Effects of Multiple Global Change Factors on Symbiotic and Asymbiotic N2 Fixation: Results Based on a Pot Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection and Analysis
2.3. Determination of Biological N2 Fixation Rate
2.4. Statistical Analysis
3. Results
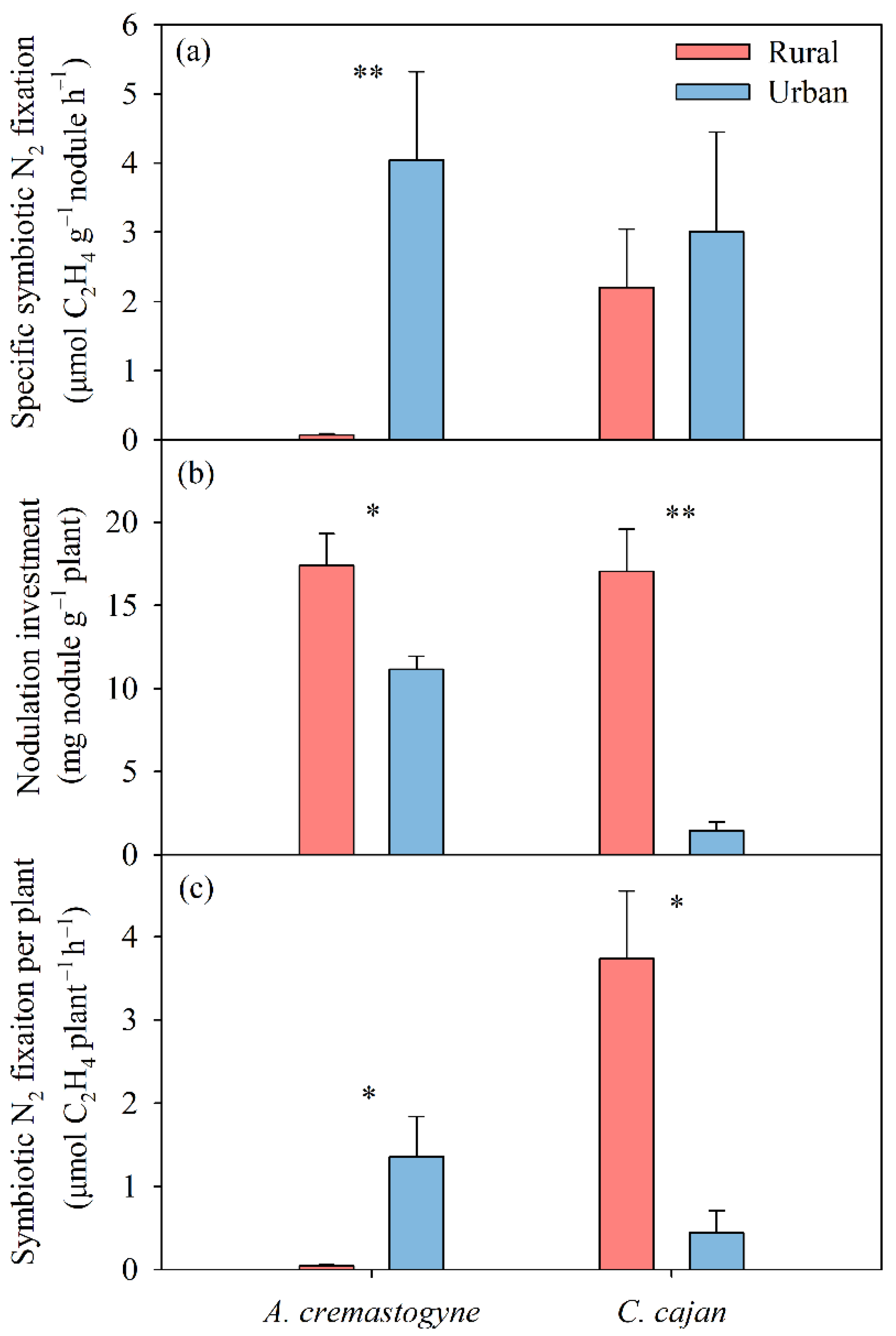
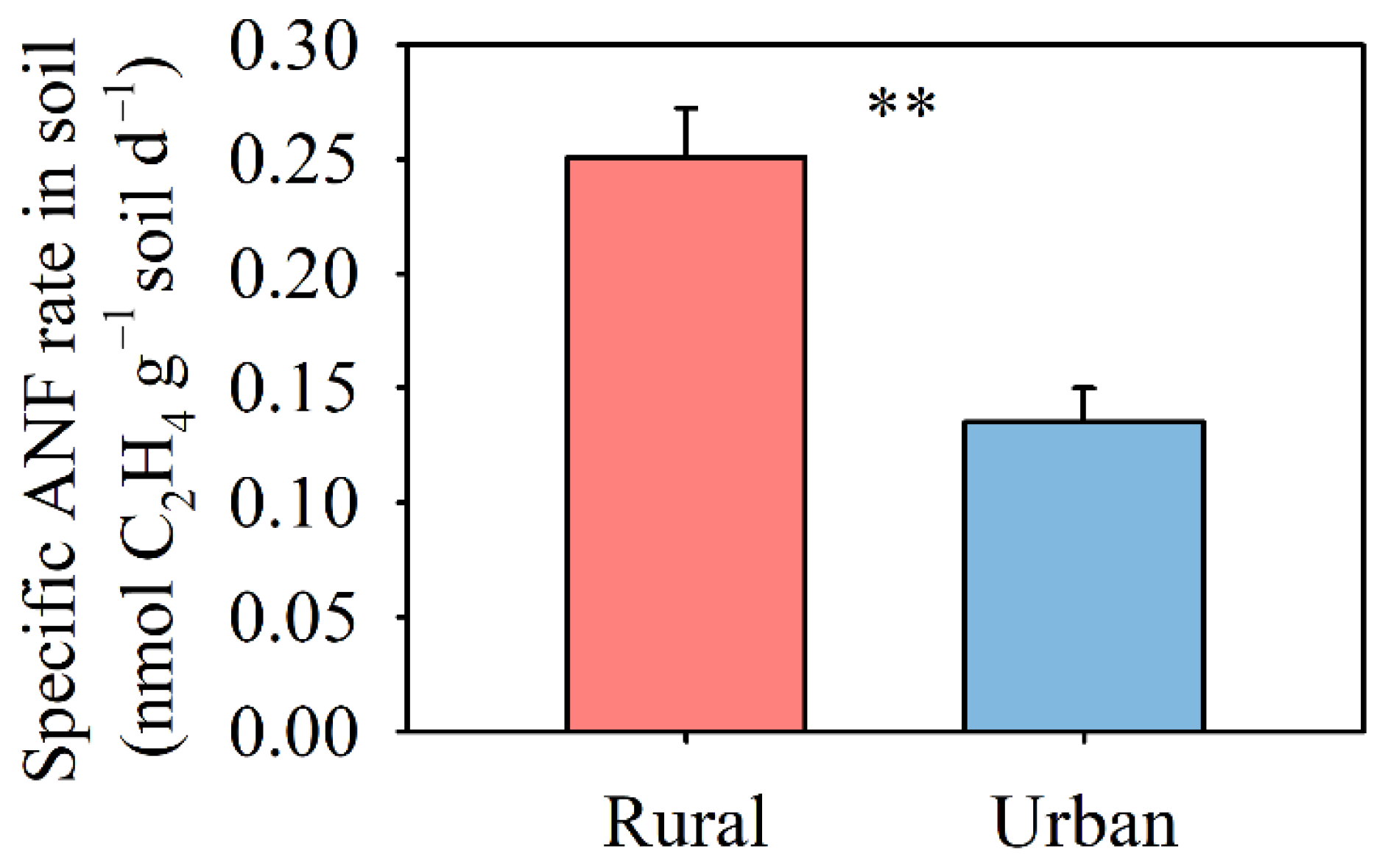
3.1. Biological N2 Fixation
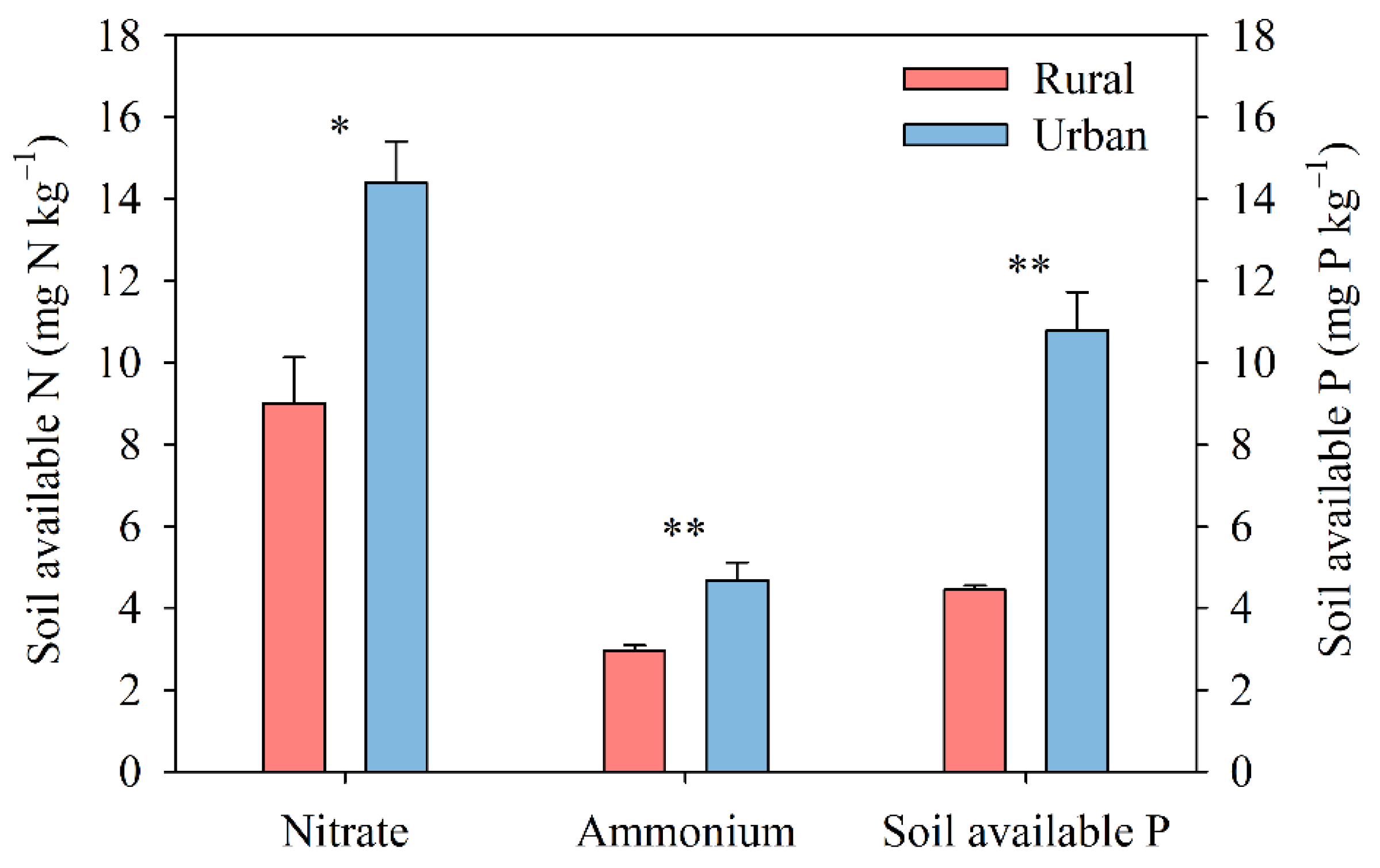
3.2. Environmental Variables and Their Correlations with Biological N2 Fixation
4. Discussion
4.1. Response of Symbiotic N2 Fixation to Multiple Global Change Factors Depends on N2-Fixing Plant Species
4.2. Decrease in Soil N2 Fixation in Response to Multiple Global Change Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional Ecology of Free-Living Nitrogen Fixation: A Contemporary Perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Kou-Giesbrecht, S.; Menge, D. Nitrogen-fixing trees could exacerbate climate change under elevated nitrogen deposition. Nat. Commun. 2019, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Lebauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhou, Z.; Zhao, P.; Luo, Y.; Ye, Q.; Zhang, K.; Song, L.; Mo, J. Effects of human disturbance activities and environmental change factors on terrestrial nitrogen fixation. Glob. Chang. Biol. 2020, 26, 6203–6217. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Q.; Xiao, K.; Wang, Z.; Wang, K. Divergent responses of biological nitrogen fixation in soil, litter and moss to temperature and moisture in a karst forest, southwest China. Soil Biol. Biochem. 2018, 118, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Zheng, M.; Chen, H.; Sun, X.; Wang, K. Topography Modulates Effects of Nitrogen Deposition on Asymbiotic N2 Fixation in Soil but not Litter or Moss in a Secondary Karst Forest. J. Geophys. Res. Biogeosci. 2019, 124, 3015–3023. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Irigoyen, J.J.; Perez, P.; Martinez-Carrasco, R.; Sanchez-Diaz, M. The use of temperature gradient tunnels for studying the combined effect of CO2, temperature and water availability in N2 fixing alfalfa plants. Ann. Appl. Biol. 2005, 146, 51–60. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, W.; Luo, Y.; Li, D.; Wang, S.; Huang, J.; Lu, X.; Mo, J. Stoichiometry controls asymbiotic nitrogen fixation and its response to nitrogen inputs in a nitrogen-saturated forest. Ecology 2018, 99, 2037–2046. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharyya, P.; Adhya, T.K. Impact of elevated CO2, flooding, and temperature interaction on heterotrophic nitrogen fixation in tropical rice soils. Biol. Fertil. Soils 2011, 47, 25–30. [Google Scholar] [CrossRef]
- Youngsteadt, E.; Dale, A.G.; Terando, A.J.; Dunn, R.R.; Frank, S.D. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob. Chang. Biol. 2015, 21, 97–105. [Google Scholar] [CrossRef]
- Tobita, H.; Uemura, A.; Kitao, M.; Kitaoka, S.; Utsugi, H. Interactive effects of elevated CO2, phosphorus deficiency, and soil drought on nodulation and nitrogenase activity in Alnus hirsuta and Alnus maximowiczii. Symbiosis 2010, 50, 59–69. [Google Scholar] [CrossRef]
- Nasto, M.K.; Winter, K.; Turner, B.L.; Cleveland, C.C. Nutrient acquisition strategies augment growth in tropical N2-fixing trees in nutrient-poor soil and under elevated CO2. Ecology 2019, 100, e02646. [Google Scholar] [CrossRef]
- Achat, D.L.; Augusto, L.; Gallet-Budynek, A.; Loustau, D. Future challenges in coupled C-N-P cycle models for terrestrial ecosystems under global change: A review. Biogeochemistry 2016, 131, 173–202. [Google Scholar] [CrossRef]
- Hupperts, S.F.; Gerber, S.; Nilsson, M.-C.; Gundale, M.J. Empirical and Earth system model estimates of boreal nitrogen fixation often differ: A pathway toward reconciliation. Glob. Chang. Biol. 2021, 27, 5711–5725. [Google Scholar] [CrossRef] [PubMed]
- Menge, D.N.L.; Lichstein, J.W.; Gregorio, A.P. Nitrogen fixation strategies can explain the latitudinal shift in nitrogen-fixing tree abundance. Ecology 2016, 95, 2236–2245. [Google Scholar] [CrossRef]
