Ecosystem Recovery in Progress? Initial Nutrient and Phytoplankton Response to Nitrogen Reduction from Sewage Treatment Upgrade in the San Francisco Bay Delta
Abstract
1. Introduction
2. Materials and Methods
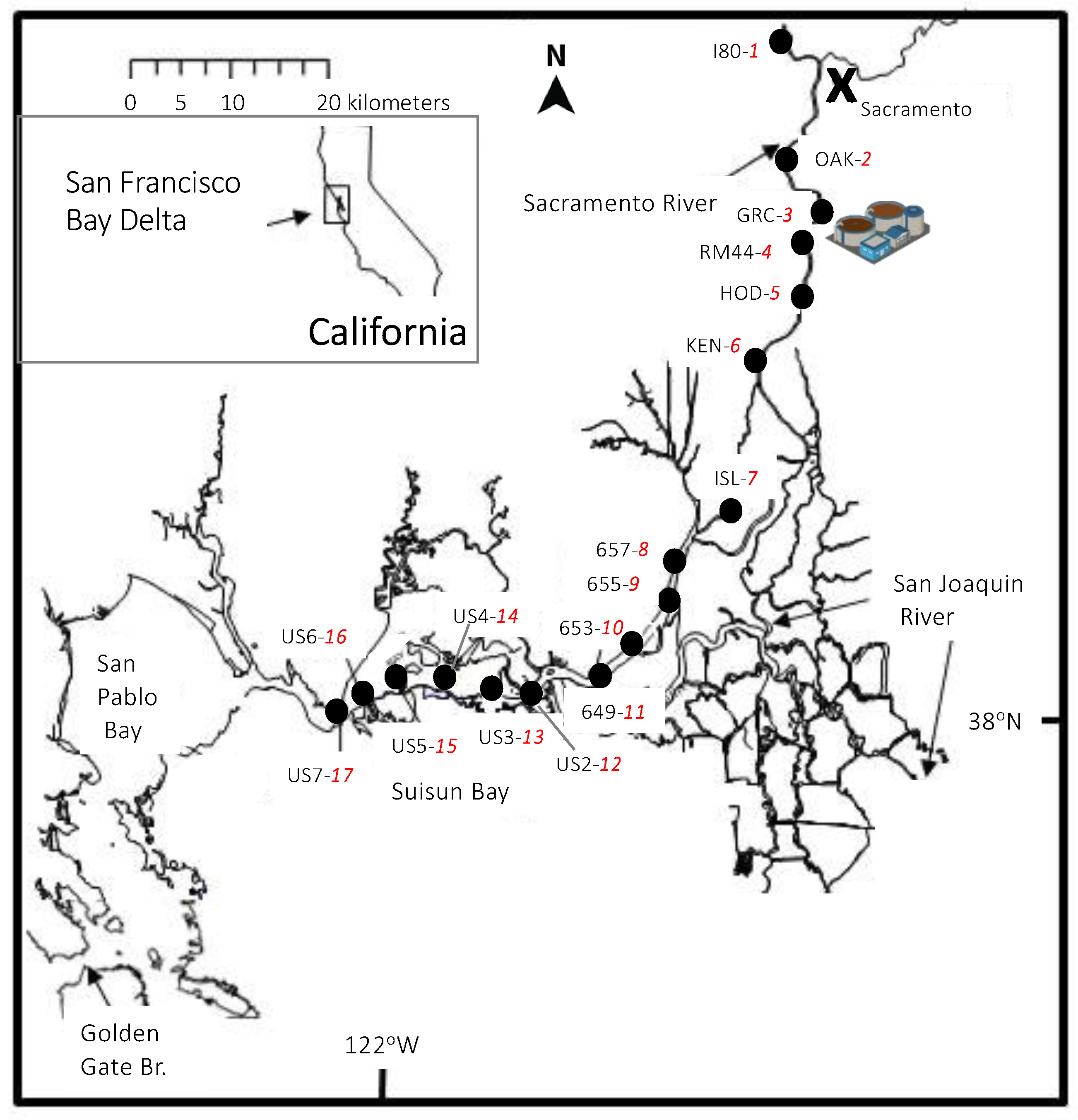
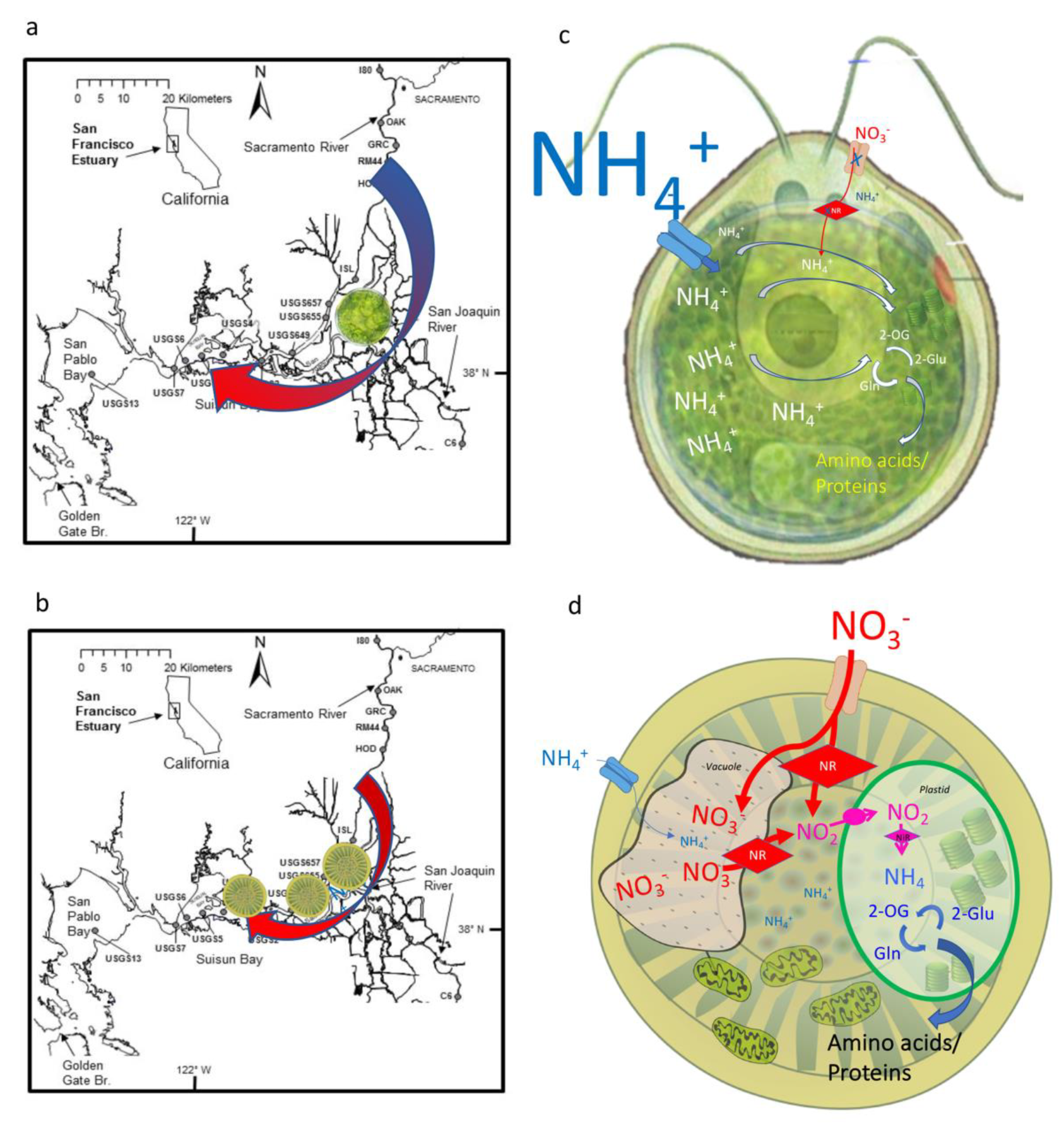
2.1. Site Description
2.2. River Flow
2.3. Sample Collection
2.4. Analytical Protocols
2.5. Photophysiological State and Irradiance Relationships
2.6. Statistical Analyses
3. Results
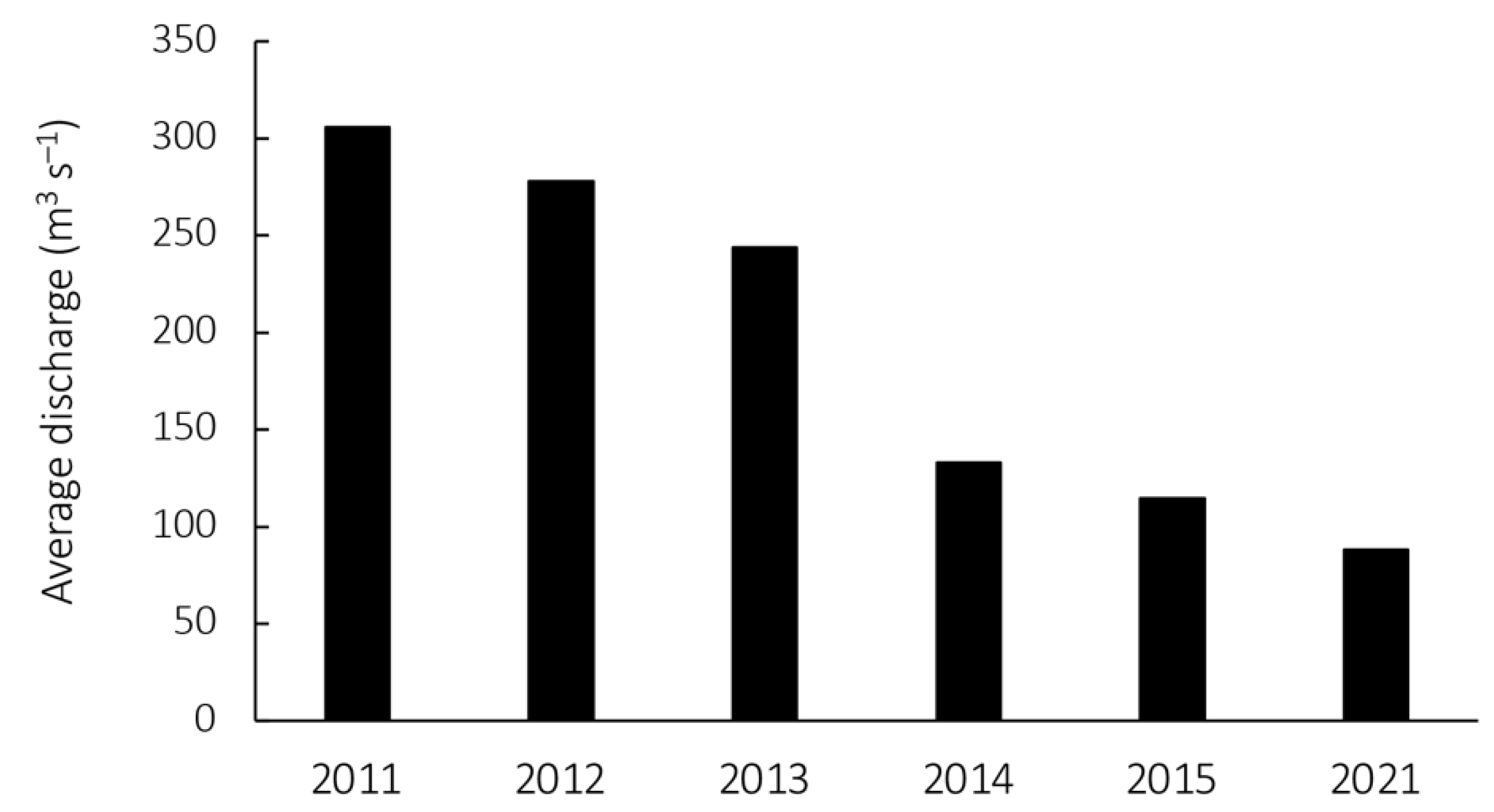
3.1. Flow Conditions
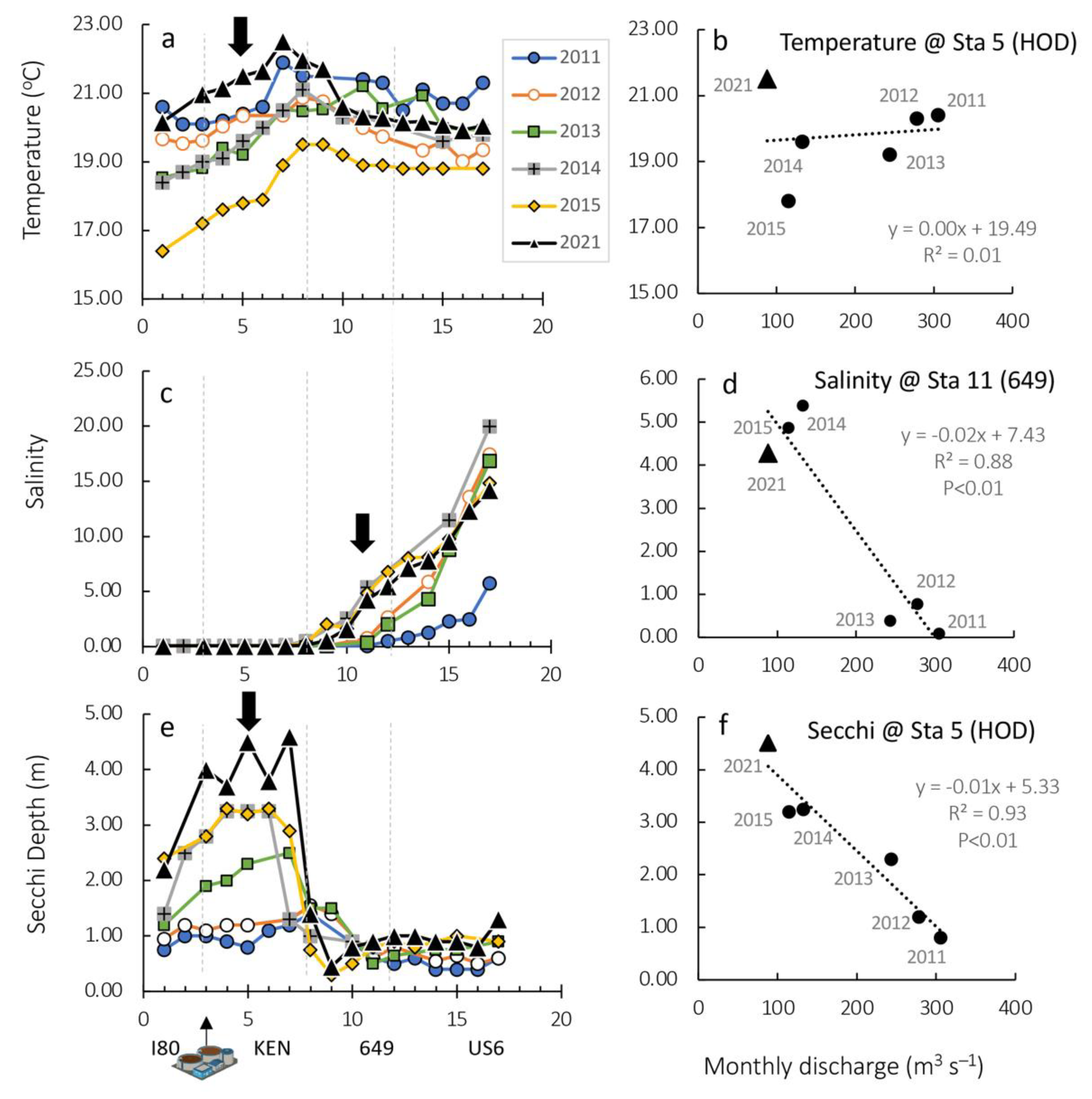
3.2. Ambient Water Column Conditions
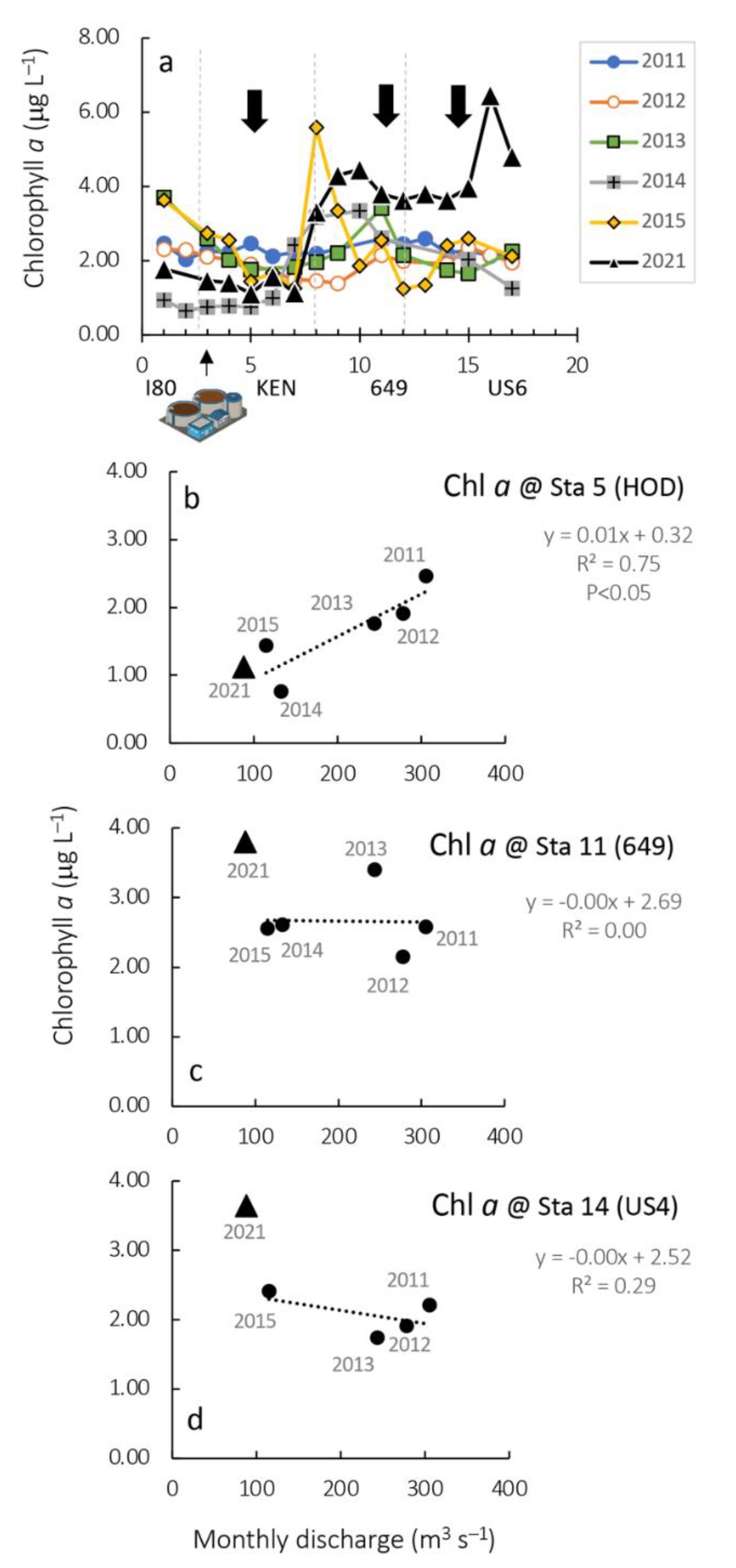
3.3. Chlorophyll a and Phytoplankton Composition
3.4. Photophysiology
4. Discussion
4.1. Major Trends and Interannual Responses
4.2. Importance of Physiological and Ecosystem Scale Experiments
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dugdale, R.C.; Wilkerson, F.P.; Hogue, V.E.; Marchi, A. The role of ammonium and nitrate in spring bloom development in San Francisco Bay. Estuar. Coast. Shelf Sci. 2007, 73, 17–29. [Google Scholar] [CrossRef]
- Sharp, J.H. Marine and aquatic communities, stress from eutrophication. In Encyclopedia of Biodiversity; Levine, S., Ed.; Academic Press: Amsterdam, The Netherlands, 2001; Volume 4, pp. 1–11. [Google Scholar]
