Abstract
Recovery efforts for the endangered Serianthes nelsonii have been deficient. To learn more about leaf development costs, the content of biomass and essential elements were determined in the supportive and laminae tissue of leaves that were constructed under different levels of incident light. The biomass required to construct a leaf in 22% light transmission was 65% of that in full sun, and light treatment did not influence the balance between supportive and laminae tissues. Concentrations of carbon, phosphorus, iron, manganese, and boron were greatest for in full-sun laminae, but those of nitrogen, potassium, calcium, magnesium, and zinc were greatest in shaded laminae. The same patterns with regard to light were exhibited in supportive tissues for carbon, nitrogen, potassium, calcium, magnesium, and zinc. In contrast, the supportive tissue phosphorus content was greatest in shaded leaves, and the light level did not influence the supportive tissue concentrations of the remaining elements. The leaf laminae consistently exhibited greater concentrations of elements with the exception of potassium and nickel, which were greater in the supportive tissues. These results indicate that the construction of full-sun S. nelsonii leaves is more costly than that of shaded leaves, and the transfer of biomass and essential elements between the supportive and laminae tissues is not substantially influenced by the developmental light level. Identifying the drivers of S. nelsonii leaf element concentrations is crucial for understanding the role of this charismatic tree in community-level processes.
1. Introduction
Serianthes nelsonii is a critically endangered tree species with an endemic range of two adjacent Mariana Islands [1]. The species was listed under the United States Endangered Species Act (ESA) in 1987 [2] and a national recovery plan was published in 1994 [3]. The global population of more than 120 mature individuals at the time of the ESA-listing [4] has declined to less than 40 today [5]. The proposed derivation of these failures in species recovery has been the decades-long funding of the same conservation practitioners and the avoidance of adaptive management research in the many funded projects [6]. The threats to this charismatic tree species include habitat loss, invasion by herbivore species, and a lack of population recruitment. These threats are common among the other threatened tree species in the region. Therefore, more research is required to better establish a foundation of understanding the needs of this imperiled species.
The petiole and rachis of pinnately compound leaves serve as thin-stem homologues, providing inexpensive and disposable stem-like functions [7]. Therefore, the plasticity of these supportive tissues may differ substantially from that of laminae tissues. The bi-pinnate compound leaf of S. nelsonii is highly plastic morphologically and anatomically when provided a range of incident light during leaf development [8]. Developing a greater understanding of this plasticity may illuminate how leaves on seedlings, saplings, and juveniles can persist in deep shade, yet leaves on emergent canopy stems of mature trees can persist in full sun. Knowledge of how incident light influences S. nelsonii plant development also enables a pragmatic application by more fully elucidating the nursery conditions that lead to the highest growth rates in a conservation nursery [6]. Continued adaptive management research designed to determine the influence of light on construction costs during leaf development may improve our understanding of the abiotic factors that influence this plant’s nutrition budgets.
The changes in leaf morphology, anatomy, and physiology that plants employ to best perform under high or low light conditions have been extensively studied [9,10,11,12,13]. Much of the research devoted to leaf-level developmental plasticity focuses exclusively on laminae responses, where investments into light harvesting capabilities are maximized in shade and investments into carboxylation capacities are maximized in full sun. Full-sun leaves are also required to invest in protective measures to mitigate high light stress [14]. This research focus on laminae traits fails to factor in the costs of supportive tissues and disallows interpretations from a whole plant perspective [11]. Within this context, the influence of developmental light level on S. nelsonii leaf construction dynamics has not been extensively studied.
The objectives of this study were to determine the quantities of biomass and essential elements required for constructing S. nelsonii leaves as influenced by incident light level. The resources needed to construct full-sun leaves were hypothesized to be greater than that for the construction of shaded leaves. The incident light level was predicted to influence laminae construction traits to a greater degree than supportive tissue construction traits. This precise information on leaf function will aid in guiding S. nelsonii conservation decisions.
2. Materials and Methods
The leaf material for this study was obtained in August 2015 from container-grown nursery plants at the University of Guam, Mangilao, Guam. Seeds collected from northern Guam under the Endangered Species Act Permit TE-84876A-0 were germinated in November 2014 under 50% shade. Vigorous seedlings were transplanted into 15 cm diameter containers in March 2015. The container medium was 60% peat and 40% horticultural perlite. A range of incident light was provided by nylon fabric shade cloth, and included 22%, 38%, 73%, or 100% of sunlight transmission. Light exclusion was provided by commercial nylon shade fabric.
The plants were irrigated by hand as needed in order to avoid root-zone oxygen deprivation caused by over-watering. The plant nutritional needs were amply met with a complete soluble fertilizer solution by adding 1 g·L−1 of a stock solution (24% nitrogen, 3.5% phosphorus, 13.2% potassium, 0.02% boron, 0.07% copper, 0.15% iron, 0.05% manganese, 0.0005% molybdenum, and 0.06% zinc). Additionally, Ca(NO3)2 was added to the solution at 0.5 g·L−1. The containers received 100 mL of the solution every week from November 2014 until March 2015, and 150 mL of the solution every week from March 2015 until August 2015. Municipal water was used for irrigation, and this source is alkaline (7.45–7.94). To address the possible unavailability of iron due to the alkaline irrigation water, an iron chelate solution was applied monthly at 100 mL per container (1.8 μM Fe as ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid).