- Menge, D.N.L.; Wolf, A.A.; Funk, J.L. Diversity of nitrogen fixation strategies in Mediterranean legumes. Nat. Plants 2015, 1, 15064. [Google Scholar] [CrossRef]
- West, J.B.; HilleRisLambers, J.; Lee, T.D.; Hobbie, S.E.; Reich, P.B. Legume species identity and soil nitrogen supply determine symbiotic nitrogen-fixation responses to elevated atmospheric [CO2]. New Phytol. 2005, 167, 523–530. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Tripler, C.E. Forest remnants along urban-rural gradients: Examining their potential for global change research. Ecosystems 2005, 8, 568–582. [Google Scholar] [CrossRef]
- Wurzburger, N.; Hedin, L.O. Taxonomic identity determines N2 fixation by canopy trees across lowland tropical forests. Ecol. Lett. 2016, 19, 62–70. [Google Scholar] [CrossRef]
- Batterman, S.A.; Wurzburger, N.; Hedin, L.O.; Austin, A. Nitrogen and phosphorus interact to control tropical symbiotic N2 fixation: A test in Inga punctata. J. Ecol. 2013, 101, 1400–1408. [Google Scholar] [CrossRef]
- Carter, M.; Gregorich, E. Soil Sampling and Methods of Analysis, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hardy, R.W.F.; Holsten, R.D.; Jackson, E.K.; Burns, R.C. The acetylene-ethylene assay for N2 fixation: Laboratory and field evaluation. Plant Physiol. 1968, 43, 1185. [Google Scholar] [CrossRef]
- Gentili, F.; Huss-Danell, K. Local and systemic effects of phosphorus and nitrogen on nodulation and nodule function in Alnus incana. J. Exp. Bot. 2003, 54, 2757–2767. [Google Scholar] [CrossRef]
- Murphy, P.M. Effect of light and atmospheric carbon dioxide concentration on nitrogen fixation by herbage legumes. Plant Soil 1986, 95, 399–409. [Google Scholar] [CrossRef]
- Menge, D.N.L.; Hedin, L.O. Nitrogen fixation in different biogeochemical niches along a 120,000-year chronosequence in New Zealand. Ecology 2009, 90, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.; Delerue, F.; Gallet-Budynek, A.; Achat, D.L. Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Glob. Biogeochem. Cycles 2013, 27, 804–815. [Google Scholar] [CrossRef]
