Age-Related Changes in Water and Nitrogen Utilization in Crop Trees and Understory Vegetation in a Hinoki Cypress Plantation Forest in Kochi City, Southern Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Litterfall
2.3. Plant Water Utilization
2.4. Statistical Analysis
3. Results
3.1. Climate
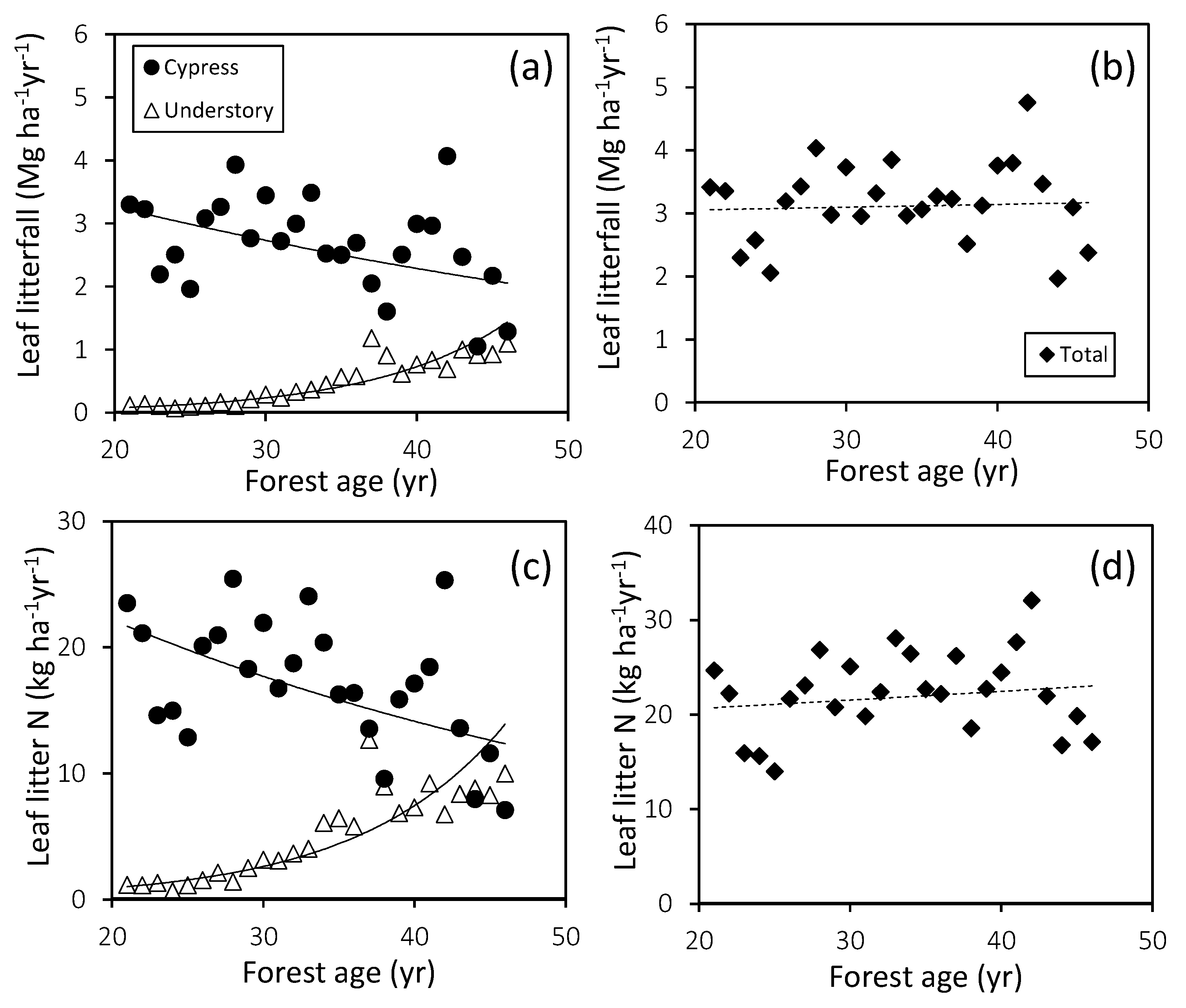
3.2. Stem Growth and Leaf Litterfall
3.3. Plant Nitrogen Utilization
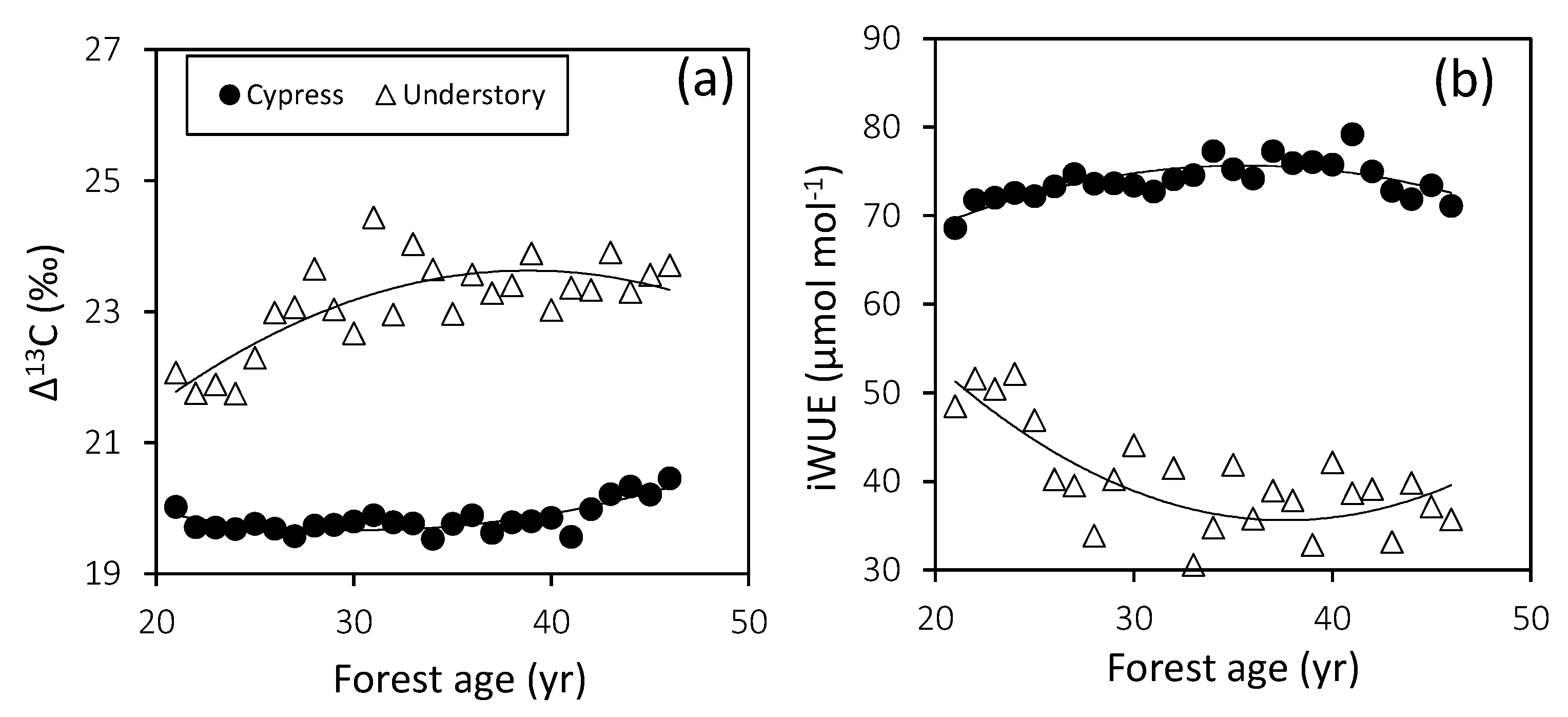
3.4. Plant Water Utilization
4. Discussion
4.1. Forest Productivity
4.2. Plant Nitrogen Utilization
4.3. Plant Water Utilization
4.4. Implication for Forest Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forest Agency. Annual Report on Forest and Forestry in Japan, Fiscal Year 2020. 2021. Available online: https://www.rinya.maff.go.jp/j/kikaku/hakusyo/R2hakusyo/index.html (accessed on 23 March 2022). (In Japanese)
- Hansen, A.J.; Spies, T.A.; Swanson, F.J.; Ohmann, J.L. Conserving biodiversity in managed forests—lessons from natural forests. Bioscience 1991, 41, 382–392. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Qin, G.Z.; Zhai, Z.; Zhou, S.C.; Tang, L.Z.; Tian, Y. Diverse understory vegetation alleviates nitrogen competition with crop trees in poplar plantations. Forests 2021, 12, 705. [Google Scholar] [CrossRef]
- Seiwa, K.; Eto, Y.; Hishita, M.; Masaka, K. Effects of thinning intensity on species diversity and timber production in a conifer (Cryptomeria japonica) plantation in Japan. J. For. Res. 2012, 17, 468–478. [Google Scholar] [CrossRef]
- Seiwa, K.; Etoh, Y.; Hisita, M.; Masaka, K.; Imaji, A.; Ueno, N.; Hasegawa, Y.; Konno, M.; Kanno, H.; Kimura, M. Roles of thinning intensity in hardwood recruitment and diversity in a conifer, Cryptomeria japonica plantation: A 5-year demographic study. For. Ecol. Manag. 2012, 269, 177–187. [Google Scholar] [CrossRef]
- Noguchi, M.; Okuda, S.; Miyamoto, K.; Itou, T.; Inagaki, Y. Composition, size structure and local variation of naturally regenerated broadleaved tree species in hinoki cypress plantations: A case study in Shikoku, south-western Japan. Forestry 2011, 84, 493–504. [Google Scholar] [CrossRef]
- Miura, S.; Ugawa, S.; Yoshinaga, S.; Yamada, T.; Hirai, K. Floor Cover Percentage Determines Splash Erosion in Chamaecyparis obtusa Forests. Soil Sci. Soc. Am. J. 2015, 79, 1782–1791. [Google Scholar] [CrossRef]