The vigorous saplings were 9 months old when their leaves were harvested (Figure 1a). The leaves were obtained from detached stem sections following a pruning treatment required to improve transplant quality, and there were six plants per light treatment. The youngest leaf that was fully expanded was selected from each plant (Figure 1b). For each leaf, all leaflets were removed from every rachilla and combined into one sample designated as laminae tissue. The petiole, rachis, and rachillae were combined into one sample designated as support tissue. The samples were dried for 48 h at 75 °C in a forced draft oven then weighed using an electronic balance (to mg). Each sample was individually milled to pass through a 20-mesh screen. The full-sunlight treatment was considered to be the control, which was compared with the samples that underwent the three shade treatments.

Figure 1.
Serianthes nelsonii plant and leaf characteristics. (a) Appearance of sapling revealing vigorous regrowth following pruning; (b) individual bipinnate compound leaf constructed under 50% sunlight transmission.
Dry combustion was employed [15] using subsamples to determine the total carbon (C) and nitrogen (N) content (FLASH EA1112 CHN Analyzer, Thermo Fisher, Waltham, MA, USA). The remainder of the tissue samples were digested by a microwave system with nitric acid and peroxide; then, essential elements were determined by inductively coupled plasma optical-emission spectrometry (ICP-OES) [16] (Spectro Genesis; SPECTRO Analytical Instruments, Kleve, Germany). These were potassium (K), phosphorus (P), calcium (Ca), magnesium (Mg), manganese (Mn), iron (Fe), zinc (Zn), boron (B), copper (Cu), and nickel (Ni). Macroelements were measured in mg·g−1 and microelements were measured in μg·g−1.
Derived variables were calculated from the concentration data. The stoichiometry variables N:P, N:K, K:P, C:N, C:P, and C:K were calculated for each replication. The total C, N, P, and K content that was required to construct the laminae and supportive tissue for each leaf was calculated by multiplying the concentration of each element by the individual leaf weight components.
Each response variable was subjected to one-way ANOVA to determine the significance among the light treatment levels (SAS Institute, Version 9.3, Cary, IN, USA). For variables that were significant, scatter plots were created to visualize the relationships, and linear or quadratic regression models were fitted as appropriate for each scatter plot, with the level of sunlight transmission as the independent variable.
3. Results
3.1. Essential Element Concentrations
Incident light level during S. nelsonii leaf construction influenced the concentrations of all six macroelements (Figure 2). Non-linear decreases in C occurred for laminae (f3,20 = 10.089, p < 0.001) and supportive (f3,20 = 4.392, p = 0.016) tissues as the light decreased from full sun to shaded conditions. Similarly, linear decreases in P occurred for laminae tissues (f3,20 = 12.901, p < 0.001) as the light decreased from full sun to shaded conditions. In contrast, linear increases in the concentrations of laminae tissues as light decreased from full sun to shaded conditions occurred for N (f3,20 = 22.434, p < 0.001), K (f3,20 = 53.518, p < 0.001), Ca (f3,20 = 7.039, p < 0.001), and Mg (f3,20 = 43.381, p = 0.002). These linear trends also occurred in concentrations of supportive tissues for N (f3,20 = 18.127, p < 0.001), P (f3,20 = 216.53, p < 0.001), K (f3,20 = 17.460, p < 0.001), Ca (f3,20 = 283.48, p < 0.001), and Mg (f3,20 = 10.967, p < 0.001). These patterns revealed that the direction and shape of the models were similar for laminae versus supportive tissue for C, N, K, Ca, and Mg (Figure 2). In contrast, P concentration exhibited slopes in opposite directions for laminae tissue versus supportive tissue. The concentration range for the two tissue types was inconsistent among the macroelements, with laminae tissues exhibiting more C, N, Ca, and Mg, but less K than the supportive tissue. The macroelement P differed from the other macroelements in this leaf trait, as the overall mean concentration was similar for the laminae versus supportive tissue.
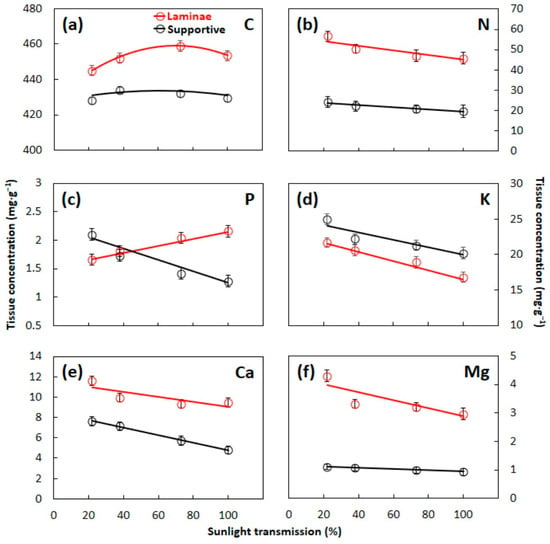
Figure 2.