- Dynarski, K.A.; Houlton, B.Z. Nutrient limitation of terrestrial free-living nitrogen fixation. New Phytol. 2018, 217, 1050–1061. [Google Scholar] [CrossRef]
- Boucho, A.C.; Carranca, C.; Redondo, R.; Calouro, F.; Madeira, M. Biomass, nodulation and N2 fixing response by subclover and pink serradela to phosphorus fertilization. Arch. Agron. Soil Sci. 2019, 65, 1431–1445. [Google Scholar] [CrossRef]
- Bytnerowicz, T.A.; Akana, P.R.; Griffin, K.L.; Menge, D.N.L. Temperature sensitivity of woody nitrogen fixation across species and growing temperatures. Nat. Plants 2022, 8, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Dijkstra, P.; Johnson, D.W.; Hinkle, C.R.; Drake, B.G. Elevated CO2 increases nitrogen fixation and decreases soil nitrogen mineralization in Florida scrub oak. Glob. Chang. Biol. 1999, 5, 781–789. [Google Scholar] [CrossRef]
- Rotaru, V.; Sinclair, T.R. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ. Exp. Bot. 2009, 66, 94–99. [Google Scholar] [CrossRef]
- Jin, J.; Tang, C.; Armstrong, R.; Sale, P. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant Soil 2012, 358, 91–104. [Google Scholar] [CrossRef]
- Taylor, B.N.; Menge, D.N.L. Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat. Plants 2018, 4, 655–661. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, L.A.; Porder, S. Light fuels while nitrogen suppresses symbiotic nitrogen fixation hotspots in neotropical canopy gap seedlings. New Phytol. 2021, 231, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Roswanjaya, Y.P.; Kohlen, W.; Stougaard, J.; Reid, D. Nitrate restricts nodule organogenesis through inhibition of cytokinin biosynthesis in Lotus japonicus. Nat. Commun. 2021, 12, 6544. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Tree species control rates of free-living nitrogen fixation in a tropical rain forest. Ecology 2008, 89, 2924–2934. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Relationships among phosphorus, molybdenum and free-living nitrogen fixation in tropical rain forests: Results from observational and experimental analyses. Biogeochemistry 2013, 114, 135–147. [Google Scholar] [CrossRef]
