Communicating Nitrogen Loss Mechanisms for Improving Nitrogen Use Efficiency Management, Focused on Global Wheat
Abstract
1. Introduction
2. Methods
2.1. Literature Review
- Search terms: nitrogen use efficiency; nitrogen cycle; nitrogen loss mechanisms; ammonia volatilization; denitrification; nitrification; immobilization; mineralization; leaching; runoff; erosion; root interaction; compaction; uptake; translocation; application; harvest; grain storage.
- Inclusion terms: soil, nitrogen, cereal crop, field, agriculture, and cereals; precision agriculture; wheat, 2010–2019.
- Exclusion terms: crop breeding, Genetically Modified Organisms (GMO), biochar, tree, medicine. Duplications, papers unrelated to the topic (e.g., rice, barley), and those targeted at nutrition were also excluded.
2.2. Focus Groups
2.3. Structuring of the Information
2.4. Causal Loop Diagrams
2.5. Graphic Design
3. Results
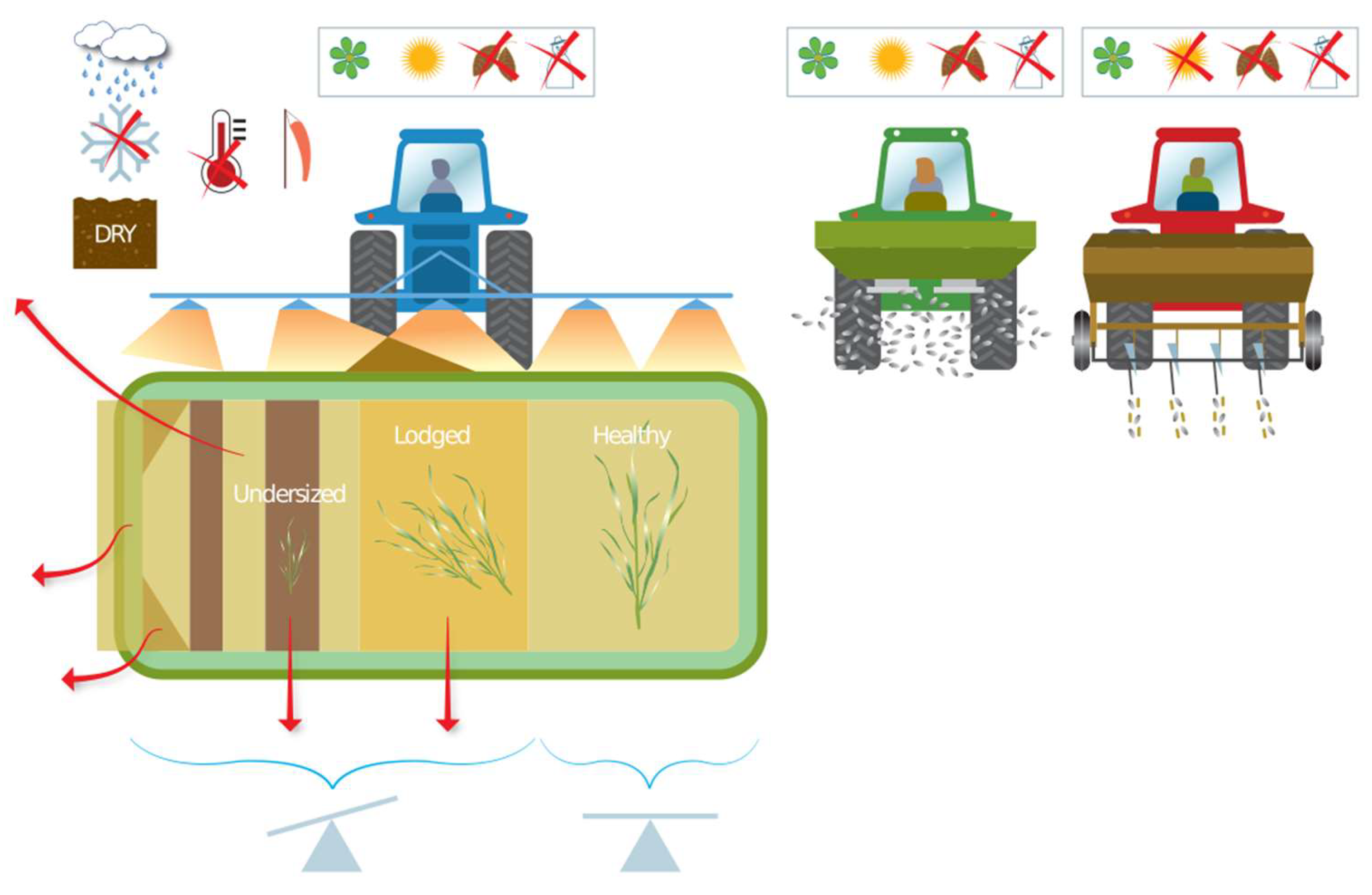
3.1. Management Control
3.2. Spatial and Temporal Variation
3.3. Data Acquisition (Method and Ease)
4. Discussion
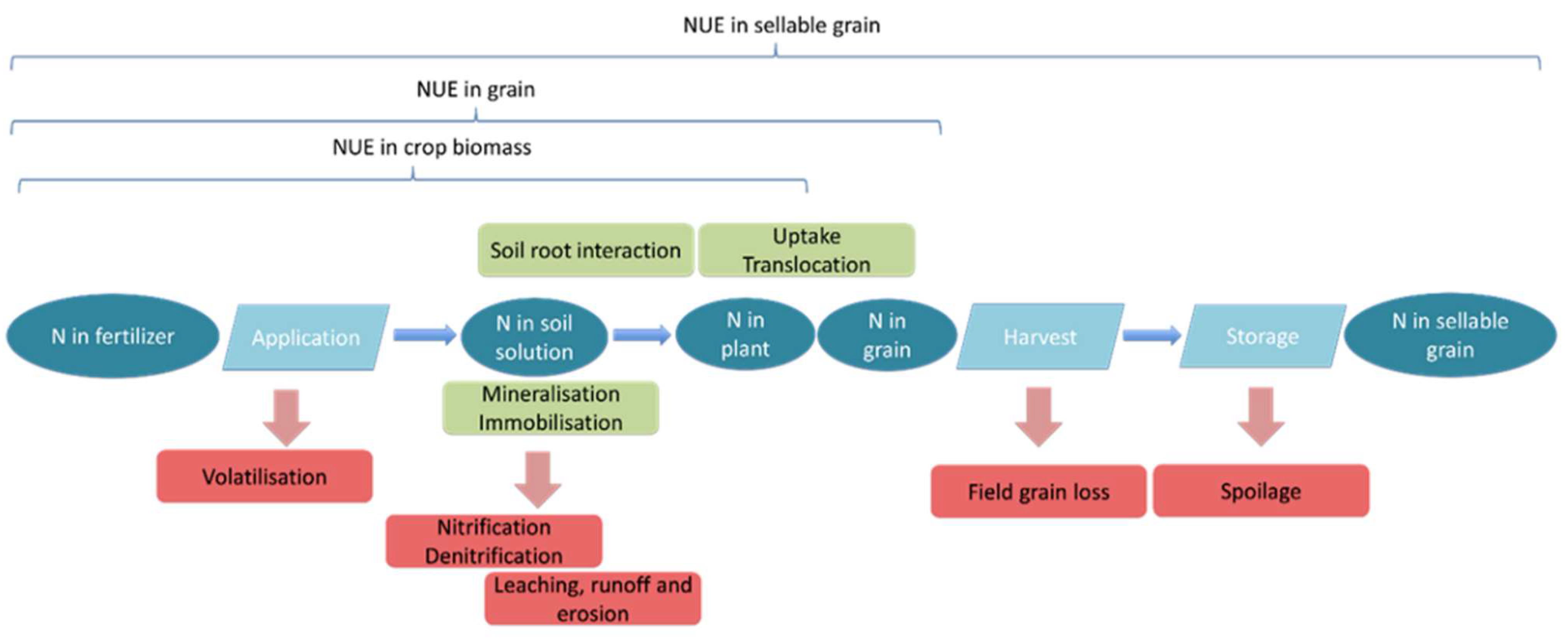
4.1. N Inputs to the System
Application
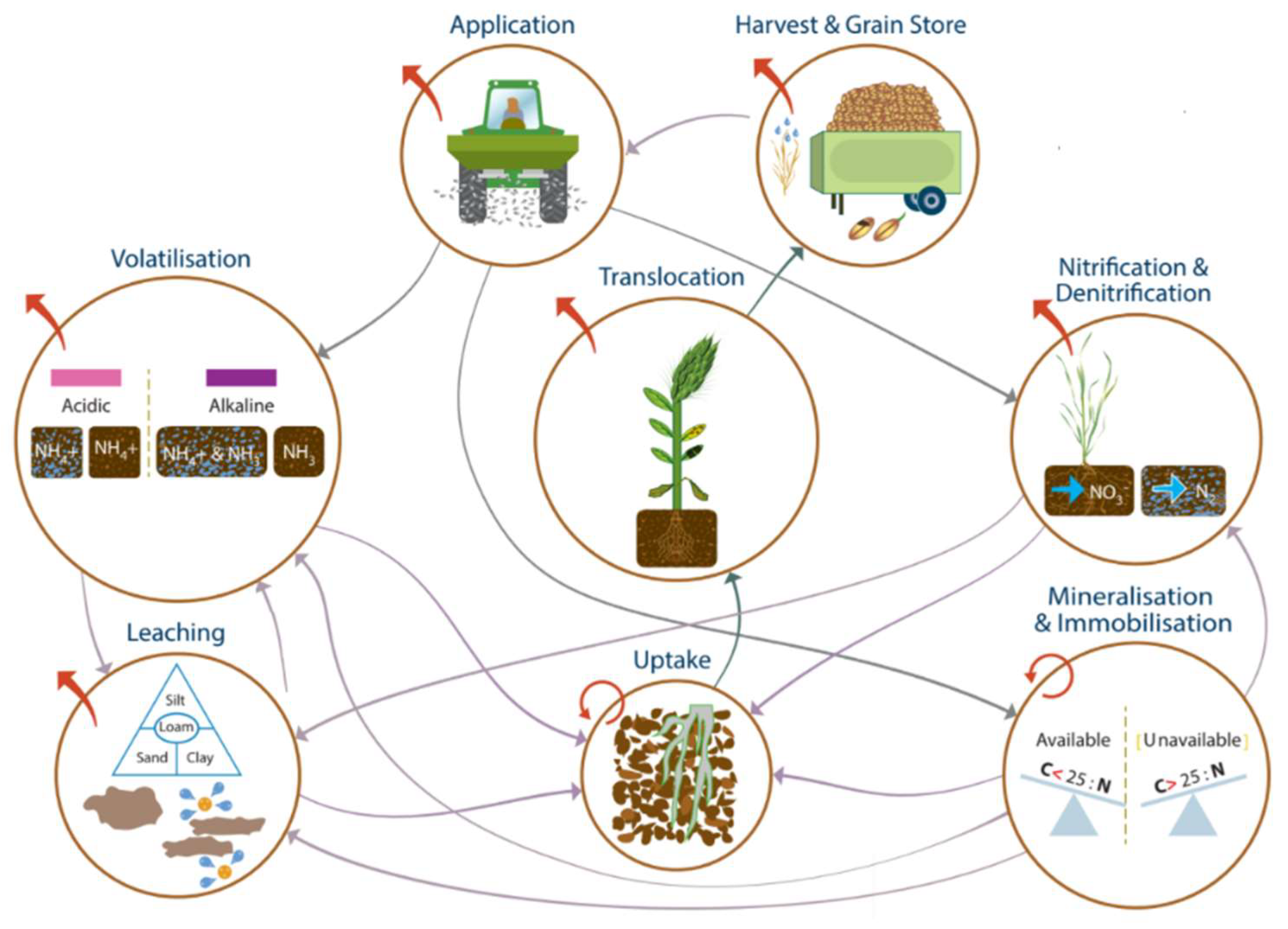
4.2. N Flows between Pools
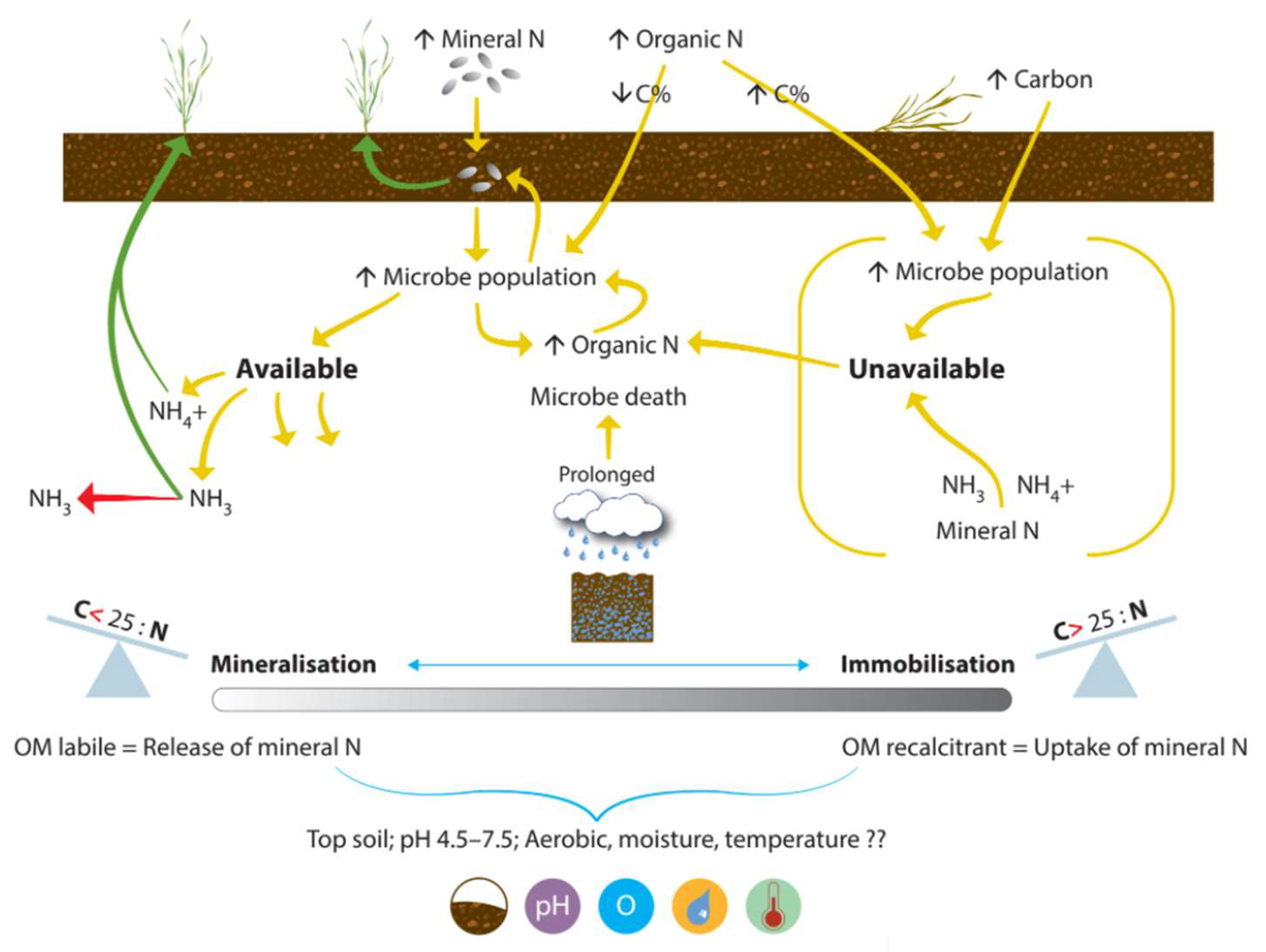
4.2.1. Mineralization and Immobilization
4.2.2. Root–Soil Interaction
4.2.3. Uptake and Translocation
4.3. N Outputs from the System
4.3.1. Volatilization
4.3.2. Nitrification/Denitrification
4.3.3. Leaching, Runoff, and Erosion
4.3.4. Harvest and Grain Storage
4.4. Identifying Priority Factors for NUE Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. World Fertilizer Trends and Outlook to 2020; FAO: Rome, Italy, 2017. [Google Scholar]
- Jenkinson, D.S. The impact of humans on the nitrogen cycle, with focus on temperate arable agriculture. Plant Soil 2001, 228, 3–15. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Need for a soil-based approach in managing nitrogen fertilizers for profitable corn production. Soil Sci. Soc. Am. J. 2006, 70, 172–182. [Google Scholar] [CrossRef]
- Gervois, S.; Ciais, P.; de Noblet-Ducoudré, N.; Brisson, N.; Vuichard, N.; Viovy, N. Carbon and water balance of European croplands throughout the 20th century. Glob. Biogeochem. Cycles 2008, 22, GB2022. [Google Scholar] [CrossRef]
- Herridge, F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Nykvis, B.; Persson, A.; Moberg, F.; Persson, L.; Cornell, S.; Rockström, J. National Environmental Performance on Planetary Boundaries; Swedish Environmental Protection Agency: Bromma, Sweden, 2013.
- Brisson, N.; Gate, P.; Gouache, D.; Charmet, G.; Oury, F.X.; Huard, F. Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crop. Res. 2010, 119, 201–212. [Google Scholar] [CrossRef]
- Oenema, O.; Bleeker, A.; Braathen, N.A.; Budňakova, M.; Bull, K.; Čermak, P.; Geupel, M.; Hicks, K.; Hoft, R.; Kozlova, N.; et al. Nitrogen in current European policies. In The European Nitrogen Assessment; Cambridge University Press: Cambridge, UK, 2011; pp. 62–81. [Google Scholar]
- Peng, S.; Buresh, R.J.; Huang, J.; Yang, J.; Zou, Y.; Zhong, X.; Wang, G.; Zhang, F. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop. Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Collins, C.; Phelan, S. CROPS COSTS AND RETURNS 2019 Crops, Environment and Land-Use Programme. Oak Park teagasc.ie. Available online: https://www.teagasc.ie/media/website/publications/2019/Crops-Costs-and-Returns-2019.pdf (accessed on 1 November 2020).
- Omara, P.; Macnack, N.; Aula, L.; Raun, B. Effect of long-term beef manure application on soil test phosphorus, organic carbon, and winter wheat yield. J. Plant Nutr. 2017, 40, 1143–1151. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Mullen, R.W.; Freeman, K.W.; Thomason, W.E.; Lukina, E.V. Improving nitrogen use efficiency in cereal grain production with optical sensing and variable rate application. Agron. J. 2002, 94, 815–820. [Google Scholar] [CrossRef]
