Abstract
Maize (Zea mays L.) is a crop widely cultivated in the state of São Paulo, and the sustainable management of nitrogen (N) nutrition is crucial to improving productivity and the environment, which calls for a reliable means of predicting potentially available soil N. A study was undertaken to evaluate and compare biological and chemical indices of potential N availability for a diverse set of 17 soils collected in the northwest region of São Paulo state. For this purpose, mineralization assays were performed at three distinct temperatures, and chemical assessments were carried out using the Illinois Soil Nitrogen Test (ISNT) and by fractionation of hydrolysable soil N. In addition, a greenhouse experiment was conducted to determine dry matter and N accumulation in the aboveground parts of maize plants. Potentially available N estimated by the incubation methods increased with increasing temperature and was strongly correlated with N uptake (r = 0.90). Hydrolysable N fractions varied widely among the soils studied and were more variable for amino sugar N than for other fractions. Potentially available N estimated by the ISNT was highly correlated with hydrolysable amino acid N and amino sugar N (r = 0.95–0.96) and also with plant dry matter accumulation (r = 0.82) and N uptake (r = 0.93). The ISNT has potential to improve fertilizer N recommendations for maize production in Brazil, provided that the test values are interpreted relative to an appropriate calibration database, planting density, and other factors affecting crop N requirement.
1. Introduction
Nitrogen (N) fertilizer recommendations in Brazil are typically based on expected crop yield, without regard to soil variations in the indigenous supply of plant-available N [1]. This leads to inefficient use of N fertilizers, enhancing the risk that underfertilization may limit yields or that overfertilization may promote N loss by leaching, with negative consequences for the environment [2]. Such difficulties can be reduced by soil testing to estimate potentially available N and thereby improve the accuracy of fertilizer N recommendations [3].
Maize (Zea mays L.) is a major crop in the state of São Paulo that is typically grown with intensive N fertilization to increase yield, although there are reports that N response can vary greatly [4,5,6]. Considering this variation, a reliable and convenient index of soil N availability would be highly useful for site-specific optimization of the fertilizer N rate. Many such indices have been proposed, but none has been widely adopted for improving fertilizer N recommendations.
Incubation assays have long been used to estimate soil N availability in humid climates that promote active microbial N cycling, the most common techniques involving direct measurement of mineral N production under aerobic or anaerobic conditions [7,8,9,10]. Aerobic assays generally allow an accurate estimation of potentially mineralizable N in tropical regions such as Brazil [11,12]; however, underestimation can occur with temperate soils due to incomplete recovery of exchangeable NH4+-N by leaching with a dilute extractant such as 0.01 M CaCl2 [13]. A more serious limitation arises from the need for a prolonged period of incubation with precise regulation of soil temperature and moisture [14], which seriously compromises the predictive value of incubation assays in production agriculture.
Nevertheless, official policy in Brazil stipulates that the incubation approach be used to determine maximum application rates for the organic amendment of cropland, with the aim of avoiding environmental damage from excessive N inputs [15]. A standardized protocol is prescribed to facilitate the comparison of independent datasets, which according to current regulations in the state of São Paulo involves aerobic incubation for 18 weeks at a constant temperature of 25–28 °C [15]. Unfortunately, the results are unlikely to represent field conditions, because (1) microbial N cycling is qualitatively and quantitatively altered when a growing plant is not present to compete for mineral N, influence soil drying and aeration, and supply root-derived C; (2) static incubations lack the natural temperature and moisture fluctuations that affect microbial N dynamics in the field; and (3) sieved surface soil is typically used, without regard to plant-available N contributed by subsurface horizons or the artifacts caused by sieving or other sample processing.
In addition to incubation assays, numerous chemical extraction methods have been proposed for the estimation of potentially mineralizable N in soils [4,9,16]. These methods have the major advantage of being much less time-consuming than incubation and thus more suitable for routine soil testing, but most have an empirical basis and have not been widely used because of low correlations with crop N uptake and/or mineral N production during soil incubations.
A more rational approach to the chemical estimation of potentially mineralizable soil N utilizes acid hydrolysis [17] to identify a specific N fraction that reflects crop responsiveness to N fertilization. Numerous attempts have been made to find such a fraction, but for years progress was prevented by fundamental flaws in the methods for the fractionation of hydrolysable soil N. After these flaws had been eliminated [18], concentrations of amino sugar N were found to be invariably higher for soils where maize was nonresponsive to N fertilization than for those showing significant fertilizer N response, whereas there was no consistent difference in the concentrations of total hydrolysable N, hydrolysable NH4+-N, or amino acid N [19]. Incubation assays performed as part of the latter study showed that mineral N production was much greater by nonresponsive than responsive soils and that a net decrease occurred in amino sugar N but not amino acid N. Subsequent work by Khan et al. [20] simplified the process for the estimation of amino sugar N, such that alkaline diffusions are performed on the soil itself, without the need for acid hydrolysis. When the resulting technique, commonly known as the Illinois Soil Nitrogen Test or ISNT, was evaluated using data from 102 on-farm N-response trials, test values were highly significant for predicting grain yields with and without optimal N fertilization, fertilizer N uptake efficiency (FNUE), and economically optimum N rate (EONR) [21]. In further evaluations with maize, the ISNT has been effective for predicting fertilizer N response in some cases [22,23,24,25,26,27] but not in others [28,29,30,31,32]. The negative findings can be attributed, at least in part, to variability in factors that would necessarily affect ISNT calibration, e.g., soil pH, drainage class, climatic conditions, crop rotation, residue C input, and plant population.