- Yoshiyama, K.; Sharp, J.H. Phytoplankton response to nutrient enrichment in an urbanized estuary: Apparent inhibition of primary production by overeutrophication. Limnol. Oceanogr. 2006, 51, 424–434. [Google Scholar] [CrossRef]
- Ball, M.D.; Arthur, J.F. Planktonic chlorophyll dynamics in the Northern San Francisco Bay and Delta. In San Francisco Bay: The Urbanized Estuary; Conomos, T.J., Ed.; Pacific Division of the American Association for the Advancement of Science: San Francisco, CA, USA, 1979; pp. 265–286. [Google Scholar]
- Jassby, A. Phytoplankton in the upper San Francisco Estuary: Recent biomass trends, their causes and their trophic significance. San Fr. Estuary Watershed Sci. 2008, 6, 1–26. Available online: https://escholarship.ord/uc/item/71h077r1 (accessed on 1 April 2022).
- Kimmerer, W.J. Open water processes of the San Francisco Estuary: From physical forcing to biological responses. San Fr. Estuary Watershed Sci. 2004, 2, 1. Available online: https://escholarship.org/uc/item/9bp499mv (accessed on 1 April 2022). [CrossRef]
- Dugdale, R.; Wilkerson, F.; Parker, A.E.; Marchi, A.; Taberski, K. River flow and ammonium discharge determine spring phytoplankton blooms in an urbanized estuary. Estuar. Coast. Shelf Sci. 2012, 115, 187–199. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Wilkerson, F.P.; Parker, A.E. A biogeochemical model of phytoplankton productivity in an urban estuary: The importance of ammonium and freshwater flow. Ecol. Modell. 2013, 263, 291–307. [Google Scholar] [CrossRef][Green Version]