- Godinot, O.; Carof, M.; Vertès, F.; Leterme, P. SyNE: An improved indicator to assess nitrogen efficiency of farming systems. Agric. Syst. 2014, 127, 41–52. [Google Scholar] [CrossRef]
- Quemada, M.; Lassaletta, L.; Jensen, L.S.; Godinot, O.; Brentrup, F.; Buckley, C.; Foray, S.; Hvid, S.K.; Oenema, J.; Richards, K.G.; et al. Exploring nitrogen indicators of farm performance among farm types across several European case studies. Agric. Syst. 2020, 177, 102689. [Google Scholar] [CrossRef]
- EUNEP (European Union, Nitrogen Expert Panel). Nitrogen Use Efficiency (NUE) an Indicator for the Utilization of Nitrogen in Food Systems; Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar]
- Winsor, G.W. Mineralisation and immobilisation of nitrogen in soil. J. Sci. Food Agric. 1958, 9, 792–801. [Google Scholar] [CrossRef]
- Chalk, P.M. Dynamics of biologically fixed N in legume-cereal rotations: A review. Aust. J. Agric. Res. 1998, 49, 303–316. [Google Scholar] [CrossRef]
- Deng, S.; Moore, J.; Tabatabai, M. Characterization of active nitrogen pools in soils under different cropping systems. Biol. Fertil. Soils 2000, 32, 302–309. [Google Scholar] [CrossRef]
- Pate, J.S. Uptake, assimilation and transport of nitrogen compounds by plants. Soil Biol. Biochem. 1973, 5, 109–119. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Velthof, G.L.; Oudendag, D.; Witzke, H.P.; Asman, W.A.H.; Klimont, Z.; Oenema, O. Integrated assessment of nitrogen losses from agriculture in EU-27 using MITERRA-EUROPE. J. Environ. Qual. 2009, 38, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, B.; Brüggemann, N.; Dannenmann, M.; Wang, Y.; Butterbach-Bahl, K. Sustaining crop productivity while reducing environmental nitrogen losses in the subtropical wheat-maize cropping systems: A comprehensive case study of nitrogen cycling and balance. Agric. Ecosyst. Environ. 2016, 231, 1–14. [Google Scholar] [CrossRef]
- Scharf, P.C.; Lory, J.A. Calibrating corn color from aerial photographs to predict sidedress nitrogen need. Agron. J. 2002, 94, 397–404. [Google Scholar] [CrossRef]
- Dhillon, N.S.; Samra, J.S.; Sadana, U.S.; Nielsen, D.R. Spatial variability of soil test values in a Typic Ustochrept. Soil Technol. 1994, 7, 163–171. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V.; Phillips, S.B.; Westerman, R.L. Effect of long-term N fertilization on soil organic C and total N in continuous wheat under conventional tillage in Oklahoma. Soil Tillage Res. 1998, 47, 323–330. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2017, 10, 51. [Google Scholar] [CrossRef]
- Peralta, N.R.; Costa, J.L.; Balzarini, M.; Franco, M.C.; Córdoba, M.; Bullock, D. Delineation of management zones to improve nitrogen management of wheat. Comput. Electron. Agric. 2015, 110, 103–113. [Google Scholar] [CrossRef]
- Haraldsson, H.V. Introduction to System Thinking and Causal Loop Diagrams; Lund University: Lund, Sweden, 2004. [Google Scholar]
- Rasmussen, P.E.; Parton, W.J. Long-term effects of residue management in wheat-fallow: I. Inputs, yield, and soil organic matter. Soil Sci. Soc. Am. J. 1994, 58, 523–530. [Google Scholar] [CrossRef]
- Elaoud, A.; Chehaibi, S. Soil compaction due to tractor traffic. J. Fail. Anal. Prev. 2011, 11, 539–545. [Google Scholar] [CrossRef]
- Do the Figures Add Up on Your Farm Machinery Investments? Farming Independent. Available online: https://www.independent.ie/business/farming/machinery/do-the-figures-add-up-on-your-farm-machinery-investments-38838101.html (accessed on 12 November 2020).
