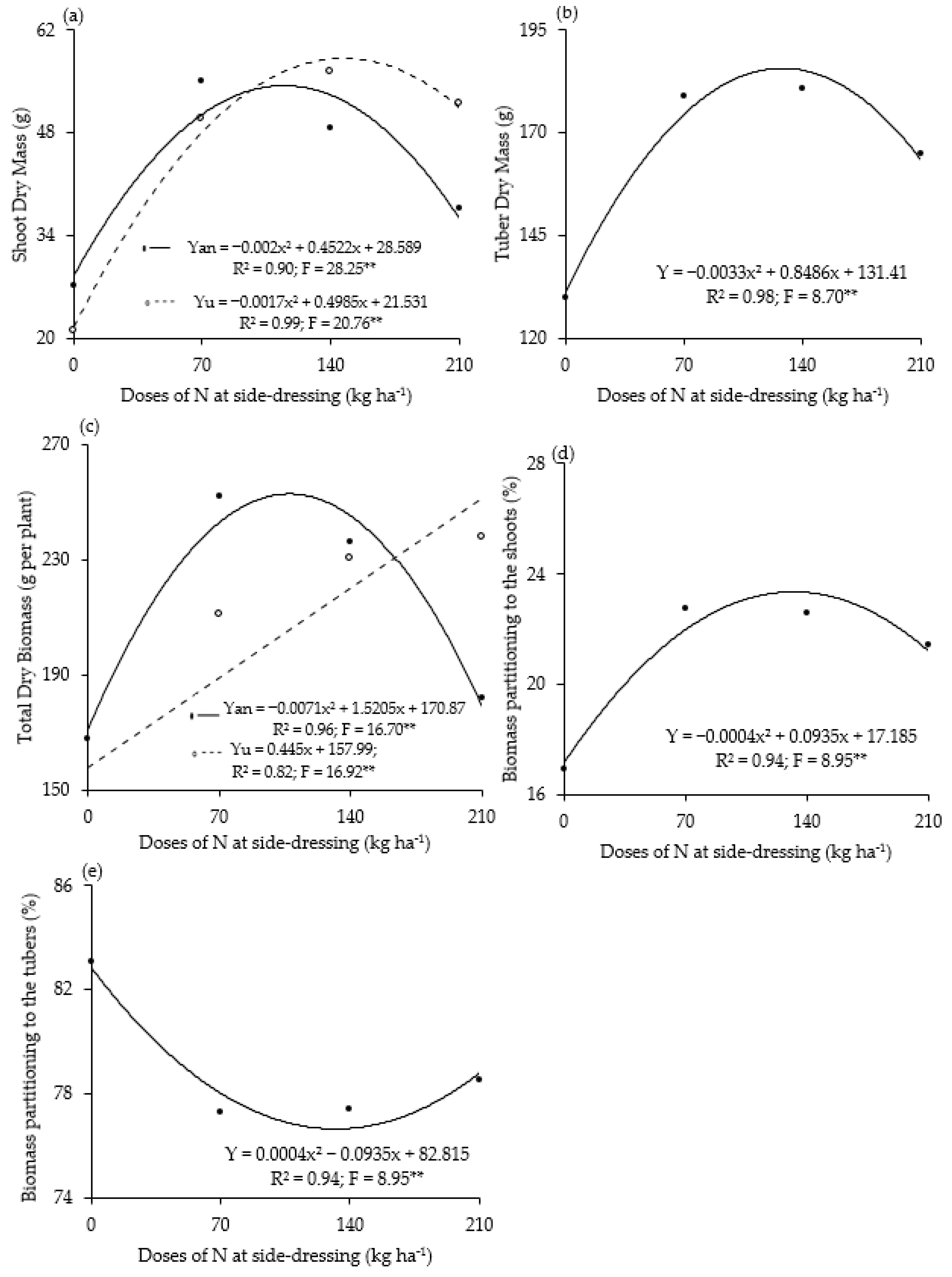
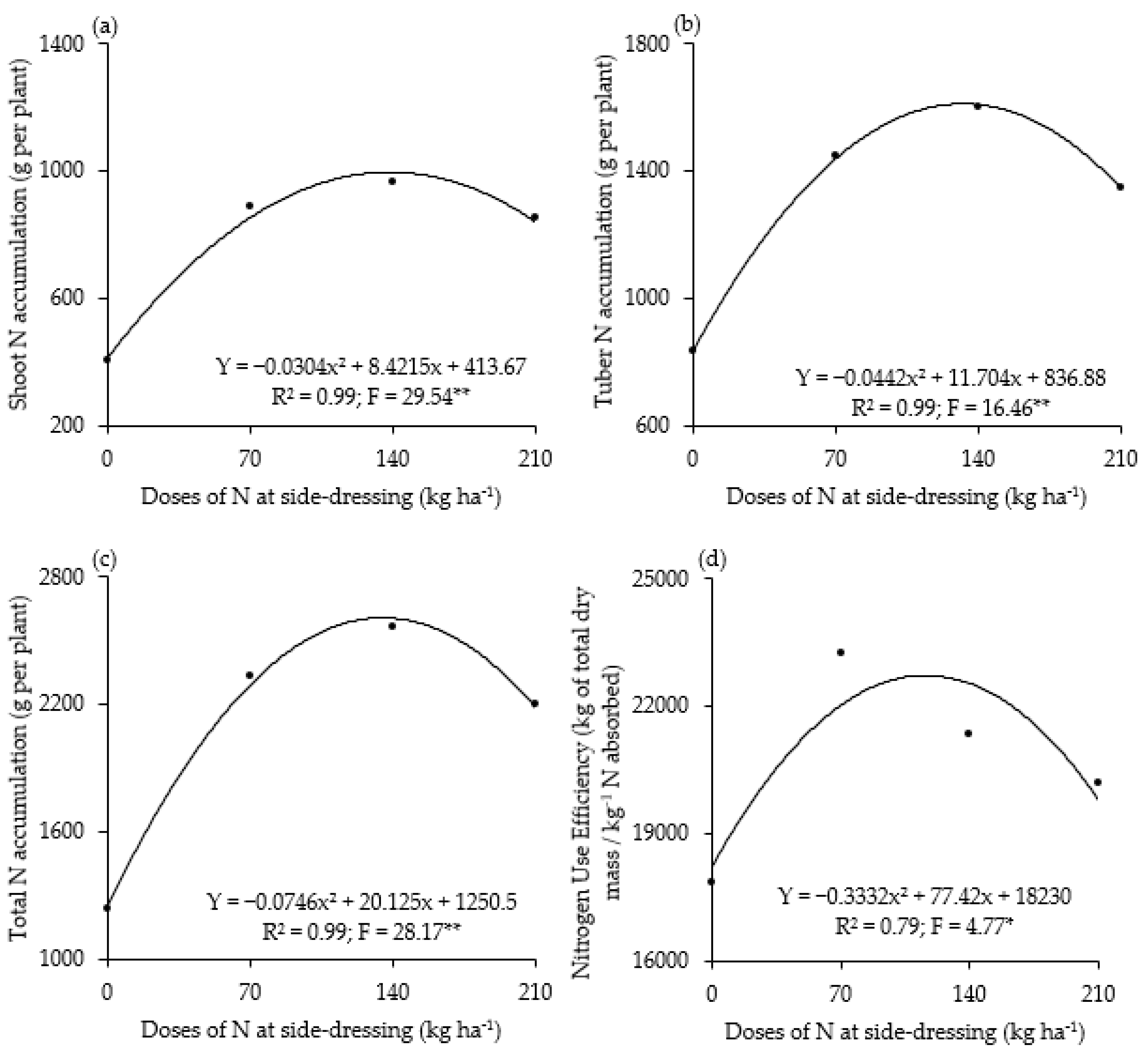
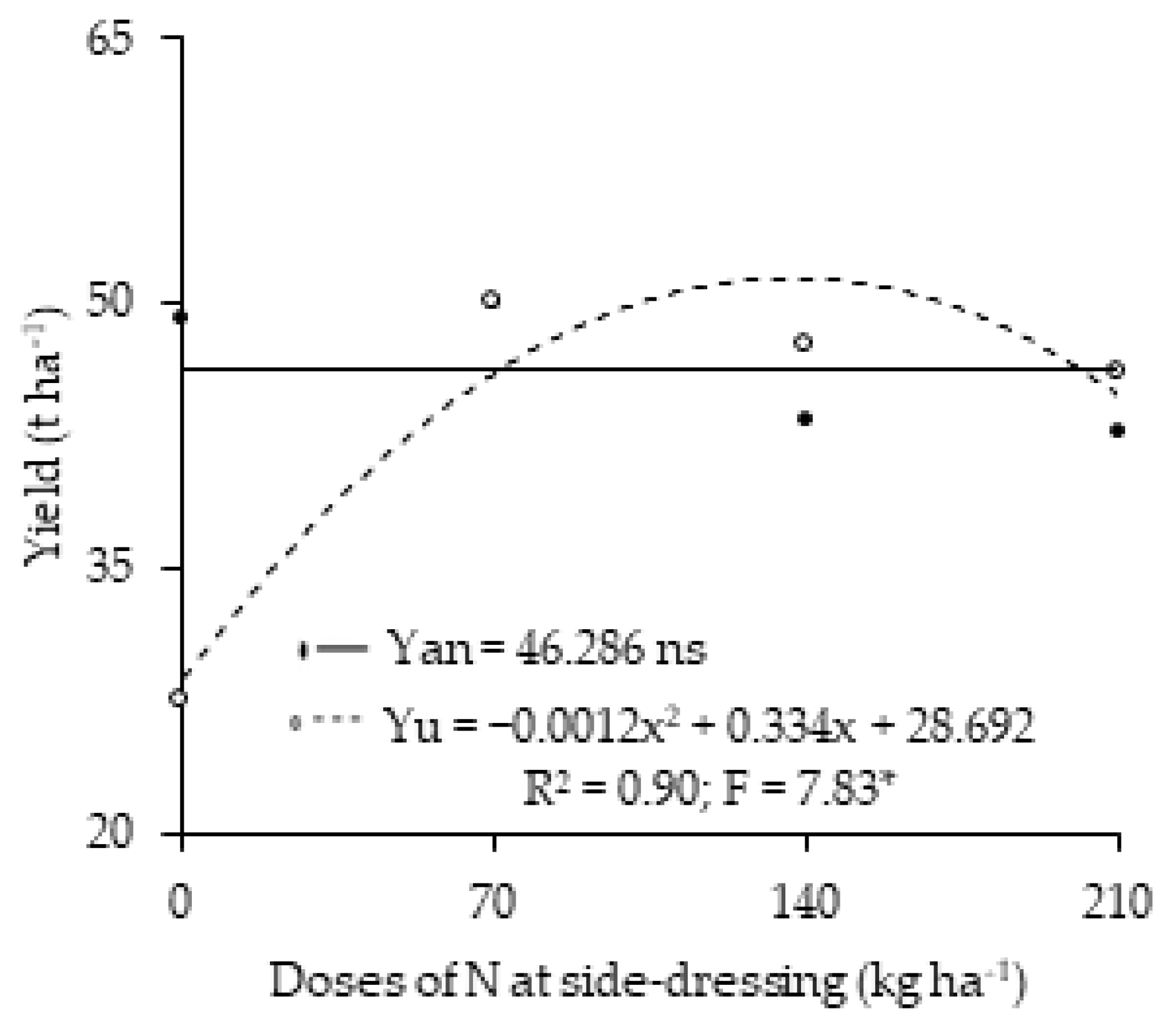
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niu, K.; Guo, H.; Liu, J. Can Food Security and Low Carbon Be Achieved Simultaneously?—An Empirical Analysis of the Mechanisms Influencing the Carbon Footprint of Potato and Corn Cultivation in Irrigation Areas. J. Integr. Agric. 2023, 22, 1230–1243. [Google Scholar] [CrossRef]
- Diaz-Gonzalez, F.A.; Vuelvas, J.; Vallejo, V.E.; Patino, D. Fertilization Rate Optimization Model for Potato Crops to Maximize Yield While Reducing Polluting Nitrogen Emissions. Ecol. Model. 2023, 485, 110498. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, H.; Chen, X.; Zhang, C.; Ma, W.; Huang, C.; Zhang, W.; Mi, G.; Miao, Y.; Li, X.; et al. Pursuing Sustainable Productivity with Millions of Smallholder Farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J.; et al. Management Strategies to Mitigate N2O Emissions in Agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef]
- Nyiraneza, J.; Cambouris, A.N.; Nelson, A.; Khakbazan, M.; Mesbah, M.; Perron, I.; Ziadi, N.; Lafond, J. Potato Yield, Net Revenue and Specific Gravity Responses to Nitrogen Fertilizer under Different Canadian Agroecozones. Agronomy 2021, 11, 1392. [Google Scholar] [CrossRef]
- Cambouris, A.N.; Luce, M.S.; Zebarth, B.J.; Ziadi, N.; Grant, C.A.; Perron, I. Potato Response to Nitrogen Sources and Rates in an Irrigated Sandy Soil. Agron. J. 2016, 108, 391–401. [Google Scholar] [CrossRef]