- Bormann, F.H.; Likens, G.R. Pattern and Process in a Forested Ecosystem; Springer: New York, NY, USA, 1979; p. 253. [Google Scholar]
- Tokuchi, N.; Fukushima, K. Long-term influence of stream water chemistry in Japanese cedar plantation after clear-cutting using the forest rotation in central Japan. For. Ecol. Manag. 2009, 257, 1768–1775. [Google Scholar] [CrossRef]
- Chiwa, M. Long-term changes in atmospheric nitrogen deposition and stream water nitrate leaching from forested watersheds in western Japan. Environ. Poll 2021, 287, 117634. [Google Scholar] [CrossRef]
- Takagi, M. Water chemistry of headwater streams under stormflow conditions in catchments covered by evergreen broadleaved forest and by coniferous plantation. Landsc. Ecol. Eng. 2015, 11, 293–302. [Google Scholar] [CrossRef]
- Mitchell, M.; Iwatsubo, G.; Ohrui, K.; Nakagawa, Y. Nitrogen saturation in Japanese forests: An evaluation. For. Ecol. Manag. 1997, 97, 39–51. [Google Scholar] [CrossRef]
- Nishina, K.; Watanabe, M.; Koshikawa, M.K.; Takamatsu, T.; Morino, Y.; Nagashima, T.; Soma, K.; Hayashi, S. Varying sensitivity of mountainous streamwater base-flow NO3− concentrations to N deposition in the northern suburbs of Tokyo. Sci. Rep. 2017, 7, 7701. [Google Scholar] [CrossRef] [PubMed]
- Tateno, R.; Fukushima, K.; Fujimaki, R.; Shimamura, T.; Ohgi, M.; Arai, H.; Ohte, N.; Tokuchi, N.; Yoshioka, T. Biomass allocation and nitrogen limitation in a Cryptomeria japonica plantation chronosequence. J. For. Res. 2009, 14, 276–285. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, L.; Xiang, W.H.; Ouyang, S.; Wu, H.L.; Lei, P.F.; Xiao, W.F.; Li, S.G.; Zeng, L.X.; Kuzyakov, Y. Increase of soil nitrogen availability and recycling with stand age of Chinese-fir plantations. For. Ecol. Manag. 2021, 480, 118643. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Peng, S.; Penuelas, J.; Zeng, H.; Piao, S. Age-related modulation of the nitrogen resorption efficiency response to growth requirements and soil nitrogen availability in a temperate pine plantation. Ecosystems 2016, 19, 698–709. [Google Scholar] [CrossRef]
- Fukushima, K.; Tateno, R.; Tokuchi, N. Soil nitrogen dynamics during stand development after clear-cutting of Japanese cedar (Cryptomeria japonica) plantations. J. For. Res. 2011, 16, 394–404. [Google Scholar] [CrossRef]