- Angus, J.F.; Van Herwaarden, A.F. Increasing water use and water use efficiency in dryland wheat. Agron. J. 2001, 93, 290–298. [Google Scholar] [CrossRef]
- McBratney, A.; Whelan, B.; Ancev, T.; Bouma, J. Future directions of precision agriculture. Precis. Agric. 2005, 6, 7–23. [Google Scholar] [CrossRef]
- Dufour, L.; Metay, A.; Talbot, G.; Dupraz, C. Assessing light competition for cereal production in temperate agroforestry systems using experimentation and crop modelling. J. Agron. Crop Sci. 2013, 199, 217–227. [Google Scholar] [CrossRef]
- Jones, C.; Olson-Rutz, K. Practices to Increase Wheat Grain Protein; Montana State University Extension; EBO206; Montana State University: Bozeman, MT, USA, 2012. [Google Scholar]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef]
- Schmidt, E.L. Nitrification in soil. Nitrogen Agric. Soils 1982, 22, 253–288. [Google Scholar]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Needham, P.; Moore, G.; Scholz, G. Soil structure decline. In Soil Guide: A Handbook for Understanding and Managing Agricultural Soils; Moore, G., Ed.; Agriculture Western Australia: South Perth, Australia, 1998; pp. 64–79. [Google Scholar]
- Angulo-Jaramillo, R.; Vandervaere, J.P.; Roulier, S.; Thony, J.L.; Gaudet, J.P.; Vauclin, M. Field measurement of soil surface hydraulic properties by disc and ring infiltrometers: A review and recent developments. Soil Tillage Res. 2000, 55, 1–29. [Google Scholar] [CrossRef]
- Godwin, R.J.; Miller, P.C.H. A review of the technologies for mapping within-field variability. Biosyst. Eng. 2003, 84, 393–407. [Google Scholar] [CrossRef]
- Hoyle, F.C. The Effect of Soluble Organic Carbon Substrates, and Environmental Modulators on Soil Microbial Function and Diversity. Ph.D. Dissertation, University of Western Australia, Perth, Australia, 2006. [Google Scholar]
- Palosuo, T.; Kersebaum, K.C.; Angulo, C.; Hlavinka, P.; Moriondo, M.; Olesen, J.E.; Patil, R.H.; Ruget, F.; Rumbaur, C.; Takáč, J.; et al. Simulation of winter wheat yield and its variability in different climates of Europe: A comparison of eight crop growth models. Eur. J. Agron. 2011, 35, 103–114. [Google Scholar] [CrossRef]
- Andersen, M.K.; Jensen, L.S. Low soil temperature effects on short-term gross N mineralisation–immobilisation turnover after incorporation of a green manure. Soil Biol. Biochem. 2001, 33, 511–521. [Google Scholar] [CrossRef]
- Mosier, A.; Syers, J.K.; Freney, J.R. (Eds.) Agriculture and the Nitrogen Cycle: Assessing the Impacts of Fertilizer Use on Food Production and the Environment; Island Press: Washington, DC, USA, 2013; Volume 65. [Google Scholar]
- Dari, B.; Rogers, C.W.; Walsh, O.S. Understanding factors controlling ammonia volatilization from fertilizer nitrogen applications. Univ. Ida. Ext. Bul 2019, 926, 1–4. [Google Scholar]
- Kroulík, M.; Kumhála, F.; Hůla, J.; Honzík, I. The evaluation of agricultural machines field trafficking intensity for different soil tillage technologies. Soil Tillage Res. 2009, 105, 171–175. [Google Scholar] [CrossRef]
- Van Veen, J.A.; Ladd, J.N.; Amato, M. Turnover of carbon and nitrogen through the microbial biomass in a sandy loam and a clay soil incubated with [14C(U)] glucose and [15N](NH4)2SO4 under different moisture regimes. Soil Biol. Biochem. 1985, 17, 747–756. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, C.M. Soil evaporation and its affecting factors under crop canopy. Commun. Soil Sci. Plant analysis 2007, 38, 259–271. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Mooney, S.J. Exploring the interacting effect of soil texture and bulk density on root system development in tomato (Solanum lycopersicum L.). Environ. Exp. Bot. 2013, 91, 38–47. [Google Scholar] [CrossRef]
- Bigelow, C.A.; Bowman, D.C.; Cassel, D.K. Nitrogen leaching in sand-based rootzones amended with inorganic soil amendments and sphagnum peat. J. Am. Soc. Hortic. Sci. 2001, 126, 151–156. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; MacDonald, J.D.; Bissonnette, N.; Bertrand, N. Ammonia volatilization following surface application of urea to tilled and no-till soils: A laboratory comparison. Soil Tillage Res. 2009, 103, 310–315. [Google Scholar] [CrossRef]
- Askegaard, M.; Olesen, J.E.; Rasmussen, I.A.; Kristensen, K. Nitrate leaching from organic arable crop rotations is mostly determined by autumn field management. Agric. Ecosyst. Environ. 2011, 142, 149–160. [Google Scholar] [CrossRef]
- Reichardt, K.; Timm, L.C. How plants absorb nutrients from the soil. In Soil, Plant and Atmosphere; Springer: Cham, Switzerland, 2020; pp. 313–330. [Google Scholar]
- Delin, S.; Nyberg, A.; Lindén, B.; Ferm, M.; Torstensson, G.; Lerenius, C.; Gruvaeus, I. Impact of crop protection on nitrogen utilisation and losses in winter wheat production. Eur. J. Agron. 2008, 28, 361–370. [Google Scholar] [CrossRef]
- O’neal, M.; Frankenberger, J.R.; Ess, D.R.; Lowenberg-Deboer, J.M. Profitability of on-farm precipitation data for nitrogen management based on crop simulation. Precis. Agric. 2004, 5, 153–178. [Google Scholar] [CrossRef]
- Curioni, G.; Chapman, D.N.; Pring, L.J.; Royal, A.C.; Metje, N. Extending TDR capability for measuring soil density and water content for field condition monitoring. J. Geotech. Geoenviron. Eng. 2018, 144, 04017111. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, M.; Wang, N. Precision agriculture—A worldwide overview. Comput. Electron. Agric. 2002, 36, 113–132. [Google Scholar] [CrossRef]
- Visual Soil Examination and Evaluation (VSEE). Teagasc. Available online: https://www.teagasc.ie/environment/soil/research/square/visual-soil-examination-and-evaluation/ (accessed on 5 September 2019).
- McDonald, N.T.; Watson, C.J.; Lalor, S.T.; Laughlin, R.J.; Wall, D.P. Evaluation of soil tests for predicting nitrogen mineralization in temperate grassland soils. Soil Sci. Soc. Am. J. 2014, 78, 1051–1064. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R. The potassium paradox: Implications for soil fertility, crop production and human health. Renew. Agric. Food Syst. 2014, 29, 3–27. [Google Scholar] [CrossRef]
- Cholpankulov, E.D.; Inchenkova, O.P.; Paredes, P.; Pereira, L.S. Cotton irrigation scheduling in Central Asia: Model calibration and validation with consideration of groundwater contribution. Irrig. Drain. J. Int. Comm. Irrig. Drain. 2008, 57, 516–532. [Google Scholar] [CrossRef]
- Ibáñez-Asensio, S.; Marques-Mateu, A.; Moreno-Ramón, H.; Balasch, S. Statistical relationships between soil colour and soil attributes in semiarid areas. Biosyst. Eng. 2013, 116, 120–129. [Google Scholar] [CrossRef]
- Gardner, J.B.; Drinkwater, L.E. The fate of nitrogen in grain cropping systems: A meta-analysis of 15N field experiments. Ecol. Appl. 2009, 19, 2167–2184. [Google Scholar] [CrossRef] [PubMed]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Godwin, R.J. Agricultural Engineering in Development: Tillage for Crop Production in Areas of Low Rainfall; FAO: Rome, Italy, 1990. [Google Scholar]
- Forrestal, P.J.; Harty, M.; Carolan, R.; Lanigan, G.J.; Watson, C.J.; Laughlin, R.J.; McNeill, G.; Chambers, B.J.; Richards, K.G. Ammonia emissions from urea, stabilized urea and calcium ammonium nitrate: Insights into loss abatement in temperate grassland. Soil Use Manag. 2016, 32, 92–100. [Google Scholar] [CrossRef]
- Ichir, L.L.; Ismaili, M. Decomposition and nitrogen dynamics of wheat residues and impact on wheat growth stages. Comptes Rendus Biol. 2002, 325, 597–604. [Google Scholar] [CrossRef]
- Miola, E.C.; Aita, C.; Rochette, P.; Chantigny, M.H.; Angers, D.A.; Bertrand, N.; Gasser, M.O. Static chamber measurements of ammonia volatilization from manured soils: Impact of deployment duration and manure characteristics. Soil Sci. Soc. Am. J. 2015, 79, 305–313. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Adler, P.B.; Stark, J.M.; Tredennick, A.T. Water and nitrogen uptake are better associated with resource availability than root biomass. Ecosphere 2017, 8, e01738. [Google Scholar] [CrossRef]
- Smith, K.A.; Jackson, D.R.; Misselbrook, T.H.; Pain, B.F.; Johnson, R.A. PA—Precision agriculture: Reduction of ammonia emission by slurry application techniques. J. Agric. Eng. Res. 2000, 77, 277–287. [Google Scholar] [CrossRef]
- Mamo, M.; Malzer, G.L.; Mulla, D.J.; Huggins, D.R.; Strock, J. Spatial and temporal variation in economically optimum nitrogen rate for corn. Agron. J. 2003, 95, 958–964. [Google Scholar] [CrossRef]
- Alley, M.M.; Scharf, P.C.; Brann, D.E.; Baethgen, W.E.; Hammons, J.L. Nitrogen Management for Winter Wheat: Principles and Recommendations; Virginia Cooperative Extension: Petersburg, VA, USA, 2009. [Google Scholar]
- Lalor, S.T.J. Cattle Slurry on Grassland-Application Methods and Nitrogen Use Efficiency. Ph.D. Dissertation, Wageningen University and Research, Wageningen, The Netherlands, 2014. [Google Scholar]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Calibration and Spreaders. CF Fertelisers. Available online: http://www.cffertilisers.co.uk/advice-services/advice/calibration-and-spreaders/ (accessed on 10 December 2020).
- Griffin, T.; Lambert, D.; Lowenberg-DeBoer, J. Economics of lightbar and auto-guidance GPS navigation technologies. Precis. Agric. 2005, 5, 581–587. [Google Scholar]
- Schwarz, J.; Herold, L.; Pölling, B. Typology of PF Technologies; FP7 Project Future Farm. Available online: http://www.futurefarm.eu/ (accessed on 2 February 2018).