In Brazil, ISNT levels were significantly related to dry matter production and N uptake by maize in a greenhouse experiment, although somewhat higher correlations were obtained for N extracted by heating with water or 0.01 M CaCl2 [16]. A significant relationship was also found in a recent study by Bettiol et al. [4] that evaluated the ISNT relative to mineral N production during incubation assays; however, Mariano et al. [33] were unable to predict sugarcane (Saccharum spp.) N requirement using the ISNT or 14 other chemical procedures for estimating soil N availability in conjunction with 21 N-response trials conducted at rainfed sites between 2006 and 2013. Climatic variability was probably a factor in the latter case, as the ISNT showed good potential when conditions allowed optimal sugarcane growth in five of these trials [3]. Another factor could have been asymbiotic N2 fixation, which has long been known to occur in the rhizosphere of sugarcane [34,35] and would be most effective in soils having limited capacity for mineral N production [36].
The work reported herein was undertaken to test the hypothesis that fertilizer N management for Brazilian maize production can be improved by assessing potentially available N. With this aim, incubation assays were performed, with and without diurnal temperature cycling, on 17 diverse soils from the northwest region of the state of São Paulo that were also analyzed by the ISNT and by fractionating acid hydrolysable N. The predictive value of each parameter was determined from correlations with dry matter production and N accumulation measured when the soils studied were used to grow maize without N fertilization in a pot experiment.
2. Materials and Methods
2.1. Soils
The Brazilian soils used were surface (0–20 cm) samples of nine Oxisols, seven Ultisols, and an Entisol obtained from cropped or uncropped sites in the state of São Paulo (Table 1). Sampling sites were chosen in order to include the most common soil types within this region, and to encompass a wide range in organic matter, texture, and other physicochemical properties (Table 2). After collecting a sample that totaled approximately 50 dm3 for each site, the soil was dried at room temperature (25–30 °C), crushed to pass through a 4 mm sieve, thoroughly homogenized, and then analyzed for chemical attributes [37] and particle size [38]. For bulk density, the soil mass was accommodated in a 5 dm3 pot and then weighed.

Table 1.
Taxonomy, vegetation, and collection sites for soils studied.

Table 2.
Physicochemical properties and fertility parameters for soils studied.
2.2. Mineralization Assays
Aerobic incubations to determine potentially mineralizable N (N0) followed the method of Stanford and Smith [40], which was modified with the use of a shorter incubation period and temperatures lower than the 35 °C adopted in their work. In addition to using a fixed temperature of 28 °C as specified by CETESB [15], each soil was incubated (3 replicates) with diurnal programming to simulate mean daily variations in soil temperature during summer (23 °C for 10 h and 28 °C for 14 h) or winter (18 °C for 10 h and 23 °C for 14 h) as previously documented for the vicinity of Jaboticabal by Volpe et al. [41].
Before initiating mineralization assays, soil reaction was eliminated as a variable by liming soils 3–6 and 8–17 to pH 5.5. For this purpose, preliminary trials were conducted to determine the quantity of lime needed for each of these soils, in which pH was measured after a 10-d incubation with different additions of a 2:1 mixture of CaCO3 and Mg5(CO3)4(OH)2, and a regression equation was developed to predict soil pH as a function of liming rate. Using the equations thereby developed, a subsample (approximately 0.5 dm3) of each soil at pH < 5.5 was thoroughly mixed with the appropriate amount of liming material, moistened to 60% water-holding capacity (WHC), and then incubated for 10 d at room temperature. The 14 pH-adjusted soils, and also 0.5 dm3 subsamples of soils 1, 2, and 7 that were moistened and incubated but not limed, were allowed to dry at room temperature, sieved to < 4 mm, and stored (4 °C) until being used for mineralization assays.
For use in performing these assays, a soil mass equivalent to 10 cm3 was mixed with 15 cm3 of acid-washed quartz sand, and the mixture was placed inside a glass leaching tube (20 cm long, 3 cm inside dia., 3.5 cm outside dia.) having a supporting disk with holes for drainage, between a 3 cm thick layer of glass wool at the bottom and a 1 cm layer at the top. Each of the three temperature regimes noted previously involved a total of 51 tubes, which were transferred to a Bio-Oxygen Demand (BOD) chamber for incubation in the dark. After 0, 3, 7, 14, 21, 28, 49, 70, and 112 d, the tubes were removed from the chamber, leaching was carried out using 100 mL of 0.01 M CaCl2, and leachates were analyzed for NH4+-N and (NO3− + NO2−)-N by steam distillation with MgO and Devarda’s alloy [42]. Before resuming incubation, samples were treated with 25 mL of a N-free nutrient solution containing CaSO4∙2H2O (0.002 M), MgSO4 (0.002 M), and KH2PO4 (0.005 M), which was drained by applying a vacuum of 40 kPa [11]. To minimize water losses while maintaining aeration, polyethylene film was applied to the top of the leaching tubes and punctured by forming 10 holes with a syringe needle.
Soil-specific data were summed to obtain (NH4+ + NO3− + NO2−)-N for each sampling date and then fitted to the exponential form of the first-order kinetic equation proposed by Stanford and Smith [40], Nm = N0(1 − e-kt), using the generalized reduced gradient algorithm of Lasdon et al. [43]. In this equation, Nm is the N mineralized at time t (mg kg−1), k is the mineralization rate constant (d−1), and t is the incubation time (d).
2.3. Soil Nitrogen Analyses
To prepare hydrolysates for soil N fractionation, duplicate 5-g soil samples were treated with 20 mL of 6 M HCl and two drops of octyl alcohol in a 50-mL bulb having a 24/40 ground glass joint for attachment to a 300 mm Liebig condenser, and heating was performed under reflux at 115 °C for 12 h. After vacuum filtration using Whatman no. 50 filter paper (GE Healthcare, Maidstone, UK), replicate hydrolysates were combined and neutralized by addition of NaOH [17]. The diffusion techniques described by Mulvaney and Khan [18] were performed (4 replicates) to determine total hydrolysable N, hydrolysable NH4+-N, (NH4+ + amino sugar)-N, and amino acid N, with titrimetric determinations of the diffused N being carried out using standard 0.01 M H2SO4 supplied by a Metrohm Model 678 EP/KF Processor equipped with a Model 665 Dosimat (Metrohm, Herisau, Switzerland) and a flat-surface electrode (Model 13-620-289; Fisher Scientific, Pittsburgh, Pennsylvania). Amino sugar N was calculated as (NH4+ + amino sugar)-N − NH4+-N, and hydrolysable unidentified N (HUN) was calculated as total hydrolysable N − (NH4+ + amino sugar)-N − amino acid N. Acid-insoluble N was determined as the difference between total soil (Kjeldahl) N and total hydrolysable N.