- Liu, K.; Du, J.; Zhong, Y.; Shen, Z.; Yu, X. The Response of Potato Tuber Yield, Nitrogen Uptake, Soil Nitrate Nitrogen to Different Nitrogen Rates in Red Soil. Sci. Rep. 2021, 11, 22506. [Google Scholar] [CrossRef]
- Abdo, A.I.; Elrys, A.S.; Abdel-Fattah, M.K.; Desoky, E.-S.M.; Huitong, L.; Wang, L. Mitigating Nitrate Accumulation in Potato Tubers under Optimum Nitrogen Fertilization with K-Humate and Calcium Chloride. J. Clean. Prod. 2020, 259, 121108. [Google Scholar] [CrossRef]
- Souza, E.F.C.; Soratto, R.P.; Fernandes, A.M.; Gupta, S.K. Performance of Conventional and Enhanced-Efficiency Nitrogen Fertilizers on Potato Tuber Mineral Composition and Marketability. J. Sci. Food Agric. 2022, 102, 3078–3087. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Fan, D.; Jiang, R.; Abbasi, N.; Song, D.; Zou, G.; Wei, D.; He, P.; He, W. Improving Potato Productivity and Mitigating Nitrogen Losses Using Enhanced-Efficiency Fertilizers: A Global Meta-Analysis. Agric. Ecosyst. Environ. 2023, 348, 108416. [Google Scholar] [CrossRef]
- Bryan, N.S.; van Grinsven, H. The Role of Nitrate in Human Health. Adv. Agron. 2013, 119, 153–182. [Google Scholar]
- Rosier, B.T.; Buetas, E.; Moya-Gonzalvez, E.M.; Artacho, A.; Mira, A. Nitrate as a Potential Prebiotic for the Oral Microbiome. Sci. Rep. 2020, 10, 12895. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in Fruits and Vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in Vegetables: Toxicity, Content, Intake and EC Regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Luz, J.M.Q.; Lana, R.M.Q.; Silva, J.R.R.D.; Castoldi, R. Yield of Potato Cultivars as a Function of Nitrogen Rates. Rev. Caatinga 2020, 33, 954–963. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Song, B.; Nie, B.; Zhang, W.; Zhao, Z. Effect of Excessive Nitrogen on Levels of Amino Acids and Sugars, and Differential Response to Post-Harvest Cold Storage in Potato (Solanum tuberosum L.) Tubers. Plant Physiol. Biochem. 2020, 157, 38–46. [Google Scholar] [CrossRef]
- Santos, H.G.d. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, DF, Brazil, 2018; ISBN 978-85-7035-800-4. [Google Scholar]
- Granja, N.P.; Feltran, J.C.; Miranda Filho, H.S.; Factor, T.L.; Lima Junior, S.; Blat, F. Batata. In Instruções Agrícolas para as Principais Culturas Econômicas; Instituto Agronômico: Campinas, SP, Brazil, 2014; pp. 54–58. [Google Scholar]
- Lorenzi, J.O.; Monteiro, D.A.; Miranda Filho, H.S. Raízes e tubérculos. In Recomendações de Adubação e Calagem para o Estado de São Paulo; Instituto Agronômico: Campinas, SP, Brazil, 1997; pp. 221–229. [Google Scholar]
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; Carmo, C.A.F.S.d.; Melo, W.J. de Análises químicas de tecido vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa Informação Tecnológica: Brasília, DF, Brazil, 2009; pp. 190–223. ISBN 978-85-7383-430-7. [Google Scholar]