- Inagaki, Y.; Miura, S.; Kohzu, A. Effects of forest type and stand age on litterfall quality and soil N dynamics in Shikoku district, southern Japan. For. Ecol. Manag. 2004, 202, 107–117. [Google Scholar] [CrossRef]
- Wu, H.L.; Xiang, W.H.; Ouyang, S.; Xiao, W.F.; Li, S.G.; Chen, L.; Lei, P.F.; Deng, X.W.; Zeng, Y.L.; Zeng, L.X.; et al. Tree growth rate and soil nutrient status determine the shift in nutrient-use strategy of Chinese fir plantations along a chronosequence. For. Ecol. Manag. 2020, 460, 117896. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Shibata, H.; Takagi, K.; Nomura, M.; Kurima, N.; Fukazawa, T.; Satoh, F.; Sasa, K. Effects of clear-cutting on nitrogen leaching and fine root dynamics in a cool-temperate forested watershed in northern Japan. For. Ecol. Manag. 2006, 225, 257–261. [Google Scholar] [CrossRef]
- Masuda, C.; Morikawa, Y.; Masaka, K.; Koga, W.; Suzuki, M.; Hayashi, S.; Tada, C.; Seiwa, K. Hardwood mixture increases stand productivity through increasing the amount of leaf nitrogen and modifying biomass allocation in a conifer plantation. For. Ecol. Manag. 2022, 504, 119835. [Google Scholar] [CrossRef]
- Högberg, P. Tansley review No 95 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L.X. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Wang, L.X.; Shaner, P.J.L.; Macko, S. Foliar δ15N patterns along successional gradients at plant community and species levels. Geophys. Res. Lett. 2007, 34, L16403. [Google Scholar] [CrossRef]
- Perakis, S.S.; Tepley, A.J.; Compton, J.E. Disturbance and topography shape nitrogen availability and δ15N over long-term forest succession. Ecosystems 2015, 18, 573–588. [Google Scholar] [CrossRef]
- Hyodo, F.; Takebayashi, Y.; Makabe, A.; Wardle, D.A.; Koba, K. Changes in stable nitrogen isotopes of plants, bulk soil and soil dissolved N during ecosystem retrogression in boreal forest. Ecol. Res. 2021, 10, 420–429. [Google Scholar] [CrossRef]
- Sun, F.F.; Kuang, Y.W.; Wen, D.Z.; Xu, Z.H.; Li, J.L.; Zuo, W.D.; Hou, E.Q. Long-term tree growth rate, water use efficiency, and tree ring nitrogen isotope composition of Pinus massoniana L. in response to global climate change and local nitrogen deposition in Southern China. J. Soils Sediments 2010, 10, 1453–1465. [Google Scholar] [CrossRef]