- Glibert, P.M.; Dugdale, R.C.; Wilkerson, F.; Parjer, A.E.; Alexander, J.; Antell, E.; Blaser, S.; Johnson, A.; Lee, J.; Lee, T.; et al. Major—But rare—Spring blooms in San Francisco Bay Delta, California, a result of the long-term drought, increased residence times, and altered nutrient loads and forms. J. Exp. Mar. Biol. Ecol. 2014, 460, 8–18. [Google Scholar] [CrossRef]
- Jungbluth, M.; Lee, C.; Patel, C.; Ignoffo, T.; Bergamaschi, B.; Kimmerer, W. Production of the copepod Pseudodiaptomus forbesi is not enhanced by ingestion of the diatom Aulacoseira granulata during a bloom. Est. Coasts 2021, 44, 1083–1099. [Google Scholar] [CrossRef]
- Sommer, T.R.; Armor, C.; Baxter, R.; Breuer, R.; Brown, L.; Chotkowski, M.; Culberson, S.; Feyrer, F.; Gingas, M.; Herbold, B.; et al. The collapse of pelagic fishes in the upper San Francisco Estuary. Fisheries 2007, 32, 270–277. [Google Scholar] [CrossRef]
- Baxter, R.; Breuer, R.; Brown, L.; Conrad, L.; Feyrer, F.; Fong, S.; Gehrts, K.; Grimaldo, L.; Herbold, B.; Hrodey, P.; et al. Interagency Ecological Program 2010 Pelagic Organism Decline Work Plan and Synthesis of Results. Interagency Ecological Program for the San Francisco Estuary. Available online: www.waterboardds.ca.gov (accessed on 3 August 2022).