The alkaline diffusion technique described by Khan et al. [20] and known as the ISNT was used to estimate potentially available N for each of the 17 soils studied in our work. For this purpose, 1 g of dried soil was treated (4 replicates) with 10 mL of 2 M NaOH in a 473-mL wide-mouth Mason jar; the jar was sealed by attaching a lid with a 60 mm (dia.) Petri dish containing 5 mL of H3BO3-indicator solution (40 g H3BO3 L−1), and heating was carried out for 5 h on an electric griddle preadjusted to provide a temperature of 50 °C. The quantity of NH3-N collected was determined by titrating the H3BO3 solution as described previously.
2.4. Greenhouse Study
After correcting soil acidity by the aforementioned addition of CaCO3 and Mg5(CO3)4(OH)2 upscaled for 20 dm3 of material, the 17 soils collected for the present project were used in a pot experiment involving a completely randomized design with four replicates. For this purpose, soil samples (4.5 dm3) were weighed into 5-dm3 pots that were transferred to a greenhouse, and each sample was treated with a nutrient solution supplying 80 (<350 g clay kg−1) or 120 (≥350 g clay kg−1) mg P dm−3, 20 mg S dm−3, 0.5 mg B dm−3, 1.0 mg Cu dm−3, 1.5 mg Zn dm−3, and 0.02 mg Mo dm−3. This solution was prepared using reagent-grade KH2PO4, MgSO4∙7H2O, H3BO3, CuSO4∙5H2O, ZnSO4∙7H2O, and (NH4)6(Mo7O24)∙4H2O, and a separate KCl solution was also prepared that was applied to increase the soil’s exchangeable K content to 5 mmolc dm−3. After fertilization, sufficient deionized water was applied to bring the soil moisture content to 60% WHC, which was maintained during a 7-d incubation at ambient temperature.
The incubated soil samples were each planted with 10 maize seeds (cv DKB 390 PRO), which was followed after 7 d by thinning to 5 plants per pot. Optimal growing conditions were maintained by daily watering to 60% of the soil’s WHC during a period when air temperature averaged 24.6 °C with a mean photoperiod of 12.26 h and 73% relative humidity. As of 38 d after planting, N deficiency symptoms were evident for all soils, and plants were harvested by cutting the stem just above the soil surface. The shoots were washed with deionized water and transferred to a forced-air oven at 65–70 °C for drying to constant mass, which was recorded as dry matter (DM) production. The dried plant samples were ground to < 0.5 mm using a Model 4 Wiley mill (Thomas Scientific, Swedesboro, New Jersey), and N concentration was determined by semimicro Kjeldahl digestion followed by steam distillation of the digests with NaOH [44]. Nitrogen uptake by the aboveground biomass was calculated as DM × N concentration [45].
2.5. Statistical Analysis
All data were evaluated for normality of residues using the Shapiro–Wilk’s test, and for homoscedasticity of variances by means of Levene’s test. The agreement between estimated and observed values obtained in mineralization assays was evaluated by analysis of variance (ANOVA) and from the coefficient of determination determined by least-squares regression, while ANOVA was also performed to compare soil- and temperature-specific N0 values and soil-specific data collected for hydrolysable N fractions, potentially mineralizable N estimated with the ISNT, and plant growth and N uptake in the pot experiment. Least significant differences were calculated at p < 0.01 for comparing soils with respect to hydrolysable N fractions, the ISNT, and plant growth and N uptake. Correlation analysis was utilized to explore relationships among the parameters studied.
3. Results
Although the soils studied were obtained from sites under very different land use (Table 1) and ranged widely in OM (13–94 g kg−1) and clay (70–700 g kg−1) contents as well as other properties (Table 2), Table 3 shows that a good fit was invariably obtained in modeling N mineralization data using the first-order kinetic model of Stanford and Smith [40], with 0.97 to 0.99 as the coefficient of determination. Given the wide range in soil properties, substantial differences were expected in mineralization capacity. This was indeed observed, as values of N0 ranged from 24 to 232 mg kg−1 and depended on incubation temperature, decreasing in the order: 23/28 °C > 28 °C > 18/23 °C. As shown by Table 3, the three temperature regimes followed the opposite order in comparing mineralization rate constants (k values).

Table 3.
Parameters obtained in fitting N mineralization data to a first-order kinetic model after triplicate incubations of 17 soils at three temperature regimes a.
For a diverse set of soils with major OM differences, there must also be extensive variation in N content. Table 4 confirms this for the soils studied, as the range was 132% larger than the mean for total N, 61% larger for hydrolysable NH4+-N, 196% larger for amino sugar N, 94% larger for amino acid N, 149% larger for HUN, and 191% larger for ISNT-N. Total recoveries by acid hydrolysis ranged from 55 to 80% of Kjeldahl N, as the distribution of different N fractions varied according to soil texture. Acid-insoluble N tended to be most abundant and HUN least abundant for the clays (soils 1–3) and sandy clays (soils 6, 9, and 13) studied, while for coarser-textured soils (≤300 g clay kg−1), the predominant trend was for amino acid N to be most abundant and amino sugar N least abundant. The three clay-textured soils were significantly higher than all the others in every hydrolysable N fraction except NH4+-N and also in total Kjeldahl N and ISNT-N. In contrast, N concentrations were more variable for the medium- and coarse-textured soils, and in some cases, higher for a loamy sand than for a sandy loam, a sandy clay loam, or a sandy clay.

Table 4.
Concentrations (mg kg−1) of hydrolysable N and potentially available N estimated by the Illinois Soil Nitrogen Test (ISNT) for the soils studied. Data reported as a mean ± standard deviation (4 replicates).