- Silva, J.A.D.; Santos, P.A.B.D.; Carvalho, L.G.D.; Moura, E.G.; Andrade, F.R. Gas Exchanges and Growth of Soybean Cultivars Submitted to Water Deficiency. Pesqui. Agropecu. Trop. 2020, 50, e58854. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, London, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Benincasa, M.M.P. Análise de Crescimento de Plantas: Noções Básicas, 2nd ed.; Funep: Jaboticabal, SP, Brazil, 2003. [Google Scholar]
- Siddiqi, M.Y.; Glass, A.D.M. Utilization Index: A Modified Approach to the Estimation and Comparision of Nutrient Utilization Efficiency in Plants. J. Plant Nutr. 2008, 4, 289–302. [Google Scholar] [CrossRef]
- Mantovani, J.R.; Cruz, M.C.P.d.; Ferreira, M.E.; Barbosa, J.C. Comparação de procedimentos de quantificação de nitrato em tecido vegetal. Pesqui. Agropecuária Bras. 2005, 40, 53–59. [Google Scholar] [CrossRef]
- IPCC. IPCC National Greenhouse Gas Inventories Programme; Eggleston, S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006; p. 664. [Google Scholar]
- Barbosa, J.C.; Maldonado Júnior, W. Experimentação Agronômica & AgroEstat: Sistema para Análises Estatísticas de Ensaios Agronômicos, 1st ed.; Gráfica Multipress Ltd.: Jaboticabal, SP, Brazil, 2015. [Google Scholar]
- IBGE—Instituto Brasileiro de Geografia e Estatística Estados/Culturas Temporárias/Batata. Available online: https://www.ibge.gov.br/estadosat/ (accessed on 28 August 2023).