Concentrations of macroelements in Serianthes nelsonii leaves as influenced by developmental light level. Supportive tissue was petiole, rachis, and all rachillae. (a) Carbon; (b) nitrogen; (c) phosphorus; (d) potassium; (e) calcium; and (f) magnesium. Markers are mean ± SE, n = 6. Equations are shown in Appendix A.
The concentrations of S. nelsonii leaf tissue microelements were generally less influenced by developmental light level than were macroelements (Figure 3). A non-linear increase in laminae tissue Fe (f3,20 = 118.90, p < 0.001) and B (f3,20 = 10.526, p < 0.001) and linear increase in laminae tissue Mn (f3,20 = 38.594, p < 0.001) occurred with increased incident light. Contrarily, a linear decrease in supportive tissue Zn (f3,20 = 10.578, p < 0.001) and Ni (f3,20 = 55.960, p < 0.001) occurred as the light level increased. The developmental light level did not significantly influence the concentrations of Cu (f3,20 = 3.017, p = 0.055) or Ni (f3,20 = 1.491, p = 0.248) in laminae tissue (Figure 3). Similarly, the contrasting light conditions did not significantly influence supportive tissue concentrations of Fe (f3,20 = 2.327, p = 0.105), Mn (f3,20 = 1.252, p < 0.318), Cu (f3,20 = 1.092, p = 0.375), or B (f3,20 = 1.119, p = 0.365). Laminae tissues contained more Fe, Mn, Zn, Cu, and B, but less Ni than supportive tissue. With the exception of Ni, incident light influenced laminae tissue microelement relations more so than supportive tissue. The concentration of Ni in supportive tissue greatly exceeded that in laminae tissue.
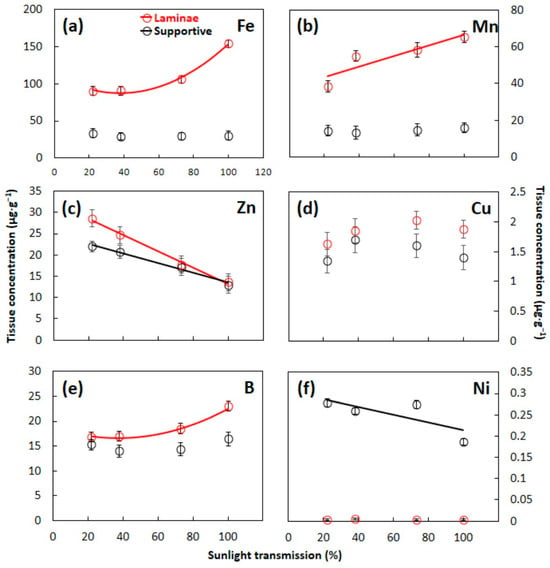
Figure 3.
Concentrations of microelements in Serianthes nelsonii leaves as influenced by developmental light level. Supportive tissue was petiole, rachis, and all rachillae. (a) Iron; (b) manganese; (c) zinc; (d) copper; (e) boron; and (f) nickel. Markers are mean ± SE, n = 6. Data series without a line were NS. Equations are shown in Appendix A.
The scaling relationships between S. nelsonii laminae and supportive tissues contrasted for C, N, P, and K concentration (Figure 4). The mean C concentration in supportive tissues was 431 mg·g−1 and did not differ throughout a range of 444–466 mg·g−1 in laminae tissue. The concentration of N in supportive tissue exhibited a positive linear relationship with the N concentration in laminae tissue. The concentration of P in supportive tissue declined in a non-linear model as the P concentration in laminae tissue increased. The rate of decline was greatest at the low end of the range of laminae tissue concentration. Finally, the concentration of K in supportive tissue increased in a non-linear model as the laminae tissue K concentration increased. The rate of increase was greatest at the upper end of the range in laminae tissue concentration.
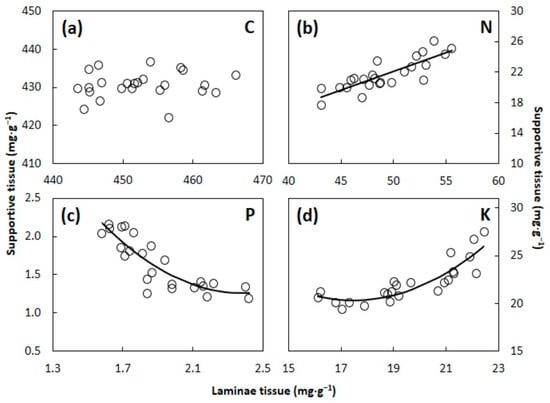
Figure 4.
The relationship of laminae versus supportive tissues for Serianthes nelsonii leaf concentrations of (a) Carbon; (b) nitrogen; (c) phosphorus; and (d) potassium. Supportive tissue was petiole, rachis, and all rachillae. Data series without a line were NS. Equations are shown in Appendix A.