Given the wide range that existed in soil concentrations of indigenous N, major differences were expected among the soils studied when maize was grown with no input of fertilizer N. This expectation is substantiated by Table 5, which shows that numerous significant differences occurred in dry matter production, plant N concentration, and plant N uptake. The latter parameter is the most useful for evaluating the biological and chemical indices of soil N availability presented in Table 3 and Table 4 and was highest for the clay-textured soil having the most OM (soil 1) and lowest for the loamy sand with the least OM (soil 17) and a sandy clay loam cropped to sugarcane (soil 14).

Table 5.
Dry matter yield, N concentration, and N uptake after growing maize in a 38 d greenhouse experiment using the soils studied. Data reported for shoots only, as a mean ± standard deviation (4 replicates).
Table 6 shows correlation coefficients for relating soil- and plant-based parameters relevant to soil N availability. Correlations were somewhat stronger for N uptake than for dry matter production, but in both cases, there were significant relationships with all of the soil correlates examined except for clay content. The strongest correlations of N uptake were obtained with ISNT-N, amino acid N, and N0 (18/23 °C) (Figure 1), while the latter parameter was correlated more strongly with these same two N fractions than with total N or the other N fractions studied. As might be expected for nonindependent variables, all of these fractions were significantly correlated with both total N and organic matter.

Table 6.
Correlations among maize growth and N uptake, soil properties, potentially mineralizable N estimated by incubation (N0) or the Illinois Soil Nitrogen Test (ISNT), and hydrolysable soil N fractions.
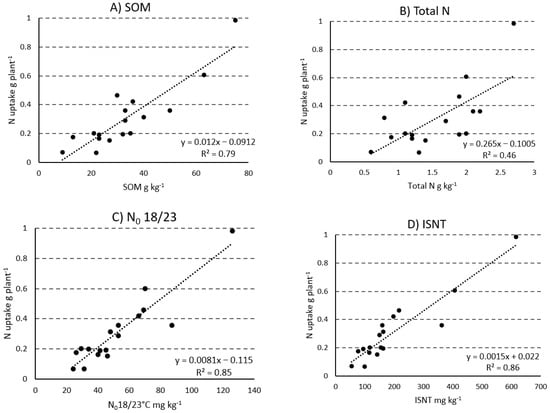
Figure 1.
Linear relationship between total N uptake by maize (Zea mays L.) and soil organic matter (A), soil total N (B), potentially available N estimated by aerobic incubation at 18/23 °C (C), and the Illinois Soil Nitrogen Test (D).
4. Discussion
The work reported was undertaken to evaluate the potential value of soil N testing for Brazilian maize production and provides encouraging evidence that fertilizer N management could be improved substantially by accounting for soil differences in the supply of indigenous N. Moreover, the findings indicate that this strategy can be successful using chemical techniques that do not require a prolonged incubation to estimate potentially mineralizable N (N0).
Despite considerable variation among N0 values estimated using the three temperature regimes adopted in our work, high coefficients of correlation were invariably obtained with dry matter production and N uptake (r ≥ 0.82), indicating that any of these regimes would enable valid estimation of potentially available N for comparisons that are only meaningful among temperature-specific N0 data. Contrary to our expectation, mineralization was more extensive with 23/28 °C temperature cycling than at a constant 28 °C, perhaps due to greater moisture loss from more rapid evaporation at the higher temperature. The implication is that N0 may be under- rather than overestimated as previously proposed [11,46] by maintaining a fixed temperature when heating is employed to maximize microbial activity, commonly at 35 °C following the procedure of Stanford and Smith [40]. That much heat would have been inappropriate in the present project as the temperature averaged 24.6 °C during the greenhouse experiment, which was very comparable to the mean temperature (25.9 °C) achieved by incubation with summer-like temperature cycling of 23/28 °C. Yet, this regime resulted in a lower correlation with dry matter production and N uptake than did either alternative (Table 6), and k values were also lower (Table 3). The latter finding has negative implications for estimating N0, because cumulative net N mineralization stabilizes more gradually with a lower k value, and thus a longer assay period is necessary for quantitative characterization [47,48]. Values of k were highest with winter-like temperatures of 18/23 °C, and this option was also advantageous for improving the correlation of N0 with N uptake.
The finding that rate constants for mineralization were maximized by incubation under winter-like conditions contrasts with previous reports of a positive relationship between incubation temperature and k values [46,49,50]. This discrepancy is due to the use of a shorter incubation period coupled with a difference in the method of modeling mineralization data, which in the present study was done to optimize data fit without the usual assumption that N0 is unaffected by incubation temperature. There was, in fact, a dramatic increase in mineral N production at higher temperatures, with no stabilization of net N mineralization within the 16-week experimental period. As a result, the model predicted a longer period for stabilization, thereby lowering the k value.
The temperature dependence of soil N mineralization reflects the thermodynamic properties of the different enzymes involved, as well as the thermal effects on the growth of the microorganisms that produce these enzymes. Consequently, the temperature adopted for incubation assays could potentially affect not only the quantity but also the chemical forms of N mineralized. The work reported provides some evidence for this view, as there were somewhat stronger correlations of N0 with the two most stable soil N fractions (HUN and AI-N) when incubations were performed at 23/28 °C rather than at 18/23 °C, while a slightly lower correlation was observed for the more labile NH4+-N fraction (Table 6). This shift in N recovery was accompanied by lower correlations with dry matter production and N uptake, indicating that the N0 increases obtained at 23/28 °C were due in part to the liberation of organic N that did not contribute to plant N uptake. The latter explanation is consistent with evidence that N0 values from laboratory incubations can overestimate the amount of N mineralized under field conditions [51,52,53], a finding normally attributed to discrepancies in soil water content and/or the use of dried and sieved soil in incubation assays.