- Lehman, P.W.; Boyer, G.; Hall, C.; Walker, S.; Gehrts, K. Distribution and toxicity of a new colonial Microcystis aeruginosa bloom in the San Francisco Bay Estuary, California. Hydrobiologia 2005, 541, 87–99. [Google Scholar] [CrossRef]
- Lehman, P.W.; Boyer, G.; Stachwell, M.; Walker, S. The influence of environmental conditions on seasonal variation of Microcystis abundance and microcystins concentration in San Francisco Estuary. Hydrobiologia 2008, 600, 187–204. [Google Scholar] [CrossRef]
- Lehman, P.W.; Kurobe, T.; Lesmeister, S.; Baxa, D.; Tung, A.; Teh, S.J. The impacts of the 2014 severe drought on the Microcystis bloom in the San Francisco Estuary. Harmful Algae 2017, 63, 94–108. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, E. Response of summer chlorophyll concentration to reduced total phosphorus concentration in the Rhine River (Netherlands) and the Sacramento-San Joaquin Delta (California, USA). Can. J. Fish. Aq. Sci. 2007, 64, 1529–1542. [Google Scholar] [CrossRef]
- Glibert, P.M.; Fullerton, D.; Burkholder, J.M.; Cornwell, J.; Kana, T.M. Ecological stoichiometry, biogeochemical cycling, invasive species, and aquatic food webs: San Francisco Estuary and comparative systems. Rev. Fish. Sci. 2011, 19, 358–417. [Google Scholar] [CrossRef]
- Parker, A.E.; Hogue, V.E.; Wilkerson, F.P.; Dugdale, R.C. Inorganic nitrogen speciation and phytoplankton growth in the high nutrient, low chlorophyll San Francisco Estuary. Est. Coast. Shelf Sci. 2012, 104–105, 91–101. [Google Scholar] [CrossRef]
- Mosier, A.C.; Francis, C.A. Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ. Microb. 2008, 10, 3002–3016. [Google Scholar] [CrossRef] [PubMed]
- Damashek, J.; Casciotti, K.L.; Francis, C.A. Variable nitrification rates across environmental gradients in turbid, nutrient-rich estuary waters of San Francisco Bay. Est. Coasts 2016, 39, 1050–1071. [Google Scholar] [CrossRef]
- Sobota, D.J.; Harrison, J.A.; Dahlgren, R.A. Influences of climate, hydrology, and land use on input and export of nitrogen in California watersheds. Biogeochemistry 2009, 94, 43–62. [Google Scholar] [CrossRef]
- Sobota, D.J.; Harrison, J.A.; Dahlgren, R.A. Linking dissolved and particulate phosphorus export in rivers draining California’s Central Valley with anthropogenic sources at the regional scale. J. Environ. Qual. 2011, 40, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Novick, E.; Senn, D. External Nutrient Loads to San Francisco Bay; Contribution No. 704 San Francisco Estuary Institute: Richmond, CA, USA, 2014. [Google Scholar]
- Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Parker, A.E.; Alexander, J.; Blaser, S.; Murasko, S. Microbial communities from San Francisco Bay Delta respond differently to oxidized and reduced nitrogen substrates—Even under conditions that would otherwise suggest nitrogen sufficiency. Front. Mar. Sci. 2014, 1, 17. [Google Scholar] [CrossRef]
- Wilkerson, F.P.; Dugdale, R.C.; Parker, A.E.; Blaser, S.B.; Pimenta, A. Nutrient uptake and primary productivity in an urban estuary: Using rate measurements to evaluate phytoplankton response to different hydrological and nutrient conditions. Aquat. Ecol. 2015, 49, 211–233. [Google Scholar] [CrossRef]
- Berg, G.M.; Driscoll, S.; Hayashi, K.; Ross, M.; Kudela, R. Variation in growth rate, carbon assimilation, and photosynthetic efficiency in response to nitrogen source and concentration in phytoplankton isolated from upper San Francisco Bay. J. Phycol. 2017, 53, 664–679. [Google Scholar] [CrossRef]
- Strong, A.L.; Mills, M.M.; Huang, I.B.; van Dijken, G.L.; Driscoll, S.E.; Berg, G.M.; Kudela, R.M.; Monismith, S.G.; Francis, C.A.; Arrigo, K.R. Response of lower Sacramento River phytoplankton to high-ammonium wastewater effluent. Elementa 2021, 9, 040. [Google Scholar] [CrossRef]
- Cloern, J.E. Use care when interpreting correlations: The ammonium example in the San Francisco Estuary. San Fran. Est. Watershed Sci. 2021, 19, 1. [Google Scholar] [CrossRef]
- Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Raven, J.A.; Dupont, C.L.; Leavitt, P.R.; Parker, A.E.; Burkholder, J.M.; Kana, T.M. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 2016, 61, 165–197. [Google Scholar] [CrossRef]
- MacIsaac, J.J.; Dugdale, R.C.; Huntsman, S.; Conway, H.L. The effect of sewage on uptake of inorganic nitrogen and carbon by natural populations of marine phytoplankton. J. Mar. Sci. 1979, 37, 51–66. [Google Scholar]
- Waiser, M.J.; Tumber, V.; Holm, J. Effluent-dominated streams. Part 1: Presence and effects of excess nitrogen and phosphorus in Wascana Creek, Saskatchewan, Canada. Environ. Tox. Chem. 2011, 30, 496–507. [Google Scholar] [CrossRef]
- Xu, J.; Glibert, P.M.; Lui, H.; Yin, K.; Yuan, X.; Chen, M.; Harrison, P.J. Nitrogen sources and rates of phytoplankton uptake in different regions of Hong Kong waters in summer. Estuaries Coasts 2012, 35, 559–571. [Google Scholar] [CrossRef]
- Swarbrick, V.J.; Simpson, G.L.; Glibert, P.M.; Leavitt, P.R. Differential stimulation and suppression of phytoplankton growth by ammonium enrichment in eutrophic hardwater lakes over 16 years. Limnol. Oceanogr. 2019, 64, S130–S149. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef] [PubMed]
- California Regional Water Quality Control Board Central Valley Region. Waste discharge requirements for the Sacramento Regional County Sanitation District, Permit. R5-2021-0019. Available online: http:///www.waterboards.ca.gov/centralvalley (accessed on 2 August 2022).
- Alpine, A.E.; Cloern, J.E. Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnol. Oceanog. 1992, 37, 946–955. [Google Scholar] [CrossRef]
- Cloern, J.E.; Dufford, R. Phytoplankton community ecology: Principles applied in San Francisco Bay. Mar. Ecol. Prog. Ser. 2005, 285, 1–28. [Google Scholar] [CrossRef]
- Cole, B.E.; Cloern, J.E. Significance of biomass and light availability to phytoplankton productivity in San Francisco Bay. Mar. Ecol. Prog. Ser. 1984, 17, 15–24. [Google Scholar] [CrossRef]
- Bever, A.J.; MacWilliams, M.L. Simulating sediment transport processes in San Pablo Bay using coupled hydrodynamic, wave, and sediment transport models. Mar. Geol. 2013, 345, 235–253. [Google Scholar] [CrossRef]
- Schoellhamer, D.H.; Wright, S.A.; Drexler, J. A conceptual model of sedimentation in the Sacramento–San Joaquin Delta. San Franc. Estuary Watershed Sci. 2012, 10. Available online: https://escholarship.org/uc/item/2652z8sq (accessed on 12 May 2022). [CrossRef][Green Version]
- Kimmerer, W.J.; Thompson, J.K. Phytoplankton growth balanced by clam and zooplankton grazing and net transport into the low-salinity zone of the San Francisco Estuary. Est. Coasts 2014, 37, 1202–1218. [Google Scholar] [CrossRef]
- Lucas, L.V.; Cloern, J.E.; Thompson, J.K.; Stacey, M.T.; Koseff, J.R. Bivalve grazing can shape phytoplankton communities. Front. Mar. Sci. 2016, 3, 14. [Google Scholar] [CrossRef]
- Huber, M. Potamocorbula amurensis (Schrenck, 1981). In World Marine Mollusca Database; Bouchet, P., Gofas, S., Rosenberg, G., Eds.; 2010; Available online: http:www.marinespecies.org/aphia.php?p=taxdetails&id=397175 (accessed on 13 May 2022).