- Shockley, J.M.; Dillon, C.R.; Stombaugh, T.S. A whole farm analysis of the influence of auto-steer navigation on net returns, risk, and production practices. J. Agric. Appl. Econ. 2011, 43, 57–75. [Google Scholar] [CrossRef]
- Blaylock, A.D.; Kaufmann, J.; Dowbenko, R.D. Nitrogen fertilizer technologies. In Proceedings of the Western Nutrient Management Conference, Salt Lake City, UT, USA, 3–4 March 2005; Volume 6, pp. 8–13. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Colaço, A.F.; Bramley, R.G. Do crop sensors promote improved nitrogen management in grain crops? Field Crop. Res. 2018, 218, 126–140. [Google Scholar] [CrossRef]
- Baral, B.R.; Pande, K.R.; Gaihre, Y.K.; Baral, K.R.; Sah, S.K.; Thapa, Y.B.; Singh, U. Increasing nitrogen use efficiency in rice through fertilizer application method under rainfed drought conditions in Nepal. Nutr. Cycl. Agroecosyst. 2020, 118, 103–114. [Google Scholar] [CrossRef]
- Black, A.S.; Sherlock, R.R.; Smith, N.P. Effect of timing of simulated rainfall on ammonia volatilization from urea, applied to soil of varyingmoisture content. J. Soil Sci. 1987, 38, 679–687. [Google Scholar] [CrossRef]
- Bouwmeester, R.J.B.; Vlek, P.L.G.; Stumpe, J.M. Effect of environmental factors on ammonia volatilization from a urea-fertilized soil. Soil Sci. Soc. Am. J. 1985, 49, 376–381. [Google Scholar] [CrossRef]
- Watson, C.J.; Kilpatrick, D.J. The effect of urea pellet size and rate of application on ammonia volatilization and soil nitrogen dynamics. Fertil. Res. 1991, 28, 163–172. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Haynes, R.J. The decomposition process: Mineralization, immobilization, humus formation. In Mineral Nitrogen in the Plant-Soil Systems; Academic Press: Orlando, FL, USA, 1986; pp. 52–126. [Google Scholar]
- Nannipieri, P.; Eldor, P. The chemical and functional characterization of soil N and its biotic components. Soil Biol. Biochem. 2009, 41, 2357–2369. [Google Scholar] [CrossRef]
- Carranca, C.; De Varennes, A.; Rolston, D.E. Variation in N-recovery of winter wheat under Mediterranean conditions studied with 15N-labelled fertilizers. Eur. J. Agron. 1999, 11, 145–155. [Google Scholar] [CrossRef]
- Sanaa, M.; Mhiri, A.; Cleemput, O.V.; Baert, L. Field study of the fate of labelled fertilizer nitrogen applied to wheat on calcareous Tunisian soils. Bull. Soc. Belg. Pedol. 1992, 42, 245–255. [Google Scholar]
- Isfan, D.; D’Avignon, A.; Lamarre, M. Nitrogen-15 fertilizer recovery in spring wheat and soil as related to the rate and time of application. In Nuclear and Related Techniques in Soil-Plant Studies for Sustainable Agriculture and Environmental Preservation, Proceedings of the Symposium of Nuclear and Related Techniques in Soil-Plant Studies on Sustainable Agriculture and Environmental Preservation, Vienna, Austria, 17–21 October 1994; International Atomic Energy Agency: Vienna, Austria, 1995. [Google Scholar]
- Stevens, W.B.; Hoeft, R.G.; Mulvaney, R.L. Fate of nitrogen-15 in a long-term nitrogen rate study: II. Nitrogen uptake efficiency. Agron. J. 2005, 97, 1046–1053. [Google Scholar] [CrossRef]
- Olson, R.V.; Murphy, L.S.; Moser, H.C.; Swallow, C.W. Fate of tagged fertilizer nitrogen applied to winter wheat. Soil Sci. Soc. Am. J. 1979, 43, 973–975. [Google Scholar] [CrossRef]
- Schindler, F.V.; Knighton, R.E. Fate of fertilizer nitrogen applied to corn as estimated by the isotopic and difference methods. Soil Sci. Soc. Am. J. 1999, 63, 1734–1740. [Google Scholar] [CrossRef]
- Sanchez, J.E.; Willson, T.C.; Kizilkaya, K.; Parker, E.; Harwood, R.R. Enhancing the mineralizable nitrogen pool through substrate diversity in long term cropping systems. Soil Sci. Soc. Am. J. 2001, 65, 1442–1447. [Google Scholar] [CrossRef]
- Maltas, A.; Charles, R.; Jeangros, B.; Sinaj, S. Effect of organic fertilizers and reduced-tillage on soil properties, crop nitrogen response and crop yield: Results of a 12-year experiment in Changins, Switzerland. Soil Tillage Res. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Prescott, C.E.; Chappell, H.N.; Vesterdal, L. Nitrogen turnover in forest floors of coastal Douglas-fir at sites differing in soil nitrogen capital. Ecology 2000, 81, 1878–1886. [Google Scholar] [CrossRef]
- Roper, M.M.; Gupta, V.V.S.R. Enhancing Non-symbiotic N Fixation in Agriculture. Open Agric. J. 2016, 10, 7–27. [Google Scholar] [CrossRef]
- Halsall, D.M.; Turner, G.L.; Gibson, A.H. Straw and xylan utilization by pure cultures of nitrogen-fixing Azospirillum spp. Appl. Environ. Microbiol. 1985, 49, 423–428. [Google Scholar] [CrossRef]
- Adl, S.M. The Ecology of Soil Decomposition; CABI: Wallingford UK, 2003. [Google Scholar]
- Herlihy, M. Nitrogen mineralisation in soils of varying texture, moisture and organic matter. Plant Soil 1979, 53, 255–267. [Google Scholar] [CrossRef]
- Mikha, M.M.; Rice, C.W.; Milliken, G.A. Carbon and nitrogen mineralization as affected by drying and wetting cycles. Soil Biol. Biochem. 2005, 37, 339–347. [Google Scholar] [CrossRef]
- Wilson, J.P.; Gallant, J.C. Terrain Analysis; Principles and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Fiez, T.E.; Miller, B.C.; Pan, W.L. Assessment of spatially variable nitrogen fertilizer management in winter wheat. J. Prod. Agric. 1994, 7, 86–93. [Google Scholar] [CrossRef]
- Burke, I.C.; Lauenroth, W.K.; Parton, W.J. Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecology 1997, 78, 1330–1340. [Google Scholar] [CrossRef]
- Mary, B.; Recous, S.; Darwis, D.; Robin, D. Interactions between decomposition of plant residues and nitrogen cycling in soil. Plant Soil 1996, 181, 71–82. [Google Scholar] [CrossRef]
- Sørensen, P.; Jensen, E.S. Mineralization-immobilization and plant uptake of nitrogen as influenced by the spatial distribution of cattle slurry in soils of different texture. Plant Soil 1995, 173, 283–291. [Google Scholar] [CrossRef]
- Wickramasinghe, K.N.; Rodgers, G.A.; Jenkinson, D.S. Transformations of nitrogen fertilizers in soil. Soil Biol. Biochem. 1985, 17, 625–630. [Google Scholar] [CrossRef]
- Azam, F. Added nitrogen interaction in the soil-plant system—A review. Pak. J. Agron. 2002, 1, 54–59. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- O’Sullivan, M.F.; Simota, C. Modelling the environmental impacts of soil compaction: A review. Soil Tillage Res. 1995, 35, 69–84. [Google Scholar] [CrossRef]
- Dutartre, P.; Bartoli, F.; Andreux, F.; Portal, J.M.; Ange, A. Influence of content and nature of organic matter on the structure of some sandy soils from West Africa. In Soil Structure/Soil Biota Interrelationships; Elsevier: Amsterdam, The Netherlands, 1993; pp. 459–478. [Google Scholar]
- Hamilton, G.; Fisher, P.; Baimbridge, M.; Bignell, J.; Sheppard, J.; Bowey, R. Managing Grey Clays: To Maximise Production and Sustainability; Department of Primary Industries and Regional Development: Perth, Australia, 2005.