Given the time required for the biological estimation of soil N availability and the difficulties inherent to simulating environmental conditions in the field, there has long been interest in a chemical alternative for predicting soil N mineralization. The present study provides reason for optimism, because N uptake was highly correlated with every soil N parameter evaluated and also with organic matter content. Among acid hydrolysable N fractions, the strongest correlation was obtained for amino acid N, which is consistent with previous studies identifying this fraction as a major source of mineralizable N [54,55,56,57,58,59,60,61].
The amino sugar fraction has also been identified as an important substrate for soil N mineralization [19] and has the major advantage over amino acid N of being decomposable by treatment with strong alkali, so quantitative estimation is much simpler. Significant correlations have been reported in several previous studies to evaluate alkaline hydrolysable N for predicting potentially mineralizable N estimated by incubation assays [62,63,64,65,66,67] and are confirmed in Table 6 by strong correlations obtained between ISNT-N and N0 values, regardless of incubation temperature. As further shown by Table 6, ISNT-N was correlated more highly with acid hydrolysable N fractions than with total N, which is consistent with evidence that soils and soil horizons differ in the proportion of total N liberated by alkaline hydrolysis [68]. A slightly higher correlation was observed for amino acid than amino sugar N, although the strongest relationship was with (NH4+ + amino sugar)-N (r = 0.98, p < 0.001) that largely constitutes alkaline hydrolysable N [20,63,69]. More importantly, the ISNT was highly predictive of soil differences in N uptake by maize during the greenhouse experiment reported, as evidenced by a correlation coefficient exceeding any obtained by incubation assays to estimate potentially mineralizable N. There is potential use for soil testing to improve fertilizer N management for Brazilian maize production, provided that ISNT levels are calibrated to crop N response through replicated field experiments and that test values are interpreted relative to planting density, crop rotation, residue management, and soil properties that affect N availability [21,25,27].
5. Conclusions
Diurnal temperature cycling can be utilized to improve the predictive value of incubation assays for estimating the crop uptake of mineralized soil N. For a tropical area such as Brazil, a winter-like cycle of 18/23 °C reduces the incubation period required to reach the mineralization threshold, but care must be taken to standardize the temperature regime so that comparable data can be obtained for reliable evaluation of potentially mineralizable N. Soil N fractionation by acid hydrolysis is predictive of mineralization capacity, but alkaline hydrolysis using the Illinois Soil N Test (ISNT) is far simpler and more convenient. Test values for a diverse set of Brazilian soils were strongly correlated with incubation estimates of potentially mineralizable N and also with N uptake by maize in a greenhouse study. The ISNT shows promise for improving maize N fertilization in Brazil.
Author Contributions
Conceptualization, F.K., M.C.P.d.C., R.L.M., S.A.K. and M.E.F.; methodology, F.K., M.C.P.d.C., R.L.M., S.A.K. and M.E.F.; software, L.B.B. and R.S.C.; validation, M.C.P.d.C., L.B.B. and R.S.C.; formal analysis, F.K., L.B.B. and R.S.C.; investigation, F.K., M.C.P.d.C. and R.L.M.; data curation, F.K., M.C.P.d.C. and L.B.B.; writing—original draft preparation, F.K., M.C.P.d.C., R.L.M. and S.A.K.; writing—review and editing, R.L.M., L.B.B. and R.S.C.; visualization, M.C.P.d.C., R.L.M. and S.A.K.; supervision, M.C.P.d.C. and R.L.M.; project administration, F.K. and M.C.P.d.C.; funding acquisition, F.K. and M.C.P.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant numbers #2010/10420-9 and #2012/09732-1.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the School of Agricultural and Veterinarian Sciences and University of Illinois staff.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aguiar, A.T.D.E.; Gonçalves, C.; Paterniani, M.E.A.G.Z.; Tucci, M.L.S.; Castro, C.E.F.D. Instruções Agrícolas Para as Principais Culturas Econômicas, Boletim 200, 7th ed.; Aguiar, A.T.D.E., Gonçalves, C., Paterniani, M.E.A.G.Z., Tucci, M.L.S., Castro, C.E.F.D., Eds.; Instituto Agronômico de Campinas: Campinas, Brazil, 2014; ISBN 0375-1538. [Google Scholar]
- Sylvestre, T.D.B.; Braos, L.B.; Batistella Filho, F.; Cruz, M.C.P.D.; Ferreira, M.E. Mineral Nitrogen Fertilization Effects on Lettuce Crop Yield and Nitrogen Leaching. Sci. Hortic. 2019, 255, 153–160. [Google Scholar] [CrossRef]