- Koba, K.; Hirobe, M.; Koyama, L.; Kohzu, A.; Tokuchi, N.; Nadelhoffer, K.; Wada, E.; Takeda, H. Natural 15N abundance of plants and soil N in a temperate coniferous forest. Ecosystems 2003, 6, 457–469. [Google Scholar] [CrossRef]
- Tateno, R.; Nakayama, M.; Yano, M.; Fukuzawa, K.; Inagaki, Y.; Koba, K.; Ugawa, S. Nitrogen source utilization in co-existing canopy tree and dwarf bamboo in a northern hardwood forest in Japan. Trees-Struct. Funct. 2020, 1047–1057. [Google Scholar] [CrossRef]
- Saurer, M.; Siegwolf, R.T.W.; Schweingruber, F.H. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Chang. Biol. 2004, 10, 2109–2120. [Google Scholar] [CrossRef]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Kubota, T.; Kagawa, A.; Abe, T.; Hosoda, I. Effects of clear-cutting, meteorological, and physiological factors on evapotranspiration in the Kamabuchi experimental watershed in northern Japan. Hydrol. Process. 2021, 35, e14111. [Google Scholar] [CrossRef]
- Saurer, M.; Spahni, R.; Frank, D.C.; Joos, F.; Leuenberger, M.; Loader, N.J.; McCarroll, D.; Gagen, M.; Poulter, B.; Siegwolf, R.T.W.; et al. Spatial variability and temporal trends in water-use efficiency of European forests. Glob. Chang. Biol. 2014, 20, 3700–3712. [Google Scholar] [CrossRef]
- Leonardi, S.; Gentilesca, T.; Guerrieri, R.; Ripullone, F.; Magnani, F.; Mencuccini, M.; Noije, T.V.; Borghetti, M. Assessing the effects of nitrogen deposition and climate on carbon isotope discrimination and intrinsic water-use efficiency of angiosperm and conifer trees under rising CO2 conditions. Glob. Chang. Biol. 2012, 18, 2925–2944. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Field, C.B.; Lin, Z.F.; Kuo, C.Y. Leaf carbon isotope and mineral-composition in subtropical plants along an irradiance cline. Oecologia 1986, 70, 520–526. [Google Scholar] [CrossRef]
- Kenzo, T.; Inoue, Y.; Yoshimura, M.; Yamashita, M.; Tanaka-Oda, A.; Ichie, T. Height-related changes in leaf photosynthetic traits in diverse Bornean tropical rain forest trees. Oecologia 2015, 177, 191–202. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; Natural Resources Conservation Service, USDA: Washington, DC, USA, 2014.