- Lucas, L.V.; Thompson, J.K.; Brown, L.R. Why are diverse relationships observed between phytoplankton biomass and transport time? Limnol. Oceanogr. 2009, 54, 381–390. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, F.; Dugdale, R.; Liu, Q.; Xue, H.; Wilkerson, F.; Chao, Y.; Zhang, Y.; Zhang, H. The interannual variabilities of chlorophyll and nutrients in San Francisco Bay: A modeling study. Ocean Dyn. 2020, 70, 1169–1186. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Phytoplankton stoichiometry. Ecol. Res. 2008, 23, 479–485. [Google Scholar] [CrossRef]
- Hillebrand, H.; Steinert, G.; Boersma, M.; Malzahn, A.; Meunier, C.L.; Plum, C.; Ptacnik, R. Goldman revisited: Faster-growing phytoplankton has lower N:P and lower stoichiometric flexibility. Limnol. Oceanogr. 2013, 58, 2076–2088. [Google Scholar] [CrossRef]
- Atwater, B.F.; Conard, S.G.; Dowden, J.N.; Hedel, C.W.; MacDonald, R.L.; Savage, W. History, landforms, and vegetation of the estuary’s tidal marshes. In San Francisco Bay: The Urbanized Estuary; Conomos, T.J., Ed.; Pacific Division of the American Association for the Advancement of Science: San Francisco, CA, USA, 1979; pp. 347–385. [Google Scholar]
- Mueller-Solger, A.B.; Jassby, A.D.; Müller-Navarra, D. Nutritional value of particulate organic matter for zooplankton (Daphnia) in a tidal freshwater system (Sacramento-San Joaquin River Delta, USA). Limnol. Oceanogr. 2002, 47, 1468–1476. [Google Scholar] [CrossRef]
- Wilkerson, F.P.; Dugdale, R.C.; Hogue, V.E.; Marchi, A. Phytoplankton blooms and nitrogen productivity in San Francisco Bay. Est. Coasts 2006, 29, 401–416. [Google Scholar] [CrossRef]
- Solórzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Bran Luebbe, Inc. Bran Luebbe AutoAnalyzer Applications: AutoAnalyzer Method No. G-172-96 Nitrate and Nitrite in Water and Seawater; Bran Luebbe, Inc.: Buffalo Grove, IL, USA, 1999. [Google Scholar]
- Bran Luebbe, Inc. Bran Luebbe AutoAnalyzer Applications: AutoAnalyzer Method No. G-175-96 Phosphate in Water and Seawater; Bran Luebbe, Inc.: Buffalo Grove, IL, USA, 1999. [Google Scholar]
- Bran Luebbe, Inc. Bran Luebbe AutoAnalyzer Applications: AutoAnalyzer Method No. G-177-96 Silicate in Water and Seawater; Bran Luebbe, Inc.: Buffalo Grove, IL, USA, 1999. [Google Scholar]
- Arar, E.J.; Collins, G.B. In Vivo Determination of Chlorophyll a and Phaeophytin a in Marine and Freshwater Phytoplankton by Fluorescence, Method 445.0. U.S. In In Vivo Determination of Chlorophyll a and Phaeophytin a in Marine and Freshwater Phytoplankton by Fluorescence, Method 445.0. U.S; Environmental Protection Agency: Washington, DC, USA, 1997. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=309417 (accessed on 22 September 2021).
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Wright, S.W. Photosynthetic pigments in the haptophyta. In The Haptophyte Algae; Green, J.C., Leadbeater, B.S.C., Eds.; Clarendon Press: Oxford, UK, 1994; pp. 111–132. [Google Scholar]
- Jeffrey, S.W.; Vesk, M. Introduction to marine phytoplankton and their pigment signatures. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; NESCO: Paris, France, 1997; pp. 37–84. [Google Scholar]
- Platt, T.; Gallegos, C.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Kimmerer, W. Long-term changes in apparent uptake of silica in the San Francisco estuary. Limnol. Oceanogr. 2005, 50, 793–798. [Google Scholar] [CrossRef]