- Otto, R.; Mulvaney, R.L.; Khan, S.A.; Trivelin, P.C.O. Quantifying Soil Nitrogen Mineralization to Improve Fertilizer Nitrogen Management of Sugarcane. Biol. Fertil. Soils 2013, 49, 893–904. [Google Scholar] [CrossRef]
- Bettiol, A.C.T.; Braos, L.B.; Lopes, I.G.; Andriolli, I.; Ferreira, M.E.; Cruz, M.C.P. Evaluation of Potentially Available Nitrogen by Biological and Chemical Methods in Soil Cultivated with Maize in Succession to Cover Crops. J. Plant Nutr. 2021, 45, 1919–1932. [Google Scholar] [CrossRef]
- Duete, R.R.C.; Muraoka, T.; Silva, E.C.D.; Ambrosano, E.J.; Trivelin, P.C.O. Acúmulo de Nitrogênio (15N) Pelos Grãos de Milho Em Função Da Fonte Nitrogenada Em Latossolo Vermelho. Bragantia 2009, 68, 463–472. [Google Scholar] [CrossRef]
- Lucas, F.T.; Borges, B.M.M.N.; Coutinho, E.L.M. Nitrogen Fertilizer Management for Maize Production under Tropical Climate. Agron. J. 2019, 111, 2031–2037. [Google Scholar] [CrossRef]
- Harmsen, G.W.; van Schreven, D.A. Mineralization of Organic Nitrogen in Soil. Adv. Agron. 1955, 7, 299–398. [Google Scholar] [CrossRef]
- Waring, S.A.; Bremner, J.M. Ammonium Production in Soil Under Waterlogged Conditions as an Index of Nitrogen Availability. Nature (London) 1964, 201, 951–952. [Google Scholar] [CrossRef]
- Keeney, D.R. Nitrogen-Availability Indices. In Methods of Soil Analysis Part 2 Rev Edn—Chemical and Microbiological Properties; Page, A.L., Ed.; Ameerican Society of Agronomy: Madison, WI, USA, 1982; pp. 711–733. [Google Scholar]
- Clark, J.D.; Fernández, F.G.; Veum, K.S.; Camberato, J.J.; Carter, P.R.; Ferguson, R.B.; Franzen, D.W.; Kaiser, D.E.; Kitchen, N.R.; Laboski, C.A.M.; et al. Predicting Economic Optimal Nitrogen Rate with the Anaerobic Potentially Mineralizable Nitrogen Test. Agron. J. 2019, 111, 3329–3338. [Google Scholar] [CrossRef]
- Yagi, R.; Ferreira, M.E.; da Cruz, M.C.P.; Barbosa, J.C. Mineralização Potencial e Líquida de Nitrogênio Em Solos. Rev. Bras. Cienc. Solo 2009, 33, 385–394. [Google Scholar] [CrossRef]
- Kuhnen, F.; Braos, L.B.; Ferreira, M.E.; da Cruz, M.C.P. Mineralization of C and N in Whey-Treated Soils and Absorption of N by Plants. J. Soil Sci. Plant Nutr. 2021, 21, 665–674. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Otto, R.; Griesheim, K.L.; Su, K.; Trivelin, P.C.O. Leaching Methods Can Underestimate Mineralization Potential of Soils. Commun. Soil Sci. Plant Anal. 2016, 47, 1701–1708. [Google Scholar] [CrossRef]
- Agehara, S.; Warncke, D.D. Soil Moisture and Temperature Effects on Nitrogen Release from Organic Nitrogen Sources. Soil Sci. Soc. Am. J. 2005, 69, 1844–1855. [Google Scholar] [CrossRef]
- CETESB. Lodo de Curtume: Critérios Para o Uso Em Áreas Agrícolas e Procedimentos Para Apresentação de Projetos; CETESB: São Paulo, Brazil, 1999.
- Braos, B.B.; Ferreira, M.E.; Cruz, M.C.P.D.; Braos, L.B.; Barbosa, J.C. Mild and Moderate Extraction Methods to Assess Potentially Available Soil Organic Nitrogen. Rev. Bras. Ciência Solo 2016, 40, e0151059. [Google Scholar] [CrossRef]
- Stevenson, F.J. Nitrogen-Organic Forms. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1185–1200. [Google Scholar]
- Mulvaney, R.L.; Khan, S.A. Diffusion Methods to Determine Different Forms of Nitrogen in Soil Hydrolysates. Soil Sci. Soc. Am. J. 2001, 65, 1284–1292. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Hoeft, R.G.; Brown, H.M. A Soil Organic Nitrogen Fraction That Reduces the Need for Nitrogen Fertilization. Soil Sci. Soc. Am. J. 2001, 65, 1164–1172. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Hoeft, R.G. A Simple Soil Test for Detecting Sites That Are Nonresponsive to Nitrogen Fertilization. Soil Sci. Soc. Am. J. 2001, 65, 1751. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Need for a Soil-Based Approach in Managing Nitrogen Fertilizers for Profitable Corn Production. Soil Sci. Soc. Am. J. 2006, 70, 172–182. [Google Scholar] [CrossRef]
- Ruffo, M.L.; Bollero, G.A.; Bullock, D.S.; Bullock, D.G. Site-Specific Production Functions for Variable Rate Corn Nitrogen Fertilization. Precis. Agric. 2006, 7, 327–342. [Google Scholar] [CrossRef]
- Klapwyk, J.H.; Ketterings, Q.M. Soil Tests for Predicting Corn Response to Nitrogen Fertilizer in New York. Agron. J. 2006, 98, 675–681. [Google Scholar] [CrossRef]
- Williams, J.D.; Crozier, C.R.; White, J.G.; Heiniger, R.W.; Sripada, R.P.; Crouse, D.A. Illinois Soil Nitrogen Test Predicts Southeastern U.S. Corn Economic Optimum Nitrogen Rates. Soil Sci. Soc. Am. J. 2007, 71, 735–744. [Google Scholar] [CrossRef]
- Williams, J.D.; Crozier, C.R.; White, J.G.; Sripada, R.P.; Crouse, D.A. Comparison of Soil Nitrogen Tests for Corn Fertilizer Recommendations in the Humid Southeastern USA. Soil Sci. Soc. Am. J. 2007, 71, 171–180. [Google Scholar] [CrossRef]