- Mitsuda, Y.; Inoue, A.; Kitahara, F.; Kadota, H.; Hirota, T. Examination of factors affecting the error of aboveground biomass estimation in overcrowded planted stands: A case study using sample felled trees from a 40-year-old Hinoki (Chamaecyparis obtusa) planted stands. Jpn. J. For. Plan. 2012, 46, 15–24, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Inagaki, Y.; Nakanishi, A.; Tange, T. A simple method for leaf and branch biomass estimation in Japanese cedar plantations. Trees-Struct. Funct. 2020, 34, 349–356. [Google Scholar] [CrossRef]
- Sakai, A.; Inagaki, Y. Changes of understory vegetation in a middle-aged hinoki cypress plantation forest in Shikoku Research Center. Ann. Rep. Shikoku Res. Cent. For. For. Prod. Res. Inst. 2018, 59, 24–25. (In Japanese) [Google Scholar]
- Inagaki, Y.; Sakai, A.; Kuramoto, S.; Kodani, E.; Yamada, T.; Kawasaki, T. Inter-annual variations of leaf-fall phenology and leaf-litter nitrogen concentration in a hinoki cypress (Chamaecyparis obtusa Endlicher) stand. Ecol. Res. 2008, 23, 965–972. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Inagaki, Y.; Nakanishi, A.; Fukata, H.; Watanabe, N. Effects of thinning on leaf biomass and stem biomass production in hinoki cypress plantations in Kochi prefecture. Bull. For. For. Prod. Res. Inst. 2021, 20, 277–285, (In Japanese with English Summary). [Google Scholar]
- Ando, T.; Hatiya, K.; Doi, K.; Kataoka, H.; Kato, Y.; Sakaguchi, K. Studies on the system of density control of sugi (Cryptomeria japonica) stand. Bull. Exp. For. Sta. 1968, 209, 1–76, (In Japanese with English Summary). [Google Scholar]
- Sumida, A.; Watanabe, T.; Miyaura, T. Interannual variability of leaf area index of an evergreen conifer stand was affected by carry-over effects from recent climate conditions. Sci. Rep. 2018, 8, 13590. [Google Scholar] [CrossRef]
- Miyamoto, K.; Okuda, S.; Inagaki, Y.; Noguchi, M.; Itou, T. Within- and between-site variations in leaf longevity in hinoki cypress (Chamaecyparis obtusa) plantations in southwestern Japan. J. For. Res. 2013, 18, 256–269. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okuda, S.; Sakai, A.; Nakanishi, A.; Shibata, S.; Fukata, H. Leaf-litter nitrogen concentration in hinoki cypress forests in relation to the time of leaf fall under different climatic conditions in Japan. Ecol. Res. 2010, 25, 429–438. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kuramoto, S.; Torii, A.; Shinomiya, Y.; Fukata, H. Effects of thinning on leaf-fall and leaf-litter nitrogen concentration in hinoki cypress (Chamaecyparis obtusa Endlicher) plantation stands in Japan. For. Ecol. Manag. 2008, 255, 1859–1867. [Google Scholar] [CrossRef]