- Ali, M.A.; Ali, M.; Sattar, M.; Ali, L. Sowing date effect on yield of different wheat varieties. J. Agric. Res. 2010, 48, 157–162. [Google Scholar]
- Shahgholi, G.; Janatkhah, J. Investigation of the effects of organic matter application on soil compaction. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Derg. 2018, 28, 175–185. [Google Scholar] [CrossRef]
- Hoad, S.P.; Russell, G.; Lucas, M.E.; Bingham, I.J. The management of wheat, barley, and oat root systems. Adv. Agron. 2001, 74, 193–246. [Google Scholar]
- Grzesiak, S.; Grzesiak, M.T.; Hura, T.; Marcińska, I.; Rzepka, A. Changes in root system structure, leaf water potential and gas exchange of maize and triticale seedlings affected by soil compaction. Environ. Exp. Bot. 2013, 88, 2–10. [Google Scholar] [CrossRef]
- Tardieu, F. Growth and functioning of roots and of root systems subjected to soil compaction. Towards a system with multiple signalling? Soil Tillage Res. 1994, 30, 217–243. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Beusen, A.H.W.; Griffioen, J.; Van Groenigen, J.W.; Hefting, M.M.; Oenema, O.; Van Puijenbroek, P.J.T.M.; Seitzinger, S.; Slomp, C.P.; Stehfest, E. Global trends and uncertainties in terrestrial denitrification and N2O emissions. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130112. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; McNeill, A.; Davidson, R.; Tester, M.; Samec, M.; Korošak, D.; Sturrock, C.; Mooney, S.J. Quantifying the effect of soil compaction on three varieties of wheat (Triticum aestivum L.) using X-ray Micro Computed Tomography (CT). Plant Soil 2012, 353, 195–208. [Google Scholar] [CrossRef]
- Soane, B.D.; Van Ouwerkerk, C. Implications of soil compaction in crop production for the quality of the environment. Soil Tillage Res. 1995, 35, 5–22. [Google Scholar] [CrossRef]
- Nadian, H.; Smith, S.E.; Alston, A.M.; Murray, R.S. The effect of soil compaction on growth and P uptake by Trifolium subterraneum: Interactions with mycorrhizal colonisation. Plant Soil 1996, 182, 39–49. [Google Scholar] [CrossRef]
- Barley, K.P. The configuration of the root system in relation to nutrient uptake. Adv. Agron. 1970, 22, 159–201. [Google Scholar]
- Atwell, B.J. The effect of soil compaction on wheat during early tillering: II. Concentrations of cell constituents. New Phytol. 1990, 115, 37–41. [Google Scholar] [CrossRef]
- Barzegar, A.R.; Asoodar, M.A.; Ansari, M. Effectiveness of sugarcane residue incorporation at different water contents and the Proctor compaction loads in reducing soil compactibility. Soil Tillage Res. 2000, 57, 167–172. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Whalley, W.R.; Dumitru, E.; Dexter, A.R. Biological effects of soil compaction. Soil Tillage Res. 1995, 35, 53–68. [Google Scholar] [CrossRef]
- Chygarev, Y.; Lodyata, S. Research of tyre rigidity in terms of ecological safety of agricultural landscapes. In Proceedings of the VII Międzynarodowe Sympozjum Ekologiczne Aspekty Mechanizacji Nawożenia, Ochrony Roślin Uprawy Gleby i Zbioru Roślin Uprawnych, Warszawa, Poland, 18–19 September 2000; pp. 171–176. [Google Scholar]
- Bakker, D.M.; Davis, R.J. Soil deformation observations in a Vertisol under field traffic. Soil Res. 1995, 33, 817–832. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.W.; Kaspar, T.C. Soil compaction and root growth: A review. Agron. J. 1994, 86, 759–766. [Google Scholar] [CrossRef]
- Bennie, A.T.P.; Botha, F.J.P. Effect of deep tillage and controlled traffic on root growth, water-use efficiency and yield of irrigated maize and wheat. Soil Tillage Res. 1986, 7, 85–95. [Google Scholar] [CrossRef]
- Nyéki, A.; Milics, G.; Kovács, A.J.; Neményi, M. Effects of soil compaction on cereal yield: A review. Cereal Res. Commun. 2017, 45, 1–22. [Google Scholar] [CrossRef]
- Farahbakhsh, F.; Hamzehzarghani, H.; Massah, A.; Tortosa, M.; Yassaie, M.; Rodriguez, V.M. Comparative metabolomics of temperature sensitive resistance to wheat streak mosaic virus (WSMV) in resistant and susceptible wheat cultivars. J. Plant Physiol. 2019, 237, 30–42. [Google Scholar] [CrossRef]
- Cott, G.M.; Jansen, M.A.; Megonigal, J.P. Uptake of organic nitrogen by coastal wetland plants under elevated CO2. Plant Soil 2020, 450, 521–535. [Google Scholar] [CrossRef]
- Gioseffi, E.; de Neergaard, A.; Schjørring, J.K. Interactions between uptake of amino acids and inorganic nitrogen in wheat plants. Biogeosciences 2012, 9, 1509–1518. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, L.; Wang, J.; Ma, J.; Zheng, N.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Fertilizer regime changes the competitive uptake of organic nitrogen by wheat and soil microorganisms: An in-situ uptake test using 13C, 15N labelling, and 13C-PLFA analysis. Soil Biol. Biochem. 2018, 125, 319–327. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol. 2008, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, S.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Use of the stable nitrogen isotope to reveal the source-sink regulation of nitrogen uptake and remobilization during grain filling phase in maize. PLoS ONE 2016, 11, e0162201. [Google Scholar] [CrossRef]
- Basche, A.D.; DeLonge, M.S. Comparing infiltration rates in soils managed with conventional and alternative farming methods: A meta-analysis. PLoS ONE 2019, 14, e0215702. [Google Scholar] [CrossRef]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Johnson, M.G.; Spokas, K. Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 2016, 142, 176–183. [Google Scholar] [CrossRef]