- Maresma, A.; Ketterings, Q.M. In-Field Variability of the Illinois Soil Nitrogen Test and Loss-on-Ignition Results for Nitrogen Management. Soil Sci. Soc. Am. J. 2017, 81, 1211–1221. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Ketterings, Q.M.; Goler, M.G.; Cherney, J.H.; Cox, W.J.; Czymmek, K.J. Illinois Soil Nitrogen Test with Organic Matter Correction for Predicting Nitrogen Responsiveness of Corn in Rotation. Soil Sci. Soc. Am. J. 2009, 73, 303–311. [Google Scholar] [CrossRef]
- Barker, D.W.; Sawyer, J.E.; Al-Kaisi, M.M.; Lundvall, J.P. Assessment of the Amino Sugar-Nitrogen Test on Iowa Soils: II. Field Correlation and Calibration. Agron. J. 2006, 98, 1352–1358. [Google Scholar] [CrossRef]
- Marriott, E.E.; Wander, M.M. Total and Labile Soil Organic Matter in Organic and Conventional Farming Systems. Soil Sci. Soc. Am. J. 2006, 70, 950–959. [Google Scholar] [CrossRef]
- Laboski, C.A.M.; Sawyer, J.E.; Walters, D.T.; Bundy, L.G.; Hoeft, R.G.; Randall, G.W.; Andraski, T.W. Evaluation of the Illinois Soil Nitrogen Test in the North Central Region of the United States. Agron. J. 2008, 100, 1070–1076. [Google Scholar] [CrossRef]
- Osterhaus, J.T.; Bundy, L.G.; Andraski, T.W. Evaluation of the Illinois Soil Nitrogen Test for Predicting Corn Nitrogen Needs. Soil Sci. Soc. Am. J. 2008, 72, 143–150. [Google Scholar] [CrossRef]
- Spargo, J.T.; Alley, M.M.; Thomason, W.E.; Nagle, S.M. Illinois Soil Nitrogen Test for Prediction of Fertilizer Nitrogen Needs of Corn in Virginia. Soil Sci. Soc. Am. J. 2009, 73, 434–442. [Google Scholar] [CrossRef]
- Mariano, E.; Trivelin, P.C.O.; Montezano, Z.F.; Cantarella, H. Soil Nitrogen Availability Indices as Predictors of Sugarcane Nitrogen Requirements. Eur. J. Agron. 2017, 89, 25–37. [Google Scholar] [CrossRef]
- Dobereiner, J. Nitrogen-Fixing Bacteria of the Genus Beijerinckia Derx in the Rhizosphere of Sugar Cane. Plant Soil 1961, 15, 211–216. [Google Scholar] [CrossRef]
- Boddey, R.M.; Urquiaga, S.; Alves, B.J.R.; Reis, V. Endophytic Nitrogen Fixation in Sugarcane: Present Knowledge and Future Applications. Plant Soil 2003, 252, 139–149. [Google Scholar] [CrossRef]
- Patra, A.K.; Le Roux, X.; Abbadie, L.; Clays-Josserand, A.; Poly, F.; Loiseau, P.; Louault, F. Effect of Microbial Activity and Nitrogen Mineralization on Free-Living Nitrogen Fixation in Permanent Grassland Soils. J. Agron. Crop Sci. 2007, 193, 153–156. [Google Scholar] [CrossRef]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropiciais; Raij, B.V., Andrade, J.C.D., Cantarella, H., Quaggio, J.A., Eds.; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- Camargo, O.A.; Moniz, A.C.; Jorge, J.A.; Valadares, J.M.A.S. Métodos de Análise Química, Mineralógica e Física de Solos Do Instituto Agronômico de Campinas; Instituto Agronômico: Campinas, Brazil, 2009; Volume 106. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy by Soil Survey Staff, 12th ed.; U.S. Department of Agriculture (USDA): Washington, DC, USA, 2014; Volume 12, ISBN 0926487221.
- Stanford, G.; Smith, S.J. Nitrogen Mineralization Potentials of Soils. Soil Sci. Soc. Am. Proc. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Volpe, C.A.; Cargnelutti Filho, A.; Cardozo, N.P. Variabilidade Temporal e Espacial Da Temperatura Do Solo Sob Diferentes Coberturas Do Solo. In Proceedings of the Congresso Brasileiro de Biometeorologia, Ribeirão Preto, Brazil, 11 April 2006; p. 4. [Google Scholar]
- Cantarella, H.; Trivelin, P.C.O. Determinação de Nitrogênio Inorgânico Em Solo Pelo Método Da Destilação a Vapor. In Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Raij, B.V., Cantarella, H., Quaggio, J.A., Eds.; Instituto Agronômico de Campinas: Campinas, Brazil, 2001; pp. 270–276. [Google Scholar]
- Lasdon, L.S.; Waren, A.D.; Jain, A.; Ratner, M. Design and Testing of a Generalized Reduced Gradient Code for Nonlinear Programming. ACM Trans. Math. Softw. 1978, 4, 34–50. [Google Scholar] [CrossRef]
- Tedesco, M.; Gianello, C.; Bissani, C. Análises de Solo, Plantas e Outros Materiais; UFRGS: Proto Alegre, Brazil, 1995. [Google Scholar]
- Ullah Sarkar, M.I.; Rahman, M.M.; Rahman, G.K.M.M.; Naher, U.A.; Ahmed, M.N. Soil Test Based Inorganic Fertilizer and Integrated Plant Nutrition System for Rice (Oryza Sativa L.) Cultivation in Inceptisols of Bangladesh. Agric. 2016, 14, 33–42. [Google Scholar] [CrossRef][Green Version]
- Wang, W.J.; Smith, C.J.; Chen, D. Towards a Standardised Procedure for Determining the Potentially Mineralisable Nitrogen of Soil. Biol. Fertil. Soils 2003, 37, 362–374. [Google Scholar] [CrossRef]
- Braos, L.B.; Ruiz, J.G.C.L.; Lopes, I.G.; Ferreira, M.E.; da Cruz, M.C.P. Mineralization of Nitrogen in Soils with Application of Acid Whey at Different PH. J. Soil Sci. Plant Nutr. 2020, 20, 1102–1108. [Google Scholar] [CrossRef]
- Lopes, I.G.; Braos, L.B.; Cruz, M.C.P.; Vidotti, R.M. Valorization of Animal Waste from Aquaculture through Composting: Nutrient Recovery and Nitrogen Mineralization. Aquaculture 2021, 531, 735859. [Google Scholar] [CrossRef]