- Fang, Y.; Koba, K.; Yoh, M.; Makabe, A.; Liu, X. Patterns of foliar δ15N and their control in Eastern Asian forests. Ecol. Res. 2013, 28, 735–748. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Koba, K.; Sasaki, Y.; Fang, Y.; Yoh, M. The natural abundance of 15N in plant and soil-available N indicates a shift of main plant N resources to NO3− from NH4+ along the N leaching gradient. Rap. Commun. Mass. Spectrom. 2010, 24, 1001–1008. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kohzu, A. Microbial immobilization and plant uptake of different N forms in three forest types in Shikoku district, southern Japan. Soil Sci. Plant Nutr. 2005, 51, 667–670. [Google Scholar] [CrossRef][Green Version]
- Inagaki, Y.; Nakanishi, A.; Fukata, H. Soil properties and nitrogen utilization of hinoki cypress as affected by strong thinning under different climatic conditions in the Shikoku and Kinki districts in Japan. J. For. Res. 2011, 16, 405–413. [Google Scholar] [CrossRef]
- Takahashi, M. Nutrient storage and stoichiometry of the forest floor organic matter in Japanese forests. Soil. Syst. 2021, 5, 51. [Google Scholar] [CrossRef]
- Poulson, S.R.; Chamberlain, C.P.; Friedland, A.J. Nitrogen isotope variation of tree-rings as a potential indicator of environmental-change. Chem. Geol. 1995, 125, 307–315. [Google Scholar] [CrossRef]
- Choi, W.J.; Lee, S.M.; Chang, S.X.; Ro, H.M. Variations of δ13C and δ15N in Pinus densiflora tree-rings and their relationship to environmental changes in eastern Korea. Water Air Soil Poll. 2005, 164, 173–187. [Google Scholar] [CrossRef]
- Yamada, T.; Yoshinaga, S.; Morisada, K.; Hirai, K. Sulfate and nitrate loads on a forest ecosystem in Kochi in southwest of Japan. Water Air Soil Poll. 2001, 130, 1115–1120. [Google Scholar] [CrossRef]
- Hishi, T.; Tateno, R.; Fukushima, K.; Fujimaki, R.; Itoh, M.; Tokuchi, N. Changes in the anatomy, morphology and mycorrhizal infection of fine root systems of Cryptomeria japonica in relation to stand ageing. Tree Physiol. 2017, 37, 61–70. [Google Scholar]
- Karizumi, N. Illustrations of Tree Roots; Seibundo-Shinkosha: Tokyo, Japan, 1979. (In Japanese) [Google Scholar]