- Souza, Á.H.C.D.; Rezende, R.; Lorenzoni, M.Z.; Santos, F.A.S.; Maller, A. Effect of Fertigation with Potassium and Nitrogen on Gas Exchange and Biomass Accumulation in Eggplant1. Pesqui. Agropecu. Trop. 2017, 47, 345–352. [Google Scholar] [CrossRef]
- Uçgun, K.; Türkeli, B.; Cansu, M.; Altindal, M. Effect of N Fertilizing on Gas Exchange, Leaf Photosynthetic Performance and Nutrient Concentrations of Sweet Cherry Cv. 0900 Ziraat. Soil Stud. 2022, 11, 78–84. [Google Scholar] [CrossRef]
- Giletto, C.M.; Reussi Calvo, N.I.; Sandaña, P.; Echeverría, H.E.; Bélanger, G. Shoot- and Tuber-Based Critical Nitrogen Dilution Curves for the Prediction of the N Status in Potato. Eur. J. Agron. 2020, 119, 126114. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, P.; Wei, Z.; Liu, J.; Hu, X.; Liu, F. Effects of Elevated CO2 and Nitrogen Supply on Leaf Gas Exchange, Plant Water Relations and Nutrient Uptake of Tomato Plants Exposed to Progressive Soil Drying. Sci. Hortic. 2022, 292, 110643. [Google Scholar] [CrossRef]
- Prado, R.d.M. 500 Perguntas e Respostas Sobre Nutrição de Plantas; FUNEP: Jaboticabal, SP, Brazil, 2014. [Google Scholar]
- Hailu, G.; Nigussie, D.; Ali, M.; Derbew, B. Nitrogen and Phosphorus Use Efficiency in Improved Potato (Solanum tuberosum L.) Cultivars in Southern Ethiopia. Am. J. Potato Res. 2017, 94, 617–631. [Google Scholar] [CrossRef]
- Xu, X.; He, P.; Qiu, S.; Zhao, S.; Ding, W.; Zhou, W. Nutrient Management Increases Potato Productivity and Reduces Environmental Risk: Evidence from China. J. Clean. Prod. 2022, 369, 133357. [Google Scholar] [CrossRef]
- Nurmanov, Y.T.; Chernenok, V.G.; Kuzdanova, R.S. Potato in Response to Nitrogen Nutrition Regime and Nitrogen Fertilization. Field Crops Res. 2019, 231, 115–121. [Google Scholar] [CrossRef]
- Nascimento, C.S.; Nascimento, C.S.; Cecílio Filho, A.B. Agronomic Performance of Rocket as a Function of Phosphorus Fertilisation in a P-Rich Soil1. Rev. Caatinga 2020, 33, 860–864. [Google Scholar] [CrossRef]
- Villa, P.M.; Sarmiento, L.; Rada, F.; Rodrigue, A.C.; Márquez, N.; Espinosa, W. Partition of biomass and nitrogen in a potato crop under three nitrogen fertilization treatments. Siembra 2020, 7, 57–68. [Google Scholar] [CrossRef]
- Elrys, A.S.; Abo El-Maati, M.F.; Abdel-Hamed, E.M.W.; Arnaout, S.M.A.I.; El-Tarabily, K.A.; Desoky, E.-S.M. Mitigate Nitrate Contamination in Potato Tubers and Increase Nitrogen Recovery by Combining Dicyandiamide, Moringa Oil and Zeolite with Nitrogen Fertilizer. Ecotoxicol. Environ. Saf. 2021, 209, 111839. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Ahmadian, A.; Ferdousi, A.; Zandi, S.; Shahmoradi, B.; Ghanbari, R.; Mahammadi, S.; Rezaee, R.; Safari, M.; Daraei, H.; et al. Effect of Washing and Cooking on Nitrate Content of Potatoes (Cv. Diamant) and Implications for Mitigating Human Health Risk in Iran. Potato Res. 2020, 63, 449–462. [Google Scholar] [CrossRef]
- Boari, F.; Cefola, M.; Di Gioia, F.; Pace, B.; Serio, F.; Cantore, V. Effect of Cooking Methods on Antioxidant Activity and Nitrate Content of Selected Wild Mediterranean Plants. Int. J. Food Sci. Nutr. 2013, 64, 870–876. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 27 August 2023).