3.2. C:N:P:K Stoichometry
The pairwise quotients of C:N:P:K stoichiometry of S. nelsonii leaf tissues were influenced by developmental light level with linear models (Figure 5). The C:N quotient exhibited a linear increase in laminae (f3,20 = 23.950, p < 0.001) and supportive (f3,20 = 16.605, p < 0.001) tissues as the developmental light level increased. The C:N values were much greater for supportive tissue as a result of the lower N concentrations when compared with those for laminae tissue. The C:P quotient exhibited a negative slope for laminae tissue but a positive slope for supportive tissue. The overall means of C:P were similar for the two tissue types. The C:K quotient exhibited a positive slope for both tissue types, and the laminae tissue C:K exceeded that of the supportive tissue. The patterns for N:P were similar to those of C:P. Unlike C:P, the overall mean for laminae tissue exceeded that for supportive tissue. The N:K quotient for supportive tissue was not influenced by the developmental light level, and the N:K for laminae tissue increased as the light level increased. The patterns for K:P were similar to those for C:P.
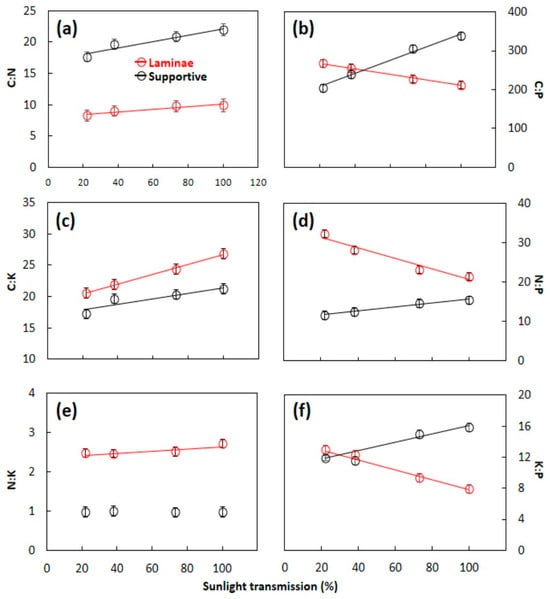
Figure 5.
The stoichiometry relationships among macroelements in Serianthes nelsonii leaves as influenced by developmental light level. Supportive tissue was petiole, rachis, and all rachillae. (a) C:N; (b) C:P; (c) C:K; (d) N:P; (e) N:K; and (f) K:P. Markers are mean ± SE, n = 6. Data series without a line were NS. Equations are shown in Appendix A.
3.3. Total Leaf Mass and Element Content
The total S. nelsonii leaf dry weight increased linearly as the developmental light level increased (Figure 6a). The laminae tissue dry weight and supportive tissue dry weight also increased linearly with an increased light level, but the slopes of the lines were less than that of total. The total leaf C, N, and K content exhibited patterns that were similar to those of total biomass (Figure 6b,c,e). The developmental light level did not influence the supportive tissue total P content, but the P in laminae tissue and total leaf tissue increased as the developmental light level increased (Figure 6d).
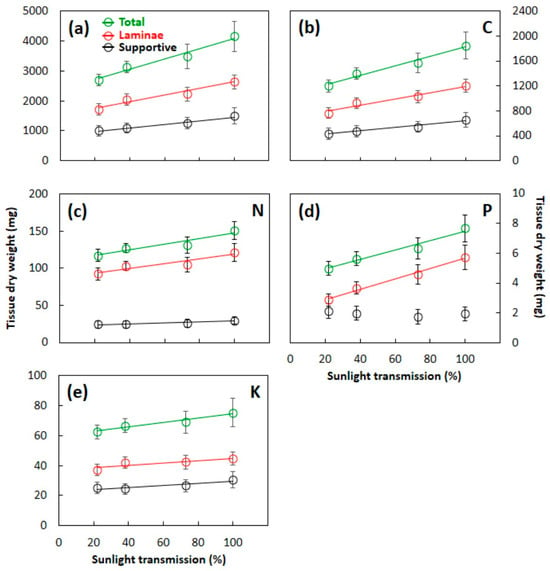
Figure 6.
Dry weight of Serianthes nelsonii leaf components as influenced by developmental light level. Supportive tissue was petiole, rachis, and all rachillae. (a) Leaf biomass; (b) carbon; (c) nitrogen; (d) phosphorus; and (e) potassium. Markers are mean ± SE, n = 6. Equations are shown in Appendix B.
Across the range of light availability from 22% to 100%, the S. nelsonii plants constructed functioning leaves with very different amounts of construction materials, confirming substantial plasticity. Acclimation to low light levels enabled the construction of whole leaves with 35% less biomass, 35% less C, 23% less N, 35% less P, and 17% less K than whole leaves under full-sun conditions.
The scaling relationships between the S. nelsonii laminae and supportive tissue dry weights contrasted among C, N, P, and K (Figure 7). As the total C, N, and K in leaf laminae tissue increased, the total C, N, and K in the supportive tissue also increased in a non-linear pattern. The rate of increase was the greatest at the upper end of the range in laminae tissue C, N, and K. The total weight of the supportive tissue P content per leaf declined as the total laminae tissue P content increased within the lower end of the range in data. This relationship reversed in the upper end of the data range, as the supportive tissue P content increased with each unit of laminae tissue P content increase. The scaling patterns of concentrations of these four macroelement concentrations (Figure 4) were dissimilar to totals (Figure 7).