- Jassby, A.D.; Cloern, J.E.; Cole, B.E. Annual primary production: Patterns and mechanisms of change in a nutrient-rich tidal ecosystem. Limnol. Oceanogr. 2002, 47, 698–712. [Google Scholar] [CrossRef]
- Carlton, J.T.; Thompson, J.K.; Schemel, L.E.; Nichols, F.H. Remarkable invasion of San Francisco Bay (California, USA) by the Asian clam, Potamocorbula amurensis. 1. Invasion and dispersal. Mar. Ecol. Prog. Ser. 1990, 66, 81–94. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T. Nonindigenous Aquatic Species in a United States Estuary: A case study of the Biological Invasions of the San Francisco Bay and Delta; U.S. Fish & Wildlife Service: Washington, DC, USA, 1995. [Google Scholar]
- Cohen, A.N.; Carlton, J.T. Accelerating invasion rate in a highly invaded estuary. Science 1998, 279, 555–558. [Google Scholar] [CrossRef]
- Brown, L.R.; Baxter, R.; Castillo, G.; Conrad, L.; Culberson, S.; Erickson, G.; Feyrer, F.; Fong, S.; Gehrts, K.; Grimaldo, L.; et al. Synthesis of Studies in the Fall Low-Salinity Zone of the San Francisco Estuary, September–December 2011: U.S. Geological Survey Scientific Investigations Report 2014–5041; U.S. Geological Survey: Reston, VA, USA, 2014. [Google Scholar]
- Ruben, A.V.; Lavaud, J.; Rousseau, B.; Guglielmi, G.; Horton, P.; Etienne, A.L. The super-excess energy dissipation in diatom algae: Comparative analysis with higher plants. Photosyn. Res. 2004, 82, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Wollenweber, B.; Handley, L.L. A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New Phytol. 1992, 121, 19–32. [Google Scholar] [CrossRef]
- Flynn, K.J.; Fasham, M.J.R.; Hipkin, C.R. Modelling the interactions between ammonium and nitrate uptake in marine phytoplankton. Phil. Trans. R Soc. Ser. B 1997, 352, 1625–1645. [Google Scholar] [CrossRef]
- Vergera, J.; Berges, J.; Falkowski, P. Diel periodicity of nitrate reductase activity and protein levels in the marine diatom Thalassiosira weissflogii (Bacillariophyceae). J. Phycol. 1998, 34, 952–961. [Google Scholar] [CrossRef]
- Flynn, K.J.; Dickson, D.M.J.; Al-Amoundi, O.A. The ratio of glutamine:glutamate in microalgae: A biomarker for N-status suitable for use at natural densities. J. Plankt. Res. 1989, 11, 165–170. [Google Scholar] [CrossRef]
- Flynn, K.J.; Jones, K.J.; Raine, R.; Richard, J.; Flynn, K. Use of intracellular amino acids as an indicator of the physiological status of natural dinoflagellate populations. Mar. Ecol. Prog. Ser. 1994, 103, 175–186. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnol. Oceanogr. 1999, 44, 556–572. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Interactions between NH4+ and NO3– uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperatures. Mar. Biol. 1999, 133, 541–551. [Google Scholar] [CrossRef]
- Parker, M.S.; Armbrust, E.V. Synergistic effects of light, temperature and nitrogen source on transcription of genes for carbon and nitrogen metabolism in the centric diatom Thalassiosira pseudonana (Bacillariophyceae). J. Phycol. 2005, 41, 1142–1153. [Google Scholar] [CrossRef]