- Huang, Y.; Gao, B.; Huang, W.; Wang, L.; Fang, X.; Xu, S.; Cui, S. Producing More Potatoes with Lower Inputs and Greenhouse Gases Emissions by Regionalized Cooperation in China. J. Clean. Prod. 2021, 299, 126883. [Google Scholar] [CrossRef]
- IEA—Instituto de Economia Agrícola Preços Agrícolas. Available online: https://www.iea.agricultura.sp.gov.br/# (accessed on 2 September 2023).


| Source | Unity | Total a |
|---|---|---|
| N fertilizer | kg ha−1 cycle−1 | 40–250 b |
| P fertilizer (P2O2) | kg ha−1 cycle−1 | 200.0 |
| K fertilizer (K2O) | kg ha−1 cycle−1 | 100.0 |
| Limestone | kg ha−1 cycle−1 | 612.5 |
| Herbicide (i.a c) | kg ha−1 cycle−1 | 1.39 |
| Fungicides (i.a c) | kg ha−1 cycle−1 | 21.19 |
| Insecticides (i.a c) | L ha−1 cycle−1 | 3.4 |
| Diesel | L ha−1 cycle−1 | 286.6 |
| Electricity-Irrigation | kwh ha−1 cycle−1 | 1205.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seno Nascimento, C.; Seno Nascimento, C.; de Jesus Pereira, B.; Soares Silva, P.H.; Pessôa da Cruz, M.C.; Bernardes Cecílio Filho, A. Enhancing Sustainability in Potato Crop Production: Mitigating Greenhouse Gas Emissions and Nitrate Accumulation in Potato Tubers through Optimized Nitrogen Fertilization. Nitrogen 2024, 5, 163-176. https://doi.org/10.3390/nitrogen5010011
Seno Nascimento C, Seno Nascimento C, de Jesus Pereira B, Soares Silva PH, Pessôa da Cruz MC, Bernardes Cecílio Filho A. Enhancing Sustainability in Potato Crop Production: Mitigating Greenhouse Gas Emissions and Nitrate Accumulation in Potato Tubers through Optimized Nitrogen Fertilization. Nitrogen. 2024; 5(1):163-176. https://doi.org/10.3390/nitrogen5010011
Chicago/Turabian StyleSeno Nascimento, Camila, Carolina Seno Nascimento, Breno de Jesus Pereira, Paulo Henrique Soares Silva, Mara Cristina Pessôa da Cruz, and Arthur Bernardes Cecílio Filho. 2024. "Enhancing Sustainability in Potato Crop Production: Mitigating Greenhouse Gas Emissions and Nitrate Accumulation in Potato Tubers through Optimized Nitrogen Fertilization" Nitrogen 5, no. 1: 163-176. https://doi.org/10.3390/nitrogen5010011
APA StyleSeno Nascimento, C., Seno Nascimento, C., de Jesus Pereira, B., Soares Silva, P. H., Pessôa da Cruz, M. C., & Bernardes Cecílio Filho, A. (2024). Enhancing Sustainability in Potato Crop Production: Mitigating Greenhouse Gas Emissions and Nitrate Accumulation in Potato Tubers through Optimized Nitrogen Fertilization. Nitrogen, 5(1), 163-176. https://doi.org/10.3390/nitrogen5010011