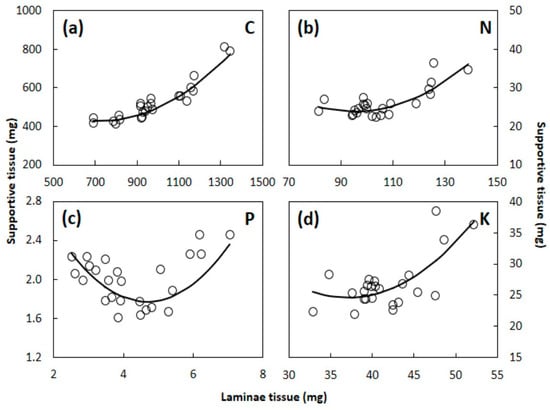
Figure 7.
The relationship of photosynthetic versus supportive tissues for Serianthes nelsonii leaf total content for (a) Carbon; (b) nitrogen; (c) phosphorus; and (d) potassium. Supportive tissue was petiole, rachis, and all rachillae. Equations are shown in Appendix B.
The dissimilarities among the macroelements in these scaling relationships can be understood in greater detail by considering the absolute amounts of these resources in the two tissue types. For example, for C, N, and K the laminae tissue contained more dry weight than supportive tissue throughout the entire range in values. About 1.7 units of C in laminae tissue occurred for each unit of C in supportive tissue (Figure 7a). In contrast, about 3.2 units of N in laminae tissue occurred for each unit of N in supportive tissue at the lower end of the range, and about 4 units of N in laminae tissue occurred for each unit of N in the supportive tissue at the upper end of the range (Figure 7b). For K, about 1.5 units were present in laminae tissue for each unit in supportive tissue (Figure 7d). The leaf construction costs for P were in sharp contrast to those of the other macroelements, with a similar amount of P contained in the two tissue types at the lowest end of the data range, but greater relative P in laminae tissue than in supportive tissue toward the upper end of the data range (Figure 7c).
4. Discussion
Heterogeneity of incident light is omnipresent in natural settings, and the in situ settings of S. nelsonii are no exception. The forest floor where seedlings and saplings of this legume tree persist receives about 6% to 7% of sunlight [8]. The saplings and juveniles of shade-tolerant plant species such as S. nelsonii may persist in deeply shaded subcanopy conditions for decades while waiting for a canopy gap to appear [17]. Yet, the emergent canopy of mature trees extends into full sunlight. The bi-pinnately compound S. nelsonii leaf is comprised of thousands of small leaflets arranged on rachillae that radiate along the length of the rachis, and this leaf design exhibits considerable morphological plasticity [8]. Understanding the drivers of the S. nelsonii leaf element concentrations is crucial for developing new knowledge concerning the role of this late-succession legume tree in community-level processes. Indeed, trees that associate with nitrogen-fixing endosymbionts may be crucial for ecosystem-level contributions [18]. This study has added to the scant literature on leaf nutrient relations of this endemic, charismatic tree species. It has also added a new case study from an under-studied region of the world [13].
4.1. Leaf Construction Needs
I hypothesized that the construction of full-sun S. nelsonii leaves would require more essential elements than for the construction of shaded leaves, in conformity with previously reported biomass studies [8]. Indeed, the concentrations and total leaf uptake of essential elements during S. nelsonii leaf construction were greatly influenced by the incident-light level in this study. The full-sun leaves required 54% more C and P, 29% more N, and 20% more K than the leaves, which developed in 22% sunlight transmission (from Figure 6). These results indicate that abiotic stressors, such as over-watering in a container nursery setting [6], may be more detrimental to S. nelsonii growth in full sun than in shaded conditions. This study was conducted with ample nutrient availability via horticultural inputs. However, in situ plants persist under nutrient-limited conditions with sympatric plants competing for those nutrients. Indeed, plasticity in response to shade may strongly depend on nutrient availability [19]. In northern Guam, P and K appear to be particularly limiting of S. nelsonii health [20]. Therefore, deficiencies in essential elements may influence S. nelsonii growth to a greater degree in full-sun growing conditions, as the shaded plants required fewer of these resources for the construction of a functional leaf to full expansion.
4.2. Laminae versus Supportive Tissue
I predicted that the incident-light level would influence laminae construction traits to a greater degree than supportive tissue construction traits. To explain, the laminae serve as the primary photosynthetic tissue of the leaf; therefore, plasticity in developmental traits may be more important than for supportive structures, which primarily serve to mechanically position the laminae and provide transport for resources from stems to laminae [21,22]. The results were in conformity with this prediction for most of the essential elements. For example, the slopes of the regression equations (Appendix A) and the range in raw data were less for the supportive tissues than for the laminae (Figure 2 and Figure 3), indicating a muted plasticity response for the construction of supportive tissues in relation to incident-light level. The two exceptions to these findings were P and Ni. Moreover, four of the microelements exhibited homeostasis under the conditions of this study whereby the incident light from 22% to full sun did not significantly influence concentrations in the supportive tissues.
In addition to greater plasticity, the leaf laminae also contained greater concentrations of most elements than the supportive tissues. Therefore, S. nelsonii laminae construction places greater demands on nutrient resource availability than supportive tissue construction. Two notable exceptions were K and Ni. Niinemets and Kull [22] also reported this pattern for K. Turgor is an integral component of mechanical integrity of supportive tissues in leaves, and one of the functions of K is the generation of turgor; therefore, a higher K content in supportive tissues likely reflects the mechanical properties of these tissues [22].