- Kume, A.; Satomura, T.; Tsuboi, N.; Chiwa, M.; Hanba, Y.T.; Nakane, K.; Horikoshi, T.; Sakugawa, H. Effects of understory vegetation on the ecophysiological characteristics of an overstory pine, Pinus densiflora. For. Ecol. Manag. 2003, 176, 195–203. [Google Scholar] [CrossRef]



| 2002 | 2017 | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Tree | 22 | (28) | 24 | (28) |
| Shrub | 17 | (20) | 14 | (16) |
| Liana | 15 | (18) | 12 | (14) |
| Herb | 17 | (20) | 14 | (16) |
| Fern | 11 | (13) | 21 | (25) |
| Total | 83 | (100) | 85 | (100) |
| 2002 | 2017 | |||
|---|---|---|---|---|
| Species Name | Height (m) | Cover Classes * | Height (m) | Cover Classes * |
| Symplocos glauca | 3.1 | 2 | 7.2 | 4 |
| Daphniphyllum macropodum | 2.4 | + | 6.3 | 1 |
| Eurya japonica | 3.6 | 1 | 5.6 | + |
| Cleyera japonica | 2.6 | + | 5.1 | 1 |
| Neolitsea sericea | 1.2 | + | 5.3 | 1 |
| Liquidambar formosana | 4.5 | 1 | ||
| Ficus erecta | 2.9 | 2 | 4.0 | 2 |
| Rubus buergeri | 0.2 | 1 | 0.2 | + |
| Ligustrum lucidum | 2.8 | 1 | 4.0 | 1 |
| Dryopteris erythrosora | 0.6 | 1 | 0.8 | 1 |
| Forest Age | Stand Density | Height | DBH | Stem Biomass | Stem Production | Leaf Biomass | Leaf Litterfall | Leaf Biomass/ Leaf Litterfall | |
|---|---|---|---|---|---|---|---|---|---|
| yr | n ha−1 | m | cm | Mg ha−1 | Mg ha−1 yr−1 | Mg ha−1 | Mg ha−1 yr−1 | yr | |
| 21–22 | 4264 | 10.9 | 9.7 | 98.8 | 5.6 | 17.7 | 3.3 | 5.4 | |
| 23–24 | 2528 | 12.1 | 11.4 | 82.1 | 6.5 | 13.7 | 2.3 | 5.8 | Thinning |
| 25–27 | 2528 | 12.9 | 12.3 | 99.9 | 5.9 | 15.6 | 2.8 | 5.6 | |
| 28–30 | 2452 | 13.3 | 13 | 113.1 | 4.4 | - | 3.4 | - | |
| 31–32 | 2302 | 14.4 | 13.6 | 124.3 | 5.6 | 14.5 | 2.9 | 5.1 | |
| 33–34 | 2189 | 15.1 | 14.3 | 135.0 | 5.3 | - | 3.0 | - | |
| 35–37 | 1774 | 15.7 | 15.3 | 129.4 | 2.9 | - | 2.4 | - | Thinning |
| 38–39 | 1623 | 16.7 | 16.2 | 137.2 | 3.9 | - | 2.1 | - | |
| 40–41 | 1547 | 17.3 | 16.9 | 148.4 | 5.6 | 12.5 | 3.0 | 4.2 | |
| 42–43 | 1472 | 18.1 | 17.8 | 161.8 | 6.7 | 13.3 | 3.3 | 4.1 | |
| 44–46 | 1057 | 19.5 | 19.6 | 147.9 | 2.3 | 11.9 | 1.5 | 7.9 | Thinning |
| Object Variable | Category | Equation | R2 | p | Annual Rate of Change (%) | Tipping Point (yr) |
|---|---|---|---|---|---|---|
| Leaf litterfall | Cypress | Ln(y) = −0.018x + 1.54 | 0.183 | 0.0289 | −1.8 | |
| Understory | Ln(y) = 0.113x − 4.82 | 0.884 | 0.0001 | 11.9 | ||
| Total | Ln(y) = 0.001x + 1.09 | 0.003 | 0.798 | 0.1 | ||