- Jean, M.-E.; Phalyvong, K.; Forest-Drolet, J.; Bellenger, J.-P. Molybdenum and phosphorus limitation of asymbiotic nitrogen fixation in forests of Eastern Canada: Influence of vegetative cover and seasonal variability. Soil Biol. Biochem. 2013, 67, 140–146. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, W.; Luo, Y.; Wan, S.; Fu, S.; Wang, S.; Liu, N.; Ye, Q.; Yan, J.; Zou, B.; et al. The Inhibitory Effects of Nitrogen Deposition on Asymbiotic Nitrogen Fixation are Divergent between a Tropical and a Temperate Forest. Ecosystems 2019, 22, 955–967. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, Z.; Luo, Y.; Zhao, P.; Mo, J. Global pattern and controls of biological nitrogen fixation under nutrient enrichment: A meta-analysis. Glob. Chang. Biol. 2019, 25, 3018–3030. [Google Scholar] [CrossRef]
- Norman, J.S.; Friesen, M.L. Complex N acquisition by soil diazotrophs: How the ability to release exoenzymes affects N fixation by terrestrial free-living diazotrophs. ISME J. 2017, 11, 315–326. [Google Scholar] [CrossRef]
| Soil Parameters | Values |
|---|---|
| pH | 7.80 ± 0.04 |
| SOC (g C kg−1) | 19.04 ± 3.32 |
| Total N (g N kg−1) | 1.68 ± 0.21 |
| Total P (g P kg−1) | 0.81 ± 0.08 |
| Exchange Ca2+ (coml kg−1) | 20.98 ± 0.61 |
| Exchange Mg2+ (coml kg−1) | 6.61 ± 0.07 |
| Available P (mg P kg−1) | 6.80 ± 1.44 |
| Available Mo (μg kg−1) | 24.96 ± 12.70 |
| Factors | Specific SNF Rate | Nodulation Investment | PNF | Specific Soil ANF Rate | ||||
|---|---|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | F Value | p Value | |
| ST | 5.15 | 0.04 | 43.36 | <0.01 | 4.38 | 0.05 | 18.07 | <0.01 |
| SP | 0.27 | 0.61 | 9.18 | <0.01 | 6.77 | 0.02 | 0.42 | 0.53 |
| ST × SP | 4.54 | 0.04 | 7.94 | 0.01 | 14.25 | <0.01 | 0.21 | 0.65 |