Leaf size per se and the compound leaf construction approach may influence the relative investments into a leaf’s supportive tissues [23]. The supportive structures in this study contained 36% to 37% of the total leaf biomass, and this trait was not influenced by the developmental light level (from Figure 5a). These relative investments into supportive tissue were much greater than what has been previously reported for simple or pinnately compound leaves [7,22,24]. Moreover, shaded leaves invested relatively more biomass into supportive tissues than full-sun leaves in some past studies [7,25]; therefore, the S. nelsonii plants in the present study did not conform to those plasticity behaviors. My results indicated that a bi-pinnate approach to leaf construction whereby leaf size is substantial but individual lamina size is minimal may result in different behavior during plasticity responses than for pinnately compound leaves in which the leaflets are larger, fewer in number, and inserted directly onto the rachis. This observation deserves further study using other bi-pinnate and tri-pinnate leaves.
Nitrogen was the only macroelement that exhibited a linear relationship between supportive tissue and laminae concentrations (Figure 4b), indicating similar relative construction demands throughout a range of incident light levels. This correlation has been reported for other tree species [21,22].
4.3. Previously Published Lamina Data
This study is the first to look at minerals and metals in the structural components of the S. nelsonii compound leaf. However, several previous studies reported essential element concentrations of green laminae tissue [20,26,27,28], and the results were highly heterogeneous among the studies. The elements N, K, Fe, Mn, and Zn exhibited two- to three-fold differences in concentrations among the studies. The fertilizer inputs in the current study were considered luxurious, and the laminae N, K, and Fe concentrations exceeded those in all previous studies. Access to root symbionts may influence legume leaf stoichiometry [18] and the available plant nutrition can influence many aspects of leaf plasticity in response to shade [19]. Clearly the concentrations of minerals and metals in the laminae of this legume tree are highly influenced by nutrient availability and access to root symbionts, and much can be learned about leaf construction with more manipulative studies that provide a range of abiotic and biotic conditions.
4.4. Conservation Implications
The recruitment of in situ S. nelsonii has been compromised for many years, and is among the reasons that the species is so threatened [6]. Additionally, decades of attempting to restore in situ populations by transplanting saplings from conservation nurseries have resulted in rapid post-transplant mortality [6]. The plasticity of plant traits is crucial for regeneration and restoration success [29,30]. The addition of this S. nelsonii leaf plasticity study to the palette of adaptive management studies has improved our understanding of leaf plasticity toward improving evidence-based decision-making.
This unique legume tree is one of more than 220,000 plant species that occur in a single country and deserve special protection protocols in each host country [31]. The tree has emerged as a symbol of cultural preservation in the face of a militaristic colonial occupying force [5,6], which places it among the tree species recognized as venerable trees [32]. The tree was described in 1919 and Red-listed as Critically Endangered in 1998 [1]. There are statutory requirements under the Endangered Species Act to have a protection plan in place, and the national recovery plan for S. nelsonii was published in 1994 [3]. However, this Mariana Island tree stands out as an example that a conservation plan is of no consequential value if the federal deciders in the permitting and funding agencies are uninterested in the successful implementation of the plan. The first large-scale nursery and out-planting project was funded in the 1990s and resulted in 100% mortality; then, numerous comparable projects were funded in the subsequent decades with the same results [6]. As of today, these same failed approaches have continued to be funded, resulting in the global population of mature individuals dwindling from more than 120 in 1994 [4] to less than 40 today [5]. This history indicates that the continued funding of propagation and out-planting projects by the same failed practitioners is not justified and resources would better propel species recovery if they were made available to capable scientists with the skills to determine the causes of the historical and ongoing plant mortality [6]. The empowered deciders have not allowed this to occur to date.
4.5. Future Directions
The data herein focused on total-dry-weight pools of resources and clearly must be considered preliminary, as continued research on this agenda is warranted. An awareness of the concentrations and stoichiometric relations of the mineral soil layer, the litter layer, roots, and stems in addition to the leaves would enable a greater understanding of the biogeochemistry of the S. nelsonii habitats. Shade tolerance is a highly complex, integrated plant trait and many responses are context-dependent. Several directions of research would yield valuable new information. First, the bulk S. nelsonii leaf concentrations reported herein do not inform how the essential elements have been used by the leaves. Teasing apart how essential elements are partitioned among biochemical pools in the leaves would address this lack of information. For species with highly plastic leaf behavior, sun leaves are constructed to minimize carboxylation limitations and shaded leaves are constructed to enhance the capture of light energy [33,34]. This tradeoff can be quantified by determining the intra-leaf location of the essential elements. Second, leaf element content is influenced by seasonal changes [35], and the horticultural setting of this study muted the influence of this environmental factor. Leaf construction dynamics of in situ plants will require a seasonal approach to fully understand how the S. nelsonii leaf is constructed. Third, S. nelsonii leaf photosynthesis research has been lacking. Chlorophyll fluorescence traits have been reported [6], but to date there have been no studies on leaf gas exchange. A greater understanding of the photosynthesis and respiration of leaf tissues under varied light conditions would greatly improve our understanding of how the leaf of this endangered tree functions to contribute to whole plant growth and productivity and how the availability of essential elements influences these processes. Fourth, leaf-level responses to light conditions may be influenced by ontogeny. Indeed, the age and size of a tree [36,37,38,39] and the age and ontogeny of a leaf [40] may affect the leaf traits that have been reported herein. The use of the youngest fully expanded leaf for this study was employed in order to standardize leaf ontogeny among the light treatments. A greater understanding of leaf function would be realized by repeating the methods used for leaves throughout the leaf expansion stages and employing them for the aging and senescing stages of individual leaves and sampled trees. Fifth, this study focused exclusively on the dry weight needs of leaf construction resources. However, the laminae area to mass ratio of a leaf may be greatly influenced by incident light, with thinner leaves constructed under shaded conditions [34,41]. These changes in leaf design greatly influence element content on an area basis, and sometimes result in more plasticity than element content on a mass basis [42]. A greater understanding of the leaf plasticity of S. nelsonii plants would be enabled by delving into leaf resource needs expressed on an area basis in addition to a biomass basis. Moreover, the use of fresh-weight in addition to dry-weight leaves may improve our understanding of global leaf scaling processes [40]. I elected to use dry-weight leaves in the present study because of their ease of measurement and because they are more often employed for large-scale analyses, e.g., in [43].