- Roques, S.; Kendall, S.; Smith, K.; Newell Price, P.; Berry, P. A Review of the Non-NPKS Nutrient Requirements of UK Cereals and Oilseed Rape; Research Review No. 78; HGCA: Kenilworth, UK, 2013. [Google Scholar]
- Fernández, F.G.; Hoeft, R.G. Managing soil pH and crop nutrients. In Illinois Agronomy Handbook, 24th ed.; University of Illinois at Urbana-Champaign: Urbana, IL, USA, 2009; pp. 91–112. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Volume 29. [Google Scholar]
- Wheat Section 4 Plant Growth and Physiology. GRDC. Available online: https://grdc.com.au/__data/assets/pdf_file/0026/370673/GrowNote-Wheat-North-04-Physiology.pdf (accessed on 5 September 2019).
- Impa, S.M.; Vennapusa, A.R.; Bheemanahalli, R.; Sabela, D.; Boyle, D.; Walia, H.; Jagadish, S.K. High night temperature induced changes in grain starch metabolism alters starch, protein, and lipid accumulation in winter wheat. Plant Cell Environ. 2020, 43, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.P.O.; Diamond, J.; Finkele, K.; Holden, N.M.; Brereton, A.J. Predicting the soil moisture conditions of Irish grasslands. Ir. J. Agric. Food Res. 2005, 44, 95–110. [Google Scholar]
- Hu, T.; Sørensen, P.; Wahlström, E.M.; Chirinda, N.; Sharif, B.; Li, X.; Olesen, J.E. Root biomass in cereals, catch crops and weeds can be reliably estimated without considering aboveground biomass. Agric. Ecosyst. Environ. 2018, 251, 141–148. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between nutrition, plant diseases and pests. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 283–298. [Google Scholar]
- FAO. The State of Food and Agriculture 2001; No. 33; Food and Agriculture Organization: Rome, Italy, 2001. [Google Scholar]
- Doyle, B.; Cummins, T.; Augustenborg, C.; Aherne, J. Ambient Atmospheric Ammonia in Ireland 2013–2014; Report 193; Environmental Protection Agency of Ireland: Wexford, Irleand, 2014. [Google Scholar]
- De Klein, C.A.; Shepherd, M.A.; van der Weerden, T.J. Nitrous oxide emissions from grazed grasslands: Interactions between the N cycle and climate change—A New Zealand case study. Curr. Opin. Environ. Sustain. 2014, 9, 131–139. [Google Scholar] [CrossRef]
- Raun, W.R.; Schepers, J.S. Nitrogen management for improved use efficiency. Nitrogen Agric. Syst. 2008, 49, 675–693. [Google Scholar]
- Fan, X.H.; Li, Y.C.; Alva, A.K. Effects of temperature and soil type on ammonia volatilization from slow-release nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2011, 42, 1111–1122. [Google Scholar] [CrossRef]
- Sommer, S.G.; Olesen, J.E.; Christensen, B.T. Effects of temperature, wind speed and air humidity on ammonia volatilization from surface applied cattle slurry. J. Agric. Sci. 1991, 117, 91–100. [Google Scholar] [CrossRef]
- Harty, M.A.; McGeough, K.L.; Carolan, R.; Müller, C.; Laughlin, R.J.; Lanigan, G.J.; Richards, K.G.; Watson, C.J. Gross nitrogen transformations in grassland soil react differently to urea stabilisers under laboratory and field conditions. Soil Biol. Biochem. 2017, 109, 23–34. [Google Scholar] [CrossRef]
- Kotlar, A.M.; de Carvalho, H.W.P.; Iversen, B.V.; van Lier, Q.D.J. Nitrate leaching from layered double hydroxides in tropical and temperate soils. Appl. Clay Sci. 2020, 184, 105365. [Google Scholar] [CrossRef]
- Tian, G.; Cai, Z.; Cao, J.; Li, X. Factors affecting ammonia volatilisation from a rice–wheat rotation system. Chemosphere 2001, 42, 123–129. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; No. 80; Food & Agriculture Organization: Rome, Italy, 2005. [Google Scholar]
- Banning, N.C.; Maccarone, L.D.; Fisk, L.M.; Murphy, D.V. Ammonia-oxidising bacteria not archaea dominate nitrification activity in semi-arid agricultural soil. Sci. Rep. 2015, 5, 11146. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Pate, J.S. An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crop. Res. 2000, 65, 211–228. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Sawyer, J.E.; Lundvall, J.; Hawkins, J.S.; Barker, D.W. Sensing Nitrogen Stress in Corn; Iowa State University: Ames, IA, USA, 2011. [Google Scholar]
- Jordan, C. The effect of fertiliser type and application rate on denitrification losses from cut grassland in Northern Ireland. Fertil. Res. 1989, 19, 45–55. [Google Scholar] [CrossRef]
- Hyde, B.P.; Hawkins, M.J.; Fanning, A.F.; Noonan, D.; Ryan, M.; O’toole, P.; Carton, O.T. Nitrous oxide emissions from a fertilized and grazed grassland in the South East of Ireland. Nutr. Cycl. Agroecosyst. 2006, 75, 187–200. [Google Scholar] [CrossRef]
- Stark, C.H.; Richards, K.G. The continuing challenge of agricultural nitrogen loss to the environment in the context of global change and advancing research. Dyn. Soil Dyn. Plant 2008, 2, 1–12. [Google Scholar]
- Firestone, M.K.; Davidson, E.A. Microbiological basis of NO and N2O production and consumption in soil. Exch. Trace Gases Between Terr. Ecosyst. Atmos. 1989, 47, 7–21. [Google Scholar]
- Verhagen, F.J.; Laanbroek, H.J. Competition for ammonium between nitrifying and heterotrophic bacteria in dual energy-limited chemostats. Appl. Environ. Microbiol. 1991, 57, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bottomley, P.J.; Myrold, D.D. Contributions of ammonia-oxidizing archaea and bacteria to nitrification in Oregon forest soils. Soil Biol. Biochem. 2015, 85, 54–62. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Campbell, C.D.; Chapman, S.J.; Freitag, T.E.; Nicol, G.W.; Singh, B.K. Multi-factorial drivers of ammonia oxidizer communities: Evidence from a national soil survey. Environ. Microbiol. 2013, 15, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Alleman, J.E. Elevated nitrite occurrence in biological wastewater treatment systems. Water Sci. Technol. 1985, 17, 409–419. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Uchiyama, I.; Toyoda, A.; Wang, Y.; Shimomura, Y.; Okubo, T.; Kurisu, F.; Hirono, Y.; Nonaka, K.; et al. An acid-tolerant ammonia-oxidizing γ-proteobacterium from soil. ISME J. 2017, 11, 1130–1141. [Google Scholar] [CrossRef]