| Environmental Variables | Rural | Urban | p Value |
|---|---|---|---|
| Annual average temperature (°C) | 20.10 | 21.94 | − |
| Annual precipitation (mm) | 1603.3 | 1548.70 | − |
| Atmosphere CO2 (ppm) | 499.5 ± 10.4 | 575.5 ± 22.1 | <0.01 |
| NO3− deposition (kg N ha−1 yr−1) | 4.16 ± 0.50 | 13.89 ± 1.09 | <0.01 |
| NH4+ deposition (kg N ha−1 yr−1) | 15.92 ± 2.92 | 17.55 ± 1.91 | 0.64 |
| DON deposition (kg N ha−1 yr−1) | 10.17 ± 2.10 | 24.75 ± 2.79 | <0.01 |
| Total N deposition (kg N ha−1 yr−1) | 30.24 ± 4.58 | 59.86 ± 4.54 | <0.01 |
| P deposition (kg P ha−1 yr−1) | 0.33 ± 0.10 | 3.64 ± 1.05 | 0.01 |
| Soil Nutrients | Specific SNF Rate | Nodulation Investment | PNF | Specific Soil ANF Rate | ||||
|---|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | r | p Value | |
| Soil NO3− | 0.34 | 0.14 | −0.48 | 0.03 | −0.33 | 0.16 | −0.30 | 0.20 |
| Soil NH4+ | 0.08 | 0.73 | −0.59 | <0.01 | −0.25 | 0.28 | −0.52 | 0.02 |
| Soil available P | 0.31 | 0.19 | −0.60 | <0.01 | −0.30 | 0.19 | −0.49 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, X.; Chen, H.; Li, D. Effects of Multiple Global Change Factors on Symbiotic and Asymbiotic N2 Fixation: Results Based on a Pot Experiment. Nitrogen 2023, 4, 159-168. https://doi.org/10.3390/nitrogen4010011
Wang Z, Sun X, Chen H, Li D. Effects of Multiple Global Change Factors on Symbiotic and Asymbiotic N2 Fixation: Results Based on a Pot Experiment. Nitrogen. 2023; 4(1):159-168. https://doi.org/10.3390/nitrogen4010011
Chicago/Turabian StyleWang, Zhenchuan, Xibin Sun, Hao Chen, and Dejun Li. 2023. "Effects of Multiple Global Change Factors on Symbiotic and Asymbiotic N2 Fixation: Results Based on a Pot Experiment" Nitrogen 4, no. 1: 159-168. https://doi.org/10.3390/nitrogen4010011
APA StyleWang, Z., Sun, X., Chen, H., & Li, D. (2023). Effects of Multiple Global Change Factors on Symbiotic and Asymbiotic N2 Fixation: Results Based on a Pot Experiment. Nitrogen, 4(1), 159-168. https://doi.org/10.3390/nitrogen4010011