5. Conclusions
Identifying the drivers of S. nelsonii leaf element concentration and stoichiometry is crucial for understanding the role of this charismatic tree in community-level biogeochemical processes. The findings of this study have revealed that the elemental costs for the construction of laminae tissues exceeded those of petiole, rachis, and rachillae tissues; the incident light level did not influence the percentage of biomass or elements invested in supportive versus laminae tissues; the percentage of biomass invested into the supportive tissues of this bi-pinnate compound leaf was greater than previous reports for simple or pinnate compound leaves; and full-sun leaves required more elemental resources for construction than shade leaves. These results may be directed toward improved conservation management decisions to better achieve species recovery.
Funding
This research was funded in part by the United States Department of Agriculture NIFA, grant number GUA0915, and by the United States Forest Service Cooperative Agreement, number 17-DG-11052021-217.
Data Availability Statement
Data available upon request.
Acknowledgments
I thank Gil Cruz, Nirmala Dongol, and Frankie Matanane for nursery maintenance.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Macroelement concentrations of Serianthes nelsonii leaves as influenced by developmental light level.
Microelement concentrations of Serianthes nelsonii leaves as influenced by developmental light level.
Scaling relationships between laminae versus supportive tissue of Serianthes nelsonii leaves for concentrations.
- Figure 4a: NS
Macroelement stoichiometry of Serianthes nelsonii leaves as influenced by developmental light level.
- Figure 5e supportive: NS
Appendix B
Models describing total dry weight of Serianthes nelsonii leaf components as influenced by developmental light level.
- Figure 6d supportive: NS
Scaling relationships between laminae versus supportive tissue of Serianthes nelsonii leaves for total content.
References
- Wiles, G.; Williams, E. Serianthes nelsonii. IUCN Red List Threat. Species 2017, e.T30437A98715973. Available online: https://doi.org/10.2305/IUCN.UK.2017-3.RLTS.T30437A98715973.en (accessed on 15 February 2024).
- United States Fish and Wildlife Service. Determination of endangered status for Serianthes nelsonii Merr. (Hayun lagu or Tronkon Guafi). Fed. Regist. 1987, 52, 4907–4910. [Google Scholar]
- United States Fish and Wildlife Service. Recovery Plan for Serianthes nelsonii; USFWS: Portland, OR, USA, 1994. [Google Scholar]
- Wiles, G.J.; Aguon, C.F.; Davis, G.W.; Grout, D.J. The status and distribution of endangered animals and plants in northern Guam. Micronesica 1995, 28, 31–49. [Google Scholar]
- Demeulenaere, E.; Ickert-Bond, S.M. Guam’s last håyun lågu tree (Serianthes nelsonii) in peril. Conserv. Sci. Practice 2023, 5, e13019. [Google Scholar] [CrossRef]
- Marler, T.E.; Musser, C.; Cascasan, A.N.J.; Cruz, G.N.; Deloso, B.E. Adaptive management lessons for Serianthes nelsonii conservation. Horticulturae 2021, 7, 43. [Google Scholar] [CrossRef]
- Niinemets, Ü. Are compound-leaved woody species inherently shade-intolerant? An analysis of species ecological requirements and foliar support costs. Plant Ecol. 1998, 134, 1–11. [Google Scholar] [CrossRef]
- Deloso, B.E.; Marler, T.E. Bi-pinnate compound Serianthes nelsonii leaf-level plasticity magnifies leaflet-level plasticity. Biology 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.S. The Adaptive Geometry of Trees; Princeton University Press: Princeton, NJ, USA, 1971; p. 144. [Google Scholar]
- Bazzaz, F.A. The physiological ecology of plant succession. Annu. Rev. Ecol. Syst. 1979, 10, 351–371. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to sun and shade: A whole-plant perspective. Aust. J. Plant Physiol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Dumas, J.B.A. Procedes de L’analyse Organique. Ann. Chim. Phys. 1831, 47, 198–205. [Google Scholar]
- Hou, X.; Jones, B.T. Inductively coupled plasma/optical emission spectrometry. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 9468–9485. [Google Scholar]
- Lin, J.; Harcombe, P.A.; Fulton, M.R.; Hall, R.W. Sapling growth and survivorship as a function of light in a mesic forest of southeast Texas, USA. Oecologia 2002, 132, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.A.; Funk, J.L.; Menge, D.N.L. The symbionts made me do it: Legumes are not hardwired for high nitrogen concentrations but incorporate more nitrogen when inoculated. New Phytol. 2017, 213, 690–699. [Google Scholar] [CrossRef]