- Kamp, A.; de Beer, D.; Nitsch, J.L.; Lavik, G.; Stief, P. Diatoms respire nitrate to survive dark and anoxic conditions. Proc. Natl. Acad. Sci. USA 2011, 108, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Poll. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Finlay, K.; Patoine, A.; Donald, D.B.; Bogard, M.; Leavitt, P.R. Experimental evidence that pollution with urea can degrade water quality in phosphorus-rich lakes of the Northern Great Plains. Limnol. Oceanogr. 2010, 55, 1213–1230. [Google Scholar] [CrossRef]
- Donald, D.B.; Bogard, M.J.; Finlay, K.; Bunting, L.; Leavitt, P.R. Phytoplankton-specific response to enrichment of phosphorus-rich surface waters with ammonium, nitrate, and urea. PLoS ONE 2013, 8, e53277. [Google Scholar] [CrossRef]
- Jensen, J.P.; Jeppesen, E.; Olrik, K.; Kristensen, P. Impact of nutrients and physical factors on the shift from cyanobacteria to chlorophyte dominance in shallow Danish lakes. Can. J. Fish. Aquat. Sci. 1994, 51, 1692–1699. [Google Scholar] [CrossRef]
- Glibert, P.M.; Berg, G.M.B. Nitrogen form, fate and phytoplankton composition. In Experimental Ecosystems and Scale: Tools for Understanding and Managing Coastal Ecosystems; Kennedy, V.S., Kemp, W.M., Peterson, J.E., Dennison, W.C., Eds.; Springer: New York, NY, USA, 2009; pp. 183–189. [Google Scholar]
- Domingues, R.B.; Barbosa, A.B.; Sommer, U.; Galvão, H.M. Ammonium, nitrate and phytoplankton interactions in a freshwater tidal estuarine zone: Potential effects of cultural eutrophication. Aquat. Sci. 2011, 73, 331–343. [Google Scholar] [CrossRef]
- Shangguan, Y.; Glibert, P.M.; Alexander, J.A.; Madden, C.J.; Murasko, S. Nutrients and phytoplankton in semi-enclosed lagoon systems in Florida Bay and their responses to changes in flow from Everglades restoration. Limnol Oceanogr. 2017, 62, S327–S347. [Google Scholar] [CrossRef]
- Shangguan, Y.; Glibert, P.M.; Alexander, J.A.; Madden, C.J.; Murasko, S. Phytoplankton community response to changing nutrients in Florida Bay: Results of mesocosm studies. J. Exp. Mar. Biol. Ecol. 2017, 494, 38–53. [Google Scholar] [CrossRef]
- Fawcett, S.E.; Ward, B.B. Phytoplankton succession and nitrogen utilization during the development of an upwelling bloom. Mar. Ecol. Progr. Ser. 2011, 428, 13–31. [Google Scholar] [CrossRef]
- Donald, D.B.; Bogard, M.J.; Finlay, K.; Leavitt, P.R. Comparative effects of urea, ammonium, and nitrate on phytoplankton abundance, community composition, and toxicity in hypereutrophic freshwaters. Limnol. Oceanogr. 2011, 56, 2161–2175. [Google Scholar] [CrossRef]
- Dai, G.-Z.; Shang, J.-L.; Qiu, B.-S. Ammonia may play an important role in the succession of cyanobacterial blooms and the distribution of common algal species in shallow freshwater lakes. Glob. Change Biol. 2012, 18, 1571–1581. [Google Scholar] [CrossRef]
- Vogt, R.J.; Rusak, J.A.; Patoine, A.; Leavitt, P.R. Differential effects of energy and mass influx on the landscape synchrony of lake ecosystems. Ecology 2011, 92, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, P.R.; Brock, C.S.; Ebel, C.; Patoine, A. Landscape-scale effects of urban nitrogen on a chain of freshwater lakes in central North America. Limnol. Oceanogr. 2006, 51, 2262–2277. [Google Scholar] [CrossRef]
- Patoine, A.; Graham, M.D.; Leavitt, P.R. Spatial variation of nitrogen fixation in lakes of the northern Great Plains. Limnol. Oceanogr. 2006, 51, 1665–1677. [Google Scholar] [CrossRef]


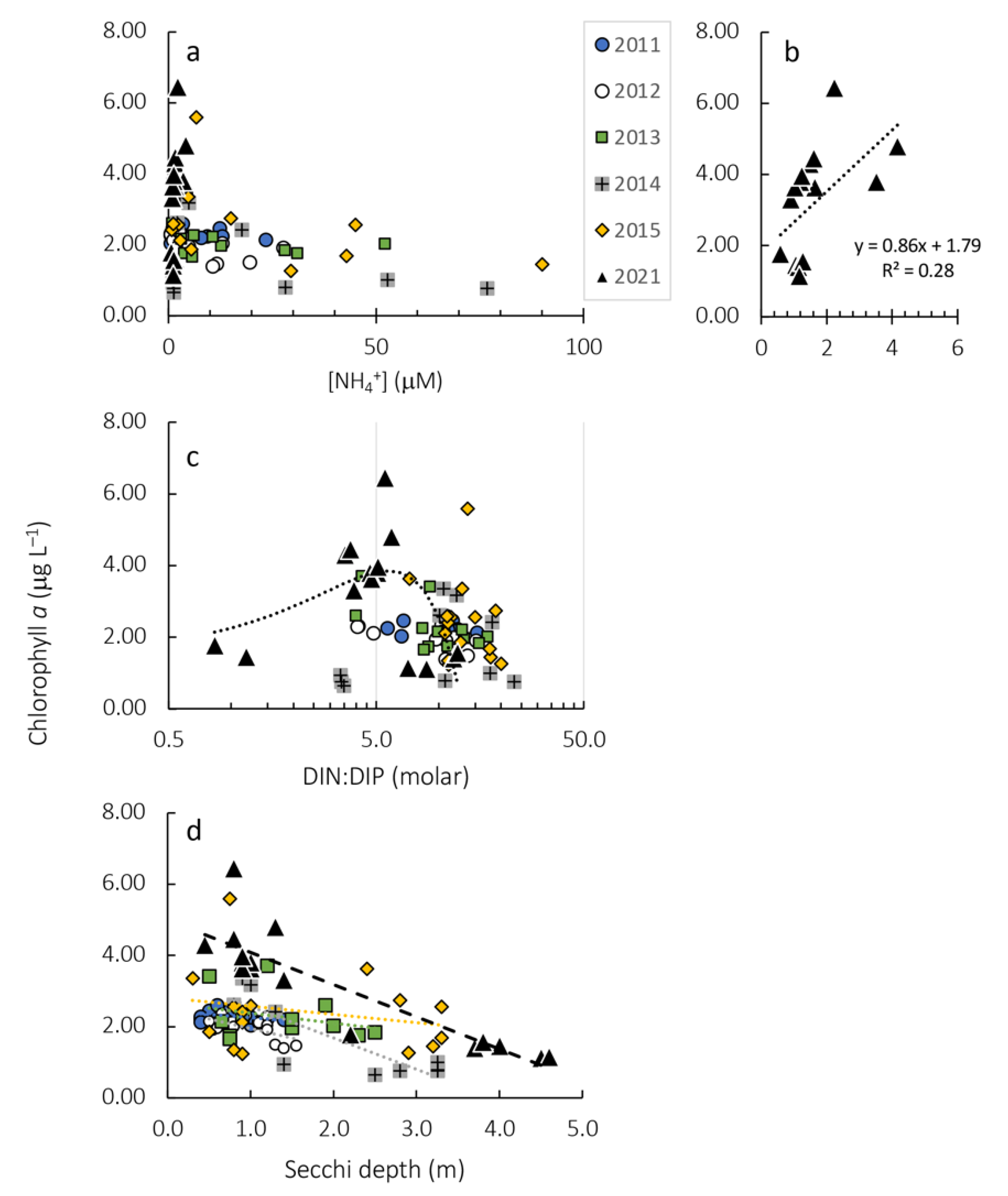