- Cogle, A.L.; Rao, K.P.C.; Yule, D.F.; Smith, G.D.; George, P.J.; Srinivasan, S.T.; Jangawad, L. Soil management for Alfisols in the semiarid tropics: Erosion, enrichment ratios and runoff. Soil Use Manag. 2002, 18, 10–17. [Google Scholar] [CrossRef][Green Version]
- Das, B.S.; Kluitenberg, G.J.; Pierzynski, G.M. Temperature dependence of nitrogen mineralization rate constant: A theoretical approach. Soil Sci. 1995, 159, 294–300. [Google Scholar] [CrossRef]
- Campbell, C.A.; Biederbeck, V.O. Influence of fluctuating temperatures and constant soil moistures on nitrogen changes in amended and unamended loam. Can. J. Soil Sci. 1972, 52, 323–336. [Google Scholar] [CrossRef]
- Chandra, P. Note on the effect of shifting temperatures on nitrification in a loam soil. Can. J. Soil Sci. 1962, 42, 314–315. [Google Scholar] [CrossRef]
- Husk, B.R.; Anderson, B.C.; Whalen, J.K.; Sanchez, J.S. Reducing nitrogen contamination from agricultural subsurface drainage with denitrification bioreactors and controlled drainage. Biosyst. Eng. 2017, 153, 52–62. [Google Scholar] [CrossRef]
- Lin, B.L.; Sakoda, A.; Shibasaki, R.; Suzuki, M. A modelling approach to global nitrate leaching caused by anthropogenic fertilisation. Water Res. 2001, 35, 1961–1968. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental Panel on Climate Change/Organization for Economic Cooperation and Development. Guidelines for National Greenhouse Gas Inventories; OECD/OCDE: Paris, France, 1997. [Google Scholar]
- Randall, G.W.; Delgado, J.A.; Schepers, J.S. Nitrogen management to protect water resources. Nitrogen Agric. Syst. 2008, 49, 911–945. [Google Scholar]
- Harmel, D.; Qian, S.; Reckhow, K.; Casebolt, P. The MANAGE database: Nutrient load and site characteristic updates and runoff concentration data. J. Environ. Qual. 2008, 37, 2403–2406. [Google Scholar] [CrossRef] [PubMed]
- Van der Werff, P.A.; Baars, A.; Oomen, G.J.M. Nutrient balances and measurement of nitrogen losses on mixed ecological farms on sandy soils in the Netherlands. Biol. Agric. Hortic. 1995, 11, 41–50. [Google Scholar] [CrossRef]
- Lind, A.M.; Debosz, K.K.; Maag, M. N-balance for mineral N on spring barley cropped sandy loam and coarse sandy soil with mineral and organic fertilizers. Acta Agric. Scand. B Plant Soil Sci. 1995, 45, 39–50. [Google Scholar] [CrossRef]
- Gaines, T.P.; Gaines, S.T. Soil texture effect on nitrate leaching in soil percolates. Commun. Soil Sci. Plant Anal. 1994, 25, 2561–2570. [Google Scholar] [CrossRef]
- Ruiz-Colmenero, M.; Bienes, R.; Eldridge, D.J.; Marques, M.J. Vegetation cover reduces erosion and enhances soil organic carbon in a vineyard in the central Spain. Catena 2013, 104, 153–160. [Google Scholar] [CrossRef]
- Meetei, T.T.; Devi, Y.B.; Chanu, T.T. Ion Exchange: The Most Important Chemical Reaction on Earth after Photosynthesis. Int. Res. J. Pure Appl. Chem 2020, 21, 31–42. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. (Eds.) Biochar: A guide to Analytical Methods; Csiro Publishing: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tahir, S.; Marschner, P. Clay addition to sandy soil reduces nutrient leaching—Effect of clay concentration and ped size. Commun. Soil Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Qafoku, N.P.; Van Ranst, E.; Noble, A.; Baert, G. Variable charge soils: Their mineralogy, chemistry and management. Adv. Agron. 2004, 84, 160–217. [Google Scholar]
- Kubotera, H. Factors influencing nitrate retention in 3 Andisol profiles in Kyushu, Japan. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Guo, P.C.; Bogring, J.; Scherer, H.W. Verhalten von Dünger-NH4 in Böden unterschiedlicher tonmineralischer Zusammensetzung im Inkubationsversuch. Z. Pflanz. Bodenkd. 1983, 146, 752–759. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Bullock, D.G. Correlation of corn and soybean grain yield with topography and soil properties. Agron. J. 2000, 92, 75–83. [Google Scholar] [CrossRef]
- Kitchen, N.R.; Drummond, S.T.; Lund, E.D.; Sudduth, K.A.; Buchleiter, G.W. Soil electrical conductivity and topography related to yield for three contrasting soil–crop systems. Agron. J. 2003, 95, 483–495. [Google Scholar] [CrossRef]
- Jiang, P.; Thelen, K.D. Effect of soil and topographic properties on crop yield in a North-Central corn–soybean cropping system. Agron. J. 2004, 96, 252–258. [Google Scholar] [CrossRef]
- Mills, W.C.; Thomas, A.W.; Langdale, G.W. Rainfall retention probabilities computed for different cropping-tillage systems. Agric. Water Manag. 1988, 15, 61–71. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef]
- Ali, M.A.; Khalid, L. Grain losses of wheat as affected by different harvesting and threshing techniques. Int. J. Res. Agric. For. 2015, 2, 20–26. [Google Scholar]
- Boxall, R.A. Post-harvest losses to insects—A world overview. Int. Biodeterior. Biodegrad. 2001, 48, 137–152. [Google Scholar] [CrossRef]
- Hollins, P.D.; Kettlewell, P.S.; Parsons, S.T.; Atkinson, M.D. The impact of supply, demand and grain quality on the UK bread and feed wheat price differential in the UK. J. Agric. Sci. 2006, 144, 411–419. [Google Scholar] [CrossRef]
- Cambi, M.; Certini, G.; Fabiano, F.; Foderi, C.; Laschi, A.; Picchio, R. Impact of wheeled and tracked tractors on soil physical properties in a mixed conifer stand. iForest Biogeosci. For. 2015, 9, 89. [Google Scholar] [CrossRef]