- Stanford, G.; Frere, N.H.; Schwaninger, D.H. Temperature Coefficient of Soil Nitrogen Mineralization. Soil Sci. 1973, 115, 321–323. [Google Scholar] [CrossRef]
- Gao, H.; Bai, J.; He, X.; Zhao, Q.; Lu, Q.; Wang, J. High Temperature and Salinity Enhance Soil Nitrogen Mineralization in a Tidal Freshwater Marsh. PLoS ONE 2014, 9, e95011. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E. Evaluation of a Method to Predict Nitrogen Mineralized from Soil Organic Matter Under Field Conditions. Soil Sci. Soc. Am. J. 1988, 52, 1027–1031. [Google Scholar] [CrossRef]
- Honeycutt, C.W. Nitrogen Mineralization from Soil Organic Matter and Crop Residues: Field Validation of Laboratory Predictions. Soil Sci. Soc. Am. J. 1999, 63, 134–141. [Google Scholar] [CrossRef]
- Zou, C.; Pearce, R.C.; Grove, J.H.; Coyne, M.S. Laboratory vs. in Situ Resin-Core Methods to Estimate Net Nitrogen Mineralization for Comparison of Rotation and Tillage Practices. J. Plant Nutr. Soil Sci. 2017, 180, 294–301. [Google Scholar] [CrossRef]
- Mohapatra, S.P. Fractions of Soil Nitrogen during Different Periods of Submergence and Their Effects on Yield and Nutrition of Wetland Rice (Oryza Sativa L.). Biol. Fertil. Soils 1988, 6, 45–49. [Google Scholar] [CrossRef]
- Sarawad, I.M.; Singh, D.; Rana, D.S.; Kumar, K.; Singh, D. Nitrogen Fractions and Their Relationships with Mineralizable N and Its Uptake by Crops in a Long-Term Fertilizer Experiment. J. indian Soc. Soil Sci. 2001, 49, 691–694. [Google Scholar]
- Jumei, L.; Shengxiu, L. Relation of Mineralizable N to Organic N Components in Dark Loessial Soils. Pedosphere 2003, 13, 279–288. [Google Scholar]
- Li, L.L.; Li, S. tian Nitrogen Mineralization from Animal Manures and Its Relation to Organic N Fractions. J. Integr. Agric. 2014, 13, 2040–2048. [Google Scholar] [CrossRef]
- Reddy, K.S.; Singh, M.; Tripathi, A.K.; Singh, M.; Saha, M.N. Changes in Amount of Organic and Inorganic Fractions of Nitrogen in an Eutrochrept Soil after Long-Term Cropping with Different Fertilizer and Organic Manure Inputs. J. Plant Nutr. Soil Sci. 2003, 166, 232–238. [Google Scholar] [CrossRef]
- Reddy, K.S.; Singh, W.A.R.M.; Tripathi, A.K.; Rao, A.S.; Sudhir, K. Contents and Depth Distribution of Nitrogen Fractions in a Kandic Paleustalf Soil Following Long-Term Cropping with Fertilizer and Farmyard Manure Applications. Agrochimica 2008, 52, 337–351. [Google Scholar]
- Wang, S.; Jin, X.; Niu, D.; Wu, F. Potentially Mineralizable Nitrogen in Sediments of the Shallow Lakes in the Middle and Lower Reaches of the Yangtze River Area in China. Appl. Geochem. 2009, 24, 1788–1792. [Google Scholar] [CrossRef]
- Pinggera, J.; Geisseler, D.; Piepho, H.P.; Joergensen, R.G.; Ludwig, B. Effect of Substrate Quality on the N Uptake Routes of Soil Microorganisms in Different Soil Depths. Pedobiologia 2015, 58, 211–218. [Google Scholar] [CrossRef]
- Bushong, J.T.; Norman, R.J.; Ross, W.J.; Slaton, N.A.; Wilson, C.E.; Gbur, E.E. Evaluation of Several Indices of Potentially Mineralizable Soil Nitrogen. Commun. Soil Sci. Plant Anal. 2007, 38, 2799–2813. [Google Scholar] [CrossRef]
- Bushong, J.T.; Roberts, T.L.; Ross, W.J.; Norman, R.J.; Slaton, N.A.; Wilson, C.E. Evaluation of Distillation and Diffusion Techniques for Estimating Hydrolyzable Amino Sugar-Nitrogen as a Means of Predicting Nitrogen Mineralization. Soil Sci. Soc. Am. J. 2008, 72, 992–999. [Google Scholar] [CrossRef]
- Sharifi, M.; Zebarth, B.J.; Burton, D.L.; Grant, C.A.; Cooper, J.M. Evaluation of Some Indices of Potentially Mineralizable Nitrogen in Soil. Soil Sci. Soc. Am. J. 2007, 71, 1233–1239. [Google Scholar] [CrossRef]
- McDonald, N.T.; Watson, C.J.; Lalor, S.T.J.; Laughlin, R.J.; Wall, D.P. Evaluation of Soil Tests for Predicting Nitrogen Mineralization in Temperate Grassland Soils. Soil Sci. Soc. Am. J. 2014, 78, 1051–1064. [Google Scholar] [CrossRef]
- Rogers, C.W.; Schroeder, K.; Rashed, A.; Roberts, T.L. Evaluation of Soil Tests for Measuring Potentially Mineralizable Soil N in Southern Idaho Soils. Soil Sci. Soc. Am. J. 2018, 82, 1279–1289. [Google Scholar] [CrossRef]
- Allen, D.E.; Bloesch, P.M.; Orton, T.G.; Schroeder, B.L.; Skocaj, D.M.; Wang, W.; Masters, B.; Moody, P.M. Nitrogen Mineralisation in Sugarcane Soils in Queensland, Australia: I. Evaluation of Soil Tests for Predicting Nitrogen Mineralisation. Soil Res. 2019, 57, 738–752. [Google Scholar] [CrossRef]
- Roberts, T.L.; Norman, R.J.; Slaton, N.A.; Wilson, C.E. Changes in Alkaline Hydrolyzable Nitrogen Distribution with Soil Depth: Fertilizer Correlation and Calibration Implications. Soil Sci. Soc. Am. J. 2009, 73, 2151–2158. [Google Scholar] [CrossRef]
- Roberts, T.L.; Norman, R.J.; Slaton, N.A.; Wilson, C.E.; Ross, W.J.; Bushong, J.T. Direct Steam Distillation as an Alternative to the Illinois Soil Nitrogen Test. Soil Sci. Soc. Am. J. 2009, 73, 1268–1275. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).