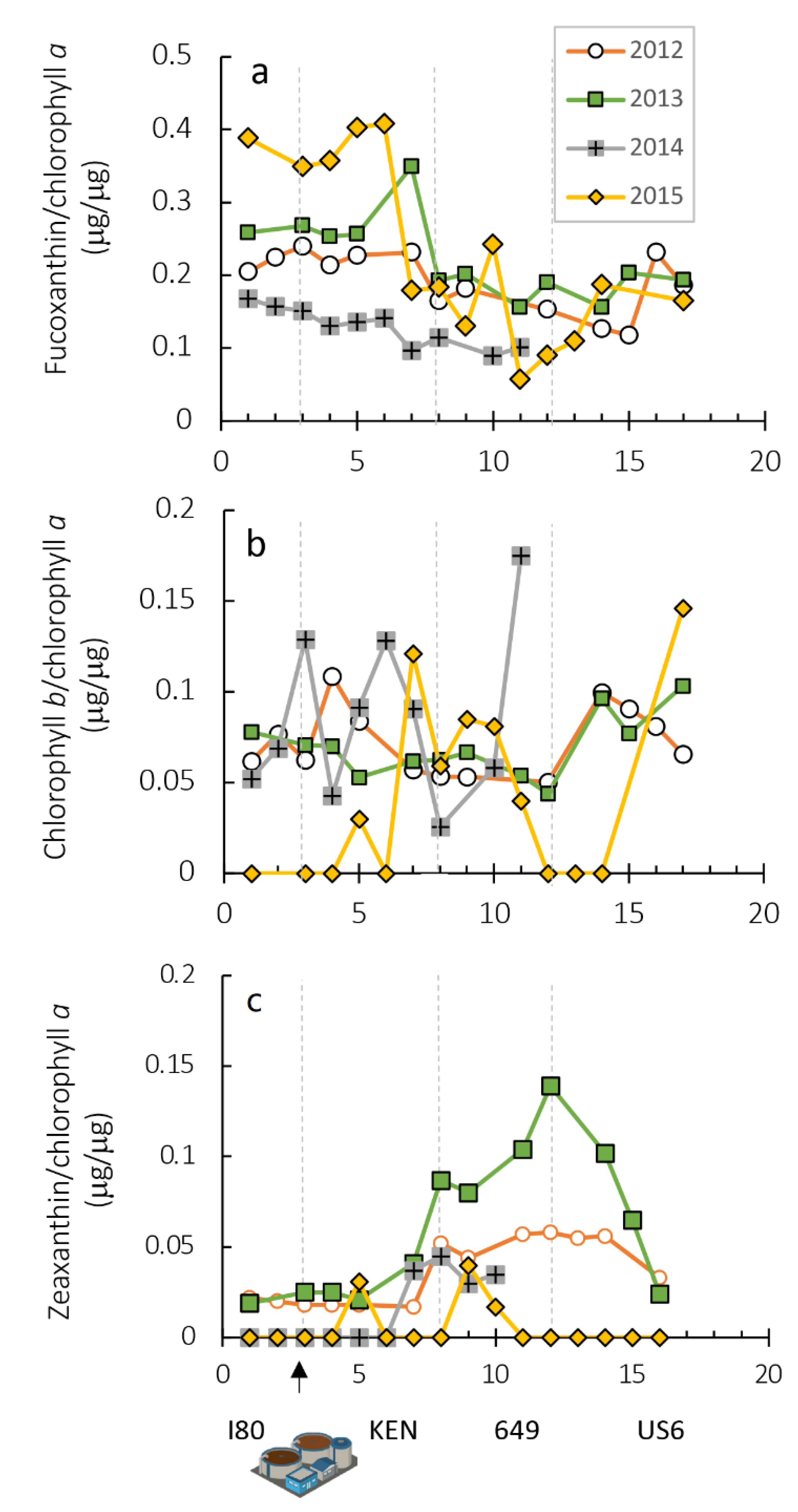
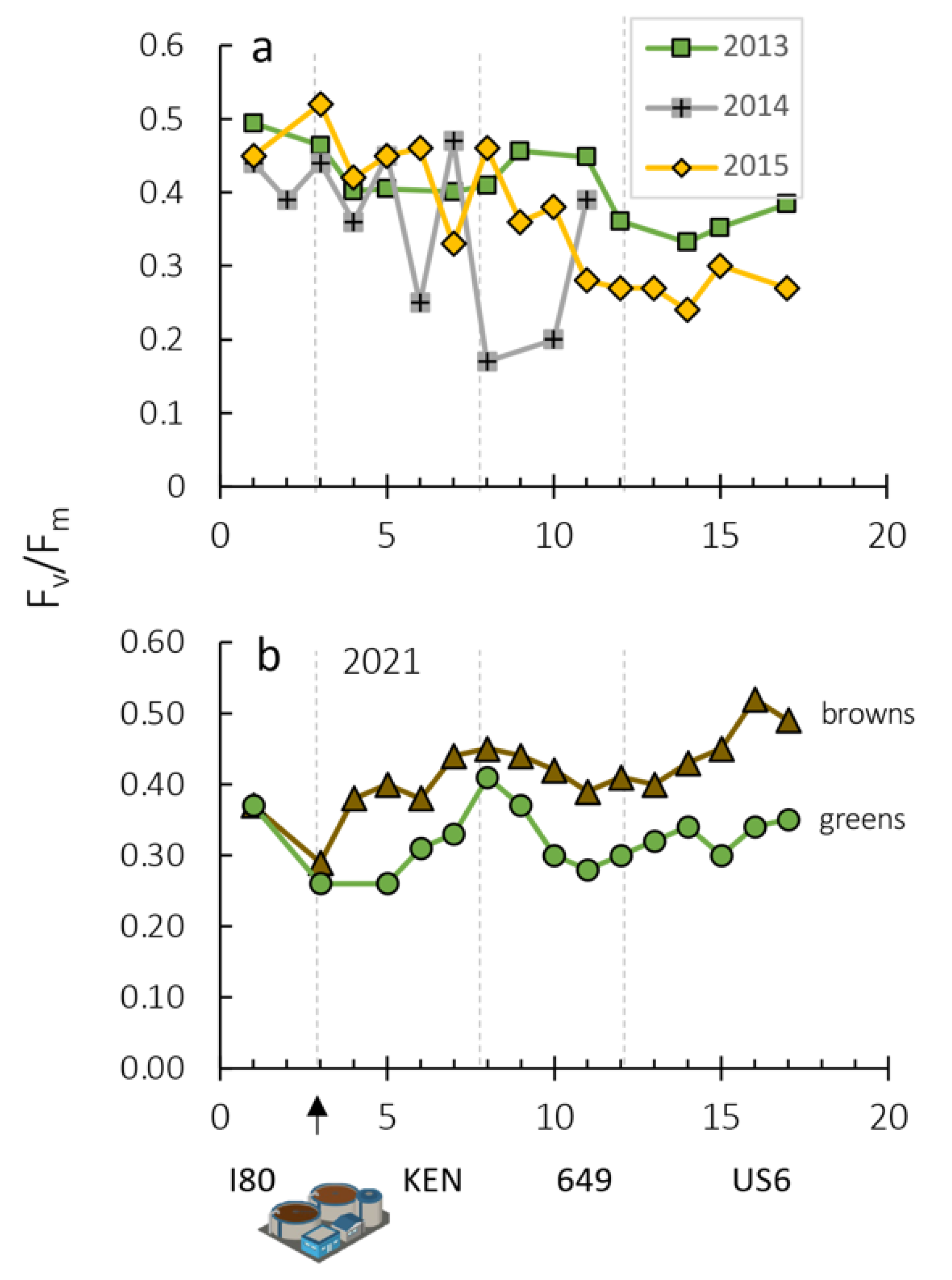
| Year | Correlation | R2 |
|---|---|---|
| 2011 | Y = −0.18x + 2.44 | 0.11 |
| 2012 | Y = −0.54x + 2.49 | 0.36 |
| 2013 | Y = −0.28x + 2.66 | 0.08 |
| 2014 | Y = −0.88x + 3.45 | 0.71 |
| 2015 | Y = −0.23x + 2.80 | 0.05 |
| 2021 | Y = −0.90x + 4.99 | 0.77 |
| Year | Correlation | R2 |
|---|---|---|
| 2011 | Y = −8.82x + 364 | 0.98 |
| 2012 | Y = −16.95x + 453 | 0.84 |
| 2013 | Y = −9.99x + 426 | 0.39 |
| 2014 | Y = −10.76x +308 | 0.84 |
| 2015 | Y = −10.27x +301 | 0.79 |
| 2021 | Y = −16.26x + 367 | 0.93 |
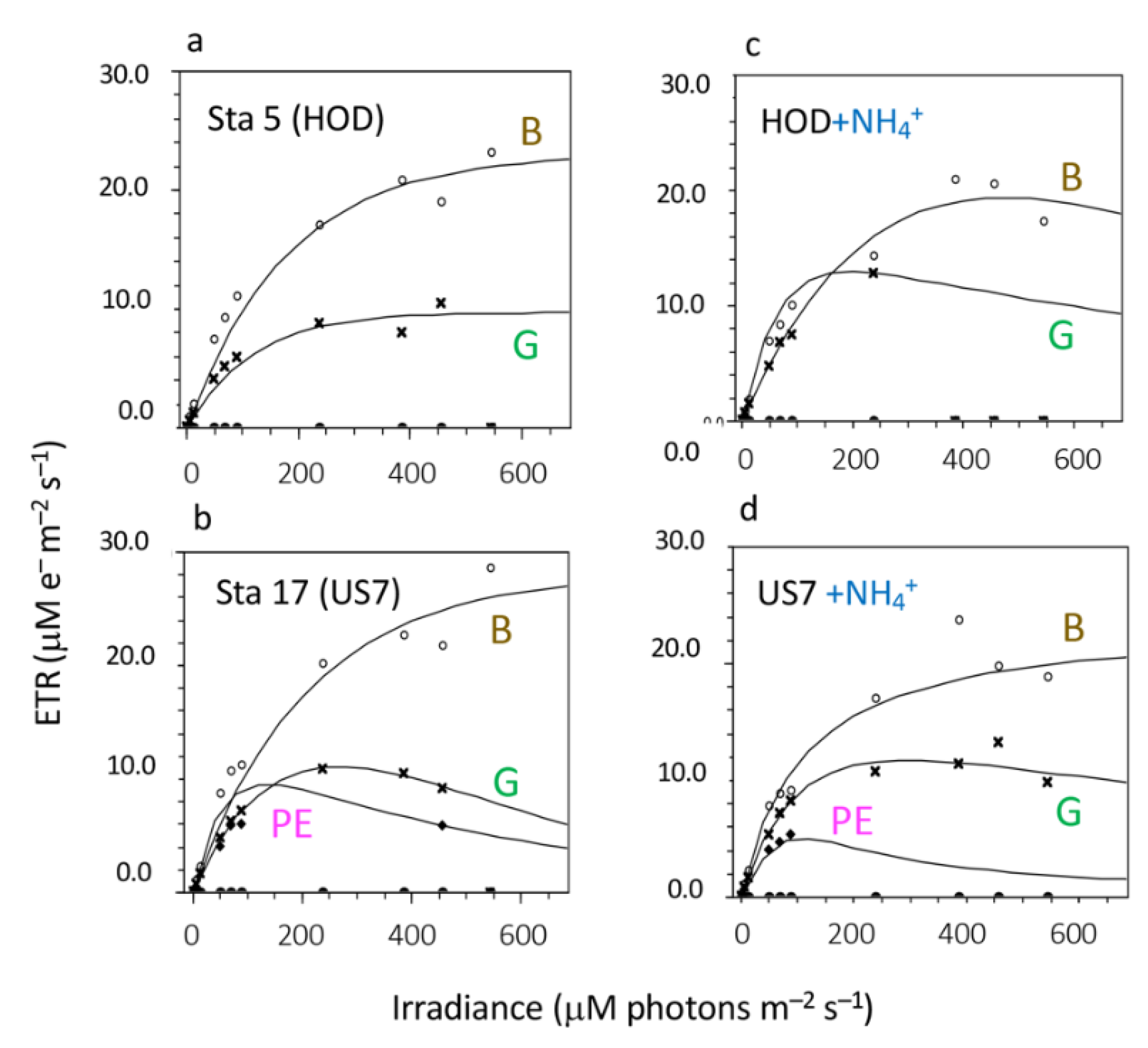
| Station | Treatment | α Brown Algae | ETRmax Brown Algae (μM e− m−2 s−1) | α Green Algae | ETRmax Green Algae (μM e− m−2 s−1) | Ambient NH4+ (μM) |
|---|---|---|---|---|---|---|
| 1 (I80) | ambient | 0.160 | 26.4 | 1.761 | 18.3 | 0.59 |
| 3 (GRC) | ambient | 0.158 | 17.5 | 0.141 | 13.4 | 1.06 |
| 4 (RM44) | ambient | 0.222 | 25.0 | 0.124 | 18.4 | 1.14 |
| +15 μM NH4+ | 0.198 | 23.9 | 0.175 | 19.7 | ||
| +30 μM NH4+ | 0.197 | 22.1 | 0.270 | 18.1 | ||
| +60 μM NH4+ | 0.185 | 21.5 | 0.155 | 17.4 | ||
| 5 (HOD) | ambient | 0.129 | 23.0 | 0.084 | 9.7 | 1.15 |
| +30 μM NH4+ | 0.111 | 19.4 | 0.160 | 8.0 | ||
| 6 (KEN) | ambient | 0.192 | 21.4 | 0.107 | 13.5 | 1.27 |
| 7 (ISL) | ambient | 0.115 | 15.5 | 0.138 | 11.5 | 1.16 |
| 8 (657) | ambient | 0.199 | 29.7 | 0.177 | 20.0 | 0.91 |
| +30 μM NH4+ | 0.184 | 30.4 | 0.163 | 18.4 | ||
| 9 (655) | ambient | 0.183 | 27.8 | 0.136 | 17.2 | 1.48 |
| 10 (653) | ambient | 0.175 | 22.6 | 0.123 | 14.3 | 1.62 |
| 11 (649) | ambient | 0.142 | 18.4 | 0.107 | 11.2 | 3.52 |
| 12 (US 2) | ambient | 0.142 | 17.7 | 0.105 | 13.3 | 1.65 |
| +30 μM NH4+ | 0.085 | 13.5 | 0.148 | 10.5 | ||
| 13 (US 3) | ambient | 0.114 | 15.4 | 0.146 | 14.4 | 1.20 |
| 14 (US 4) | ambient | 0.111 | 15.6 | 0.166 | 13.6 | 1.03 |
| 15 (US 5) | ambient | 0.116 | 14.8 | 0.175 | 10.8 | 1.24 |
| 16 (US 6) | ambient | 0.145 | 20.8 | 0.099 | 10.9 | 2.23 |
| 17 (US 7) | ambient | 0.134 | 28.1 | 0.112 | 11.1 | 4.17 |
| +30 μM NH4+ | 0.142 | 20.9 | 0.090 | 12.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Parker, A.E. Ecosystem Recovery in Progress? Initial Nutrient and Phytoplankton Response to Nitrogen Reduction from Sewage Treatment Upgrade in the San Francisco Bay Delta. Nitrogen 2022, 3, 569-591. https://doi.org/10.3390/nitrogen3040037
Glibert PM, Wilkerson FP, Dugdale RC, Parker AE. Ecosystem Recovery in Progress? Initial Nutrient and Phytoplankton Response to Nitrogen Reduction from Sewage Treatment Upgrade in the San Francisco Bay Delta. Nitrogen. 2022; 3(4):569-591. https://doi.org/10.3390/nitrogen3040037
Chicago/Turabian StyleGlibert, Patricia M., Frances P. Wilkerson, Richard C. Dugdale, and Alexander E. Parker. 2022. "Ecosystem Recovery in Progress? Initial Nutrient and Phytoplankton Response to Nitrogen Reduction from Sewage Treatment Upgrade in the San Francisco Bay Delta" Nitrogen 3, no. 4: 569-591. https://doi.org/10.3390/nitrogen3040037
APA StyleGlibert, P. M., Wilkerson, F. P., Dugdale, R. C., & Parker, A. E. (2022). Ecosystem Recovery in Progress? Initial Nutrient and Phytoplankton Response to Nitrogen Reduction from Sewage Treatment Upgrade in the San Francisco Bay Delta. Nitrogen, 3(4), 569-591. https://doi.org/10.3390/nitrogen3040037