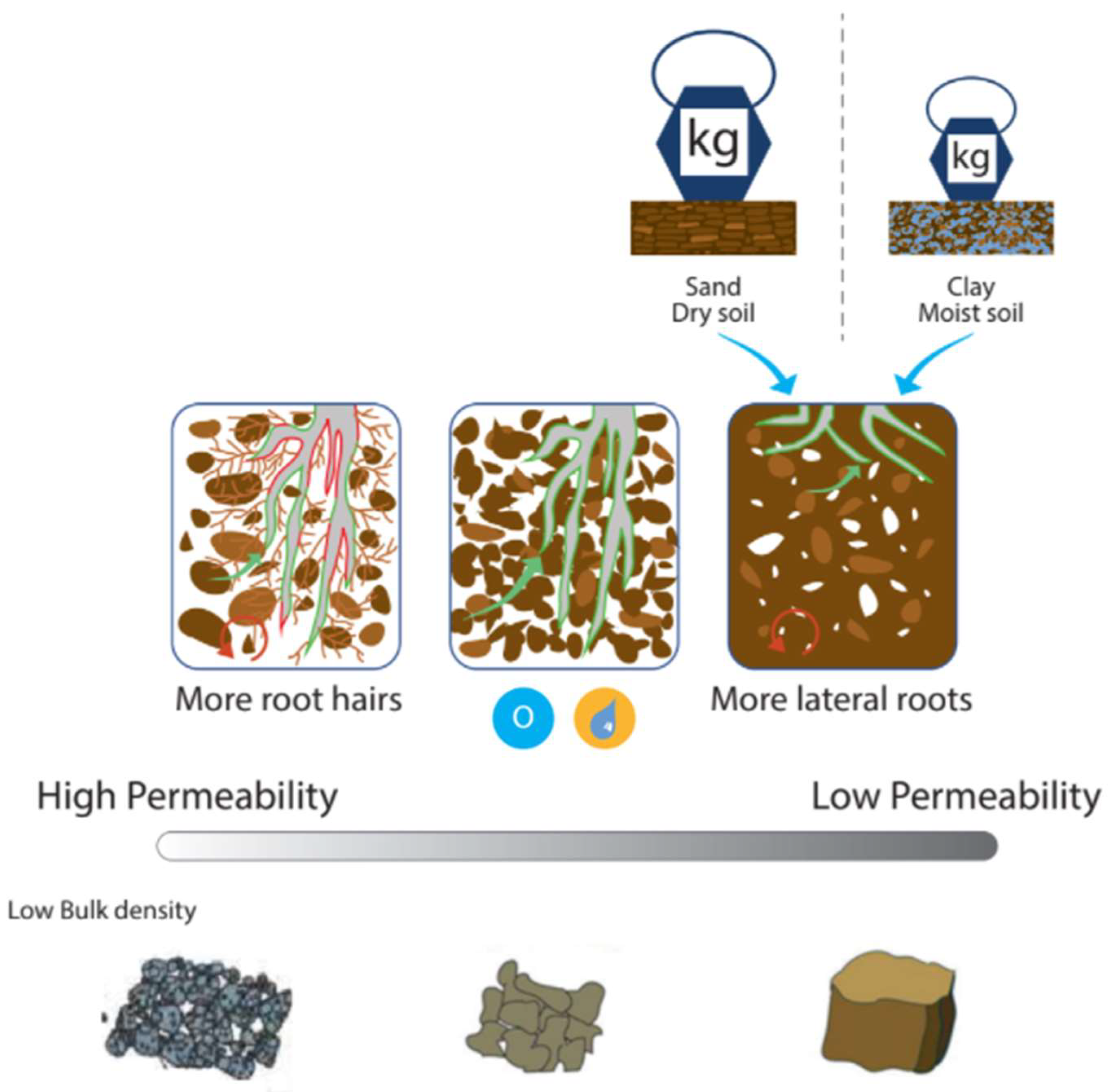
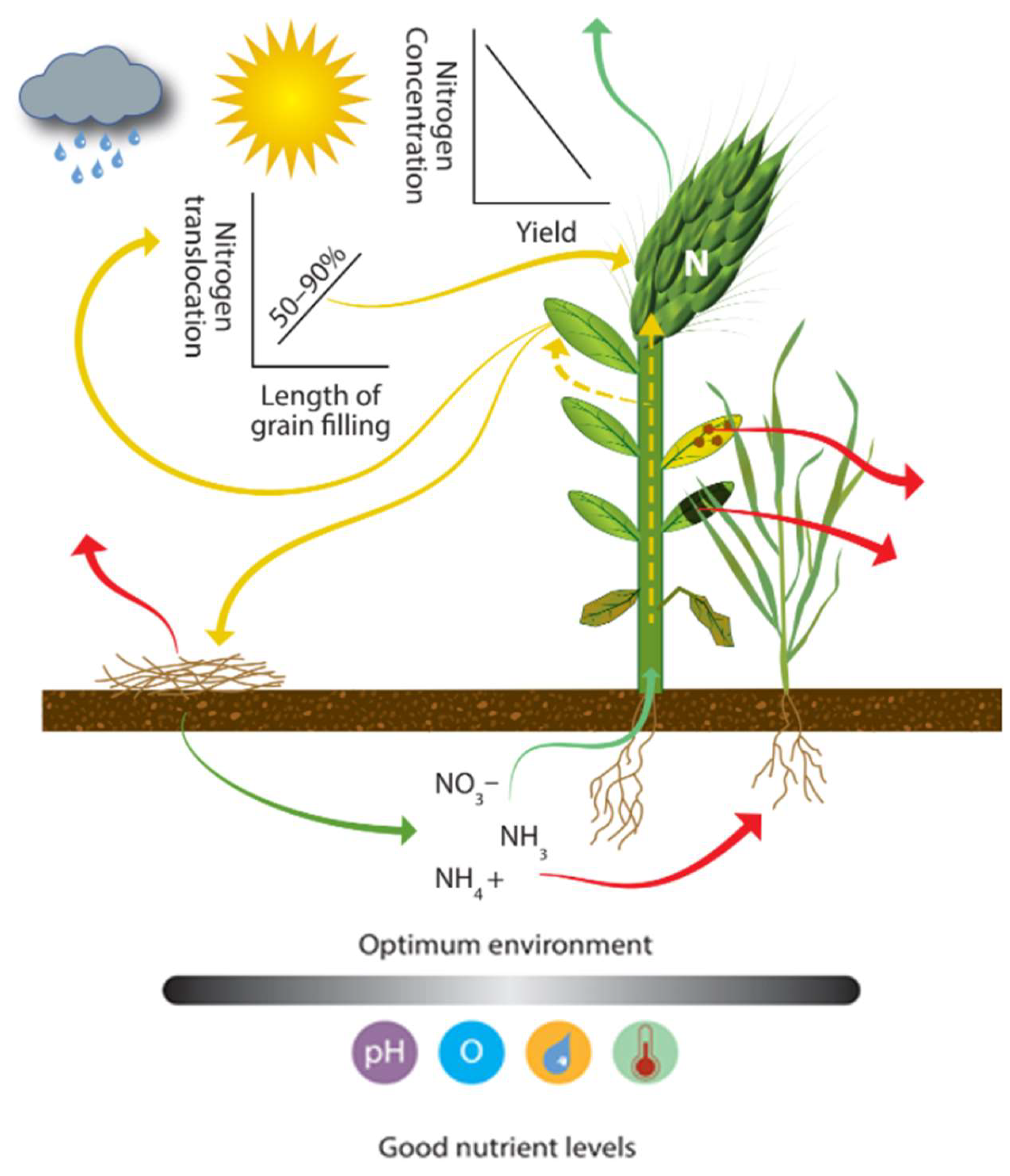
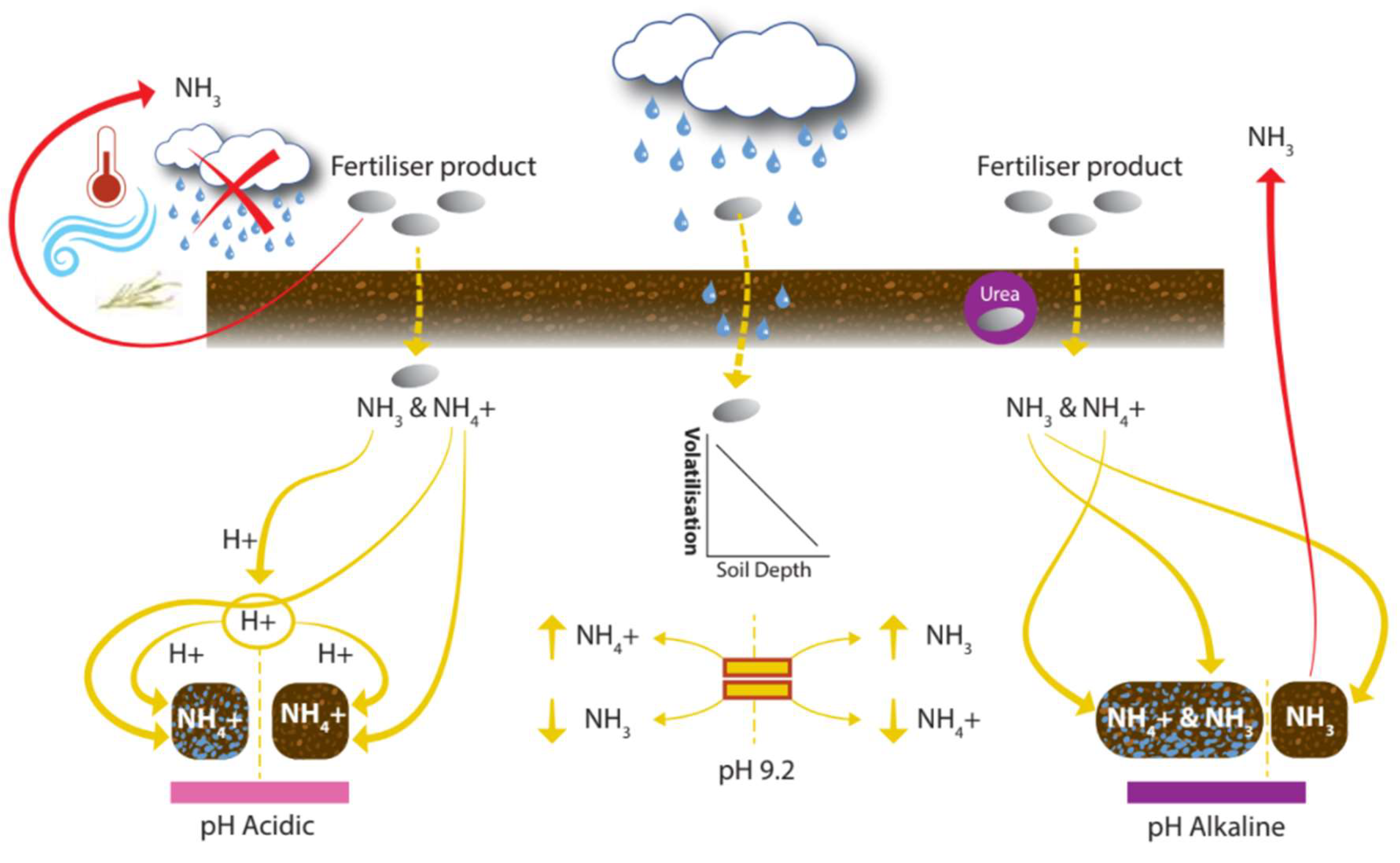
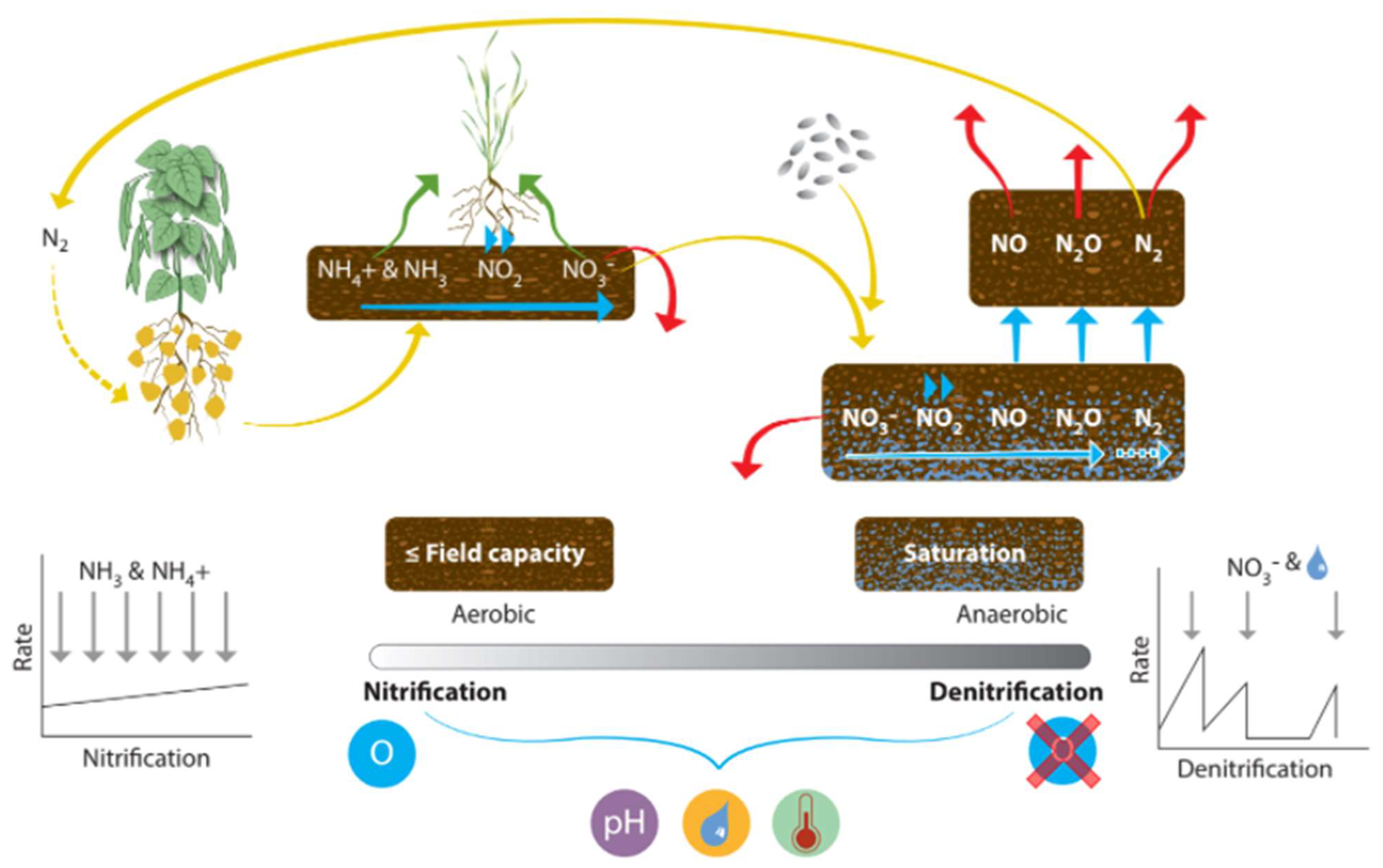
| Category | Property | Spatial Variability | Temporal Variability | Possibility of Control | Data Collection | Method | Reference | |
|---|---|---|---|---|---|---|---|---|
| Management | Social | Field machinery availability | - | - | - | 1 | Query | [29,30,31] |
| Storage and drying facility | - | - | - | 1 | ||||
| Fertilizer applications | - | - | - | 1 | Query/record | |||
| Other field processes/applications | - | - | - | 1 | ||||
| Expected economic return/cashflow | - | - | - | 1 | ||||
| Legislation | - | - | - | 1 | Published | |||
| Weather and climate | Rain | Low | Yes | No | 1–2 | Observation/predicted/simple sensor | [32,33,34,35,36] | |
| Wind | Low | Yes | No | 1–2 | ||||
| Temperature | Low | Yes | No | 1–2 | ||||
| Light intensity | Low | Yes | No | 1–2 | ||||
| Soil | Structure | Soil moisture or water logging | Yes | Yes | Partially | 2–3 | Simple sensor/field test | [29,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] |
| Available soil air | Yes | Yes | Yes | |||||
| Soil resistance resilience? | Yes | Low | Yes | |||||
| Infiltration rate | Yes | Low | Yes | |||||
| Evapotranspiration | Yes | Yes | Partially | 2–4 | Field/lab test | |||
| Available nutrients | CNR | Yes | Yes | Partially | 4 | Lab test | ||
| N species | Yes | Yes | Partially | |||||
| Soluble C | Yes | Yes | Yes | |||||
| Other nutrients | Yes | Yes | Yes | |||||
| pH | Yes | Low | Yes | 2–3 | Simple field test | |||
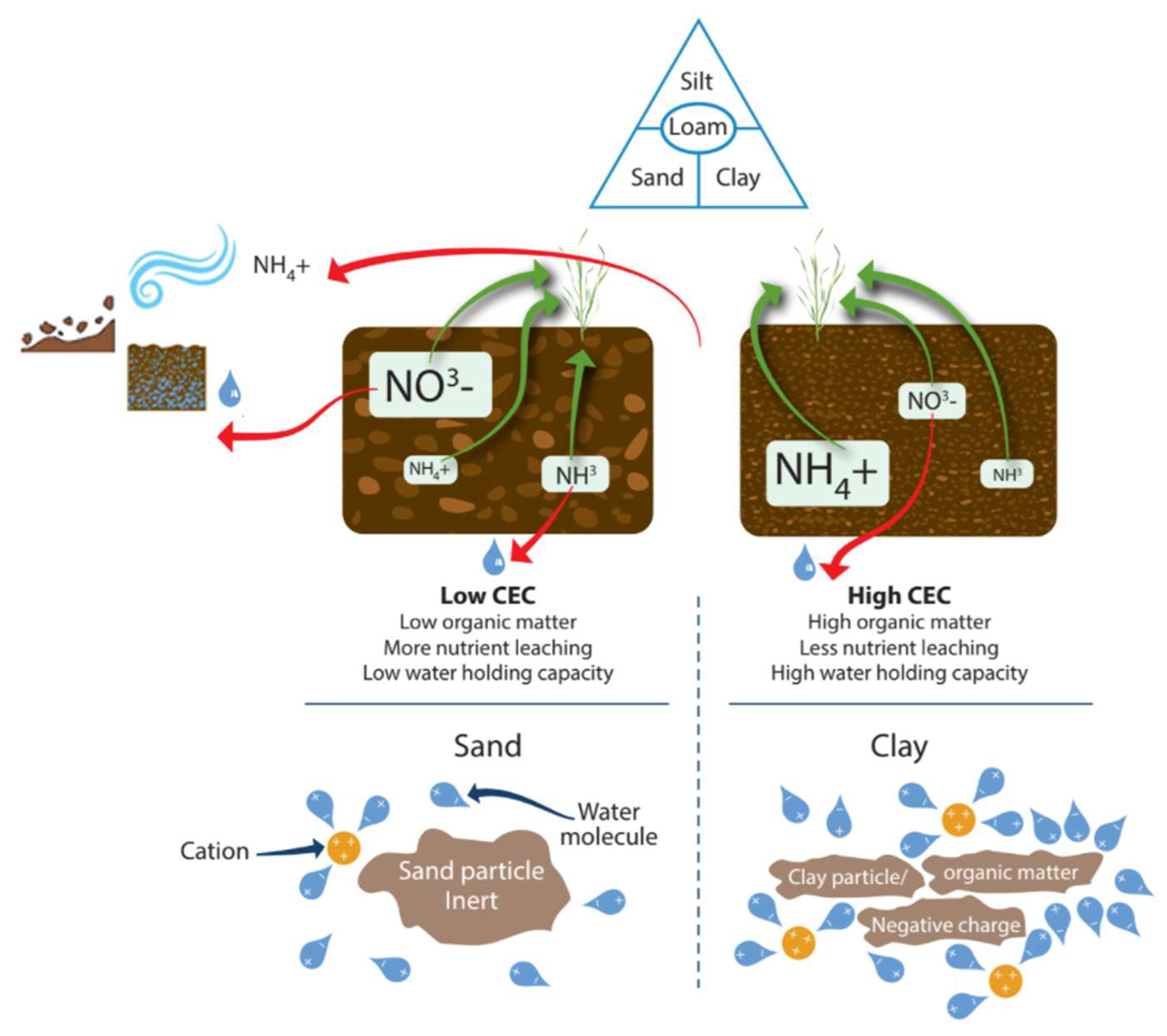
| Texture (CEC) | Yes | No | No | 1–4 | Observation/lab test | |||
| OM (CEC) | Yes | Low | Yes | 1–4 | ||||
| Field | Topography | Yes | No | No | 1–2 | Observation/simple sensor | [41,52,53] | |
| Relative topography (elevation) | Yes | No | No | 1–4 | ||||
| Soil depth | Yes | No | No | 1–3 | Query/field test/sensor | |||
| Crop residue | Low | Yes | Yes | 1 | Observation | |||
| Crop | Crop density | Yes | Yes | Yes | 1–2 | Observation/simple sensor | [41,43,49,54] | |
| Growth stage | Low | Yes | Partially | 1 | Observation | |||
| Root biomass | Yes | Yes | Partially | 4 | Field/lab test | |||
| Anomalous | Weeds | Yes | Yes | Yes | 1–2 | Observation/query/record | [43,55] | |
| Disease | Yes | Yes | Yes | 1–2 | ||||
| Pests | Yes | Yes | Yes | 1–2 | ||||
| Yield | Previous yield | Yes | Yes | Partially | 1 | Query | [56] | |
| Manageable Cause of NUE Reduction | Risk If Not Managed/Typical Risk | Relevant Soil Variable Property | Management Solution | Implications Derived from Focus Groups | |
|---|---|---|---|---|---|
| Inputs | Missing target area (field boundary; management unit) | High Low | None |
| Importance: high Concern: low |
| Overlap/underlap of swaths/spray causing wrong application rate | High Medium | None |
| Importance: mid Concern: low | |
| Choice of fertilizer for the soil, plant and environmental conditions | Medium Medium | Texture pH |
| Importance: high Concern: variable from high to low | |
| Flow between pools | CNR >25–30:1 encouraging immobilization | High High | CNR SOM |
| Importance: mid Concern: low |
| pH too low or high, encouraging immobilization and restricting uptake | High Medium to High | pH |
| Importance: high Concern: mid | |
| Poor root development to capture water and nutrient resources for the plant throughout the growing season | High Medium to High | SOM Bulk density Microbial biomass Earthworms |
| Importance: high Concern: high | |
| Pests and disease | High Low | Nutrient levels Compaction |
| Importance: high Concern: high | |
| Micronutrient availability | Medium Medium | pH Micronutrients |
| Importance: high Concern: high | |
| Outputs | pH determines N species and loss mechanism | High Medium | pH |
| Importance: high Concern: low |
| Low buffering capacity | Medium Medium | SOM CEC |
| Importance: mid Concern: mid | |
| Anaerobic conditions causing denitrification | High Medium | Soil structure Soil moisture SOM Earthworms |
| Importance: high Concern: low | |
| Runoff and erosion losses | Medium Medium | Capping Bulk density Earthworms SOM Compaction |
| Importance: mid Concern: mid/high | |
| Poor nutrient retention | Medium Medium | CEC SOM |
| Importance: high Concern: high | |
| Inefficient harvesting | High Low | None |
| Importance: high Concern: low | |
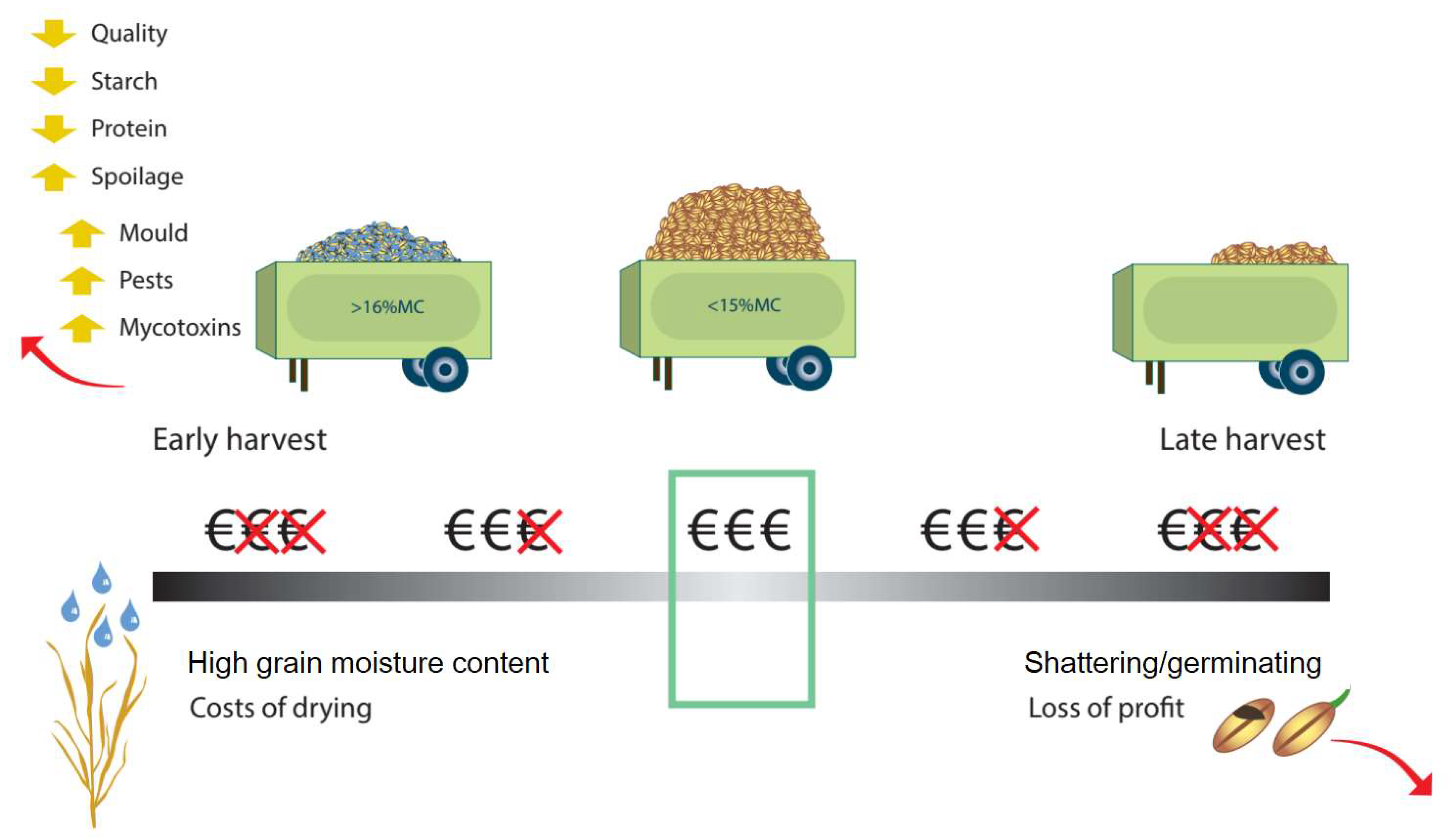
| Harvest timing losses | High Medium | Soil water content |
| Importance: high Concern: low | |
| Storage losses | Medium Low | None |
| Importance: high Concern: mid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whetton, R.L.; Harty, M.A.; Holden, N.M. Communicating Nitrogen Loss Mechanisms for Improving Nitrogen Use Efficiency Management, Focused on Global Wheat. Nitrogen 2022, 3, 213-246. https://doi.org/10.3390/nitrogen3020016
Whetton RL, Harty MA, Holden NM. Communicating Nitrogen Loss Mechanisms for Improving Nitrogen Use Efficiency Management, Focused on Global Wheat. Nitrogen. 2022; 3(2):213-246. https://doi.org/10.3390/nitrogen3020016
Chicago/Turabian StyleWhetton, Rebecca L., Mary A. Harty, and Nicholas M. Holden. 2022. "Communicating Nitrogen Loss Mechanisms for Improving Nitrogen Use Efficiency Management, Focused on Global Wheat" Nitrogen 3, no. 2: 213-246. https://doi.org/10.3390/nitrogen3020016
APA StyleWhetton, R. L., Harty, M. A., & Holden, N. M. (2022). Communicating Nitrogen Loss Mechanisms for Improving Nitrogen Use Efficiency Management, Focused on Global Wheat. Nitrogen, 3(2), 213-246. https://doi.org/10.3390/nitrogen3020016