- Portsmuth, A.; Niinemets, U. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 21, 61–77. [Google Scholar] [CrossRef]
- Marler, T.E. Leaf elemental concentrations, stoichiometry, and resorption in Guam’s coastal karst forests. Diversity 2021, 13, 545. [Google Scholar] [CrossRef]
- Niinemets, Ü. Differences in chemical composition relative to functional differentiation between petioles and laminas of Fraxinus excelsior. Tree Physiol. 1999, 19, 39–45. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, O. Biomass investment in leaf lamina versus lamina support in relation to growth irradiance and leaf size in temperate deciduous trees. Tree Physiol. 1999, 19, 349–358. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Portsmuth, A.; Tena, D.; Tobias, M.; Matesanz, S.; Valladares, F. Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Annals Bot. 2007, 100, 283–303. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Fleck, S. Petiole mechanics, leaf inclination, morphology, and investment in support in relation to light availability in the canopy of Liriodendron tulipifera. Oecologia 2002, 132, 21–33. [Google Scholar] [CrossRef]
- Sims, D.A.; Gebauer, R.L.E.; Pearcy, R.W. Scaling sun and shade photosynthetic acclimation of Alocasia macrorrhiza to whole-plant performance—II. Simulation of carbon balance and growth at different photon flux densities. Plant Cell Environ. 1994, 17, 889–900. [Google Scholar] [CrossRef]
- Marler, T.E. NPK fertilization of Serianthes plants influences growth and stoichiometry of leaf nutrients. Horticulturae 2022, 8, 717. [Google Scholar] [CrossRef]
- Marler, T.E. Soil from Serianthes rhizosphere influences growth and leaf nutrient content of Serianthes plants. Agronomy 2022, 12, 1938. [Google Scholar] [CrossRef]
- Marler, T.E. Foliar nutrition of Serianthes nelsonii seedlings as a conservation tool. HortScience 2022, 57, 389–390. [Google Scholar] [CrossRef]
- Lawson, S.S.; Michler, C.H. Afforestation, restoration and regeneration—Not all trees are created equal. J. For. Res. 2014, 25, 3–20. [Google Scholar] [CrossRef]
- Löf, M.; Madsen, P.; Metslaid, M.; Witzell, J.; Jacobs, D.F. Restoring forests: Regeneration and ecosystem function for the future. New For. 2019, 50, 139–152. [Google Scholar] [CrossRef]
- Antonelli, A.; Fry, C.; Smith, R.J.; Eden, J.; Govaerts, R.H.A.; Kersey, P.; Nic Lughadha, E.; Onstein, R.E.; Simmonds, M.S.J.; Zizka, A.; et al. State of the World’s Plants and Fungi 2023; Royal Botanic Gardens, Kew: Richmond, UK, 2023. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Laurance, W.; Franklin, J. Global decline in large old trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Kull, O.; Tenhunen, J.D. An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiol. 1998, 18, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Yu, Z.-C.; Zheng, X.-T.; He, W.; Lin, W.; Yan, G.-Z.; Zhu, H.; Peng, C.-L. Different responses of macro- and microelement contents of 41 subtropical plants to environmental changes in the wet and dry seasons. J. Plant Ecol. 2023, 16, rtad027. [Google Scholar] [CrossRef]
- Lusk, C.H. Leaf area and growth of juvenile temperate evergreens in low light: Species of contrasting shade tolerance change rank during ontogeny. Funct. Ecol. 2004, 18, 820–828. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Hurtado, V.H.; Poorter, L. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol. 2006, 20, 207–216. [Google Scholar] [CrossRef]
- Li, X.-B.; Liu, C.-C.; Chen, J.-X.; Zhang, M.-M.; Zhang, J.-H.; Tong, Z.-K.; Yang, Q. Leaf plasticity of the subtropical evergreen tree Phoebe bournei increases with ontogeny in response to sun and shade. Forests 2023, 14, 1683. [Google Scholar] [CrossRef]
- Barton, K.E. The ontogenetic dimension of plant functional ecology. Funct. Ecol. 2024, 38, 1–16. [Google Scholar] [CrossRef]
- Guo, X.; Schrader, J.; Shi, P.; Jiao, Y.; Miao, Q.; Xue, J.; Niklas, K.J. Leaf-age and petiole biomass play significant roles in leaf scaling theory. Front. Plant Sci. 2023, 14, 1322245. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, L.; Bongers, F. Light-dependent leaf trait variation in 43 tropical dry forest tree species. Am. J. Bot. 2007, 94, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).