| Leaf litter N | Cypress | Ln(y) = −0.022x + 1.25 | 0.265 | 0.0071 | −2.2 | |
| Understory | Ln(y) = 0.104x − 4.45 | 0.858 | 0.0001 | 11.0 | ||
| Total | Ln(y) = 0.004x + 0.64 | 0.026 | 0.435 | 0.4 | ||
| Leaf N concentration | Cypress | y = −0.027x + 7.31 | 0.121 | 0.0815 | ||
| Cypress * | y = −0.040x + 7.60 | 0.507 | 0.0001 | |||
| Understory | y = −0.0152x2 + 0.926x − 2.14 | 0.438 | 0.0013 | 30 | ||
| Total | y = 0.021x + 6.35 | 0.059 | 0.229 | |||
| δ15N | Cypress | y = −0.0037x2 + 0.187x − 5.38 | 0.871 | 0.0001 | 26 | |
| Understory | y = 0.0036x2 − 0.332x + 5.60 | 0.813 | 0.0001 | 45 | ||
| Total | y = −0.002x2 + 0.098x − 4.19 | 0.401 | 0.0027 | 28 | ||
| δ13C | Cypress | y = −0.003x2 + 0.125x − 28.62 | 0.885 | 0.0001 | 25 | |
| Understory | y = 0.006x2 − 0.459x − 21.70 | 0.745 | 0.0001 | 41 | ||
| Total | y = −0.003x2 + 0.141 − 28.54 | 0.898 | 0.0001 | 21 | ||
| Δ13C | Cypress | y = 0.003x2 − 0.159 + 22.07 | 0.652 | 0.0001 | 30 | |
| Understory | y = −0.006x2 + 0.451x + 14.86 | 0.620 | 0.0001 | 39 | ||
| iWUE | Cypress | y = −0.028x2 + 2.013x + 39.89 | 0.582 | 0.0001 | 36 | |
| Understory | y = 0.057x2 − 4.266x + 115.9 | 0.516 | 0.0002 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inagaki, Y.; Miyamoto, K.; Sakai, A. Age-Related Changes in Water and Nitrogen Utilization in Crop Trees and Understory Vegetation in a Hinoki Cypress Plantation Forest in Kochi City, Southern Japan. Nitrogen 2022, 3, 247-259. https://doi.org/10.3390/nitrogen3020017
Inagaki Y, Miyamoto K, Sakai A. Age-Related Changes in Water and Nitrogen Utilization in Crop Trees and Understory Vegetation in a Hinoki Cypress Plantation Forest in Kochi City, Southern Japan. Nitrogen. 2022; 3(2):247-259. https://doi.org/10.3390/nitrogen3020017
Chicago/Turabian StyleInagaki, Yoshiyuki, Kazuki Miyamoto, and Atsushi Sakai. 2022. "Age-Related Changes in Water and Nitrogen Utilization in Crop Trees and Understory Vegetation in a Hinoki Cypress Plantation Forest in Kochi City, Southern Japan" Nitrogen 3, no. 2: 247-259. https://doi.org/10.3390/nitrogen3020017
APA StyleInagaki, Y., Miyamoto, K., & Sakai, A. (2022). Age-Related Changes in Water and Nitrogen Utilization in Crop Trees and Understory Vegetation in a Hinoki Cypress Plantation Forest in Kochi City, Southern Japan. Nitrogen, 3(2), 247-259. https://doi.org/10.3390/nitrogen3020017





