Abstract
Nitrogen (N) losses are a major environmental issue. Globally, crop N fertilizer applications are excessive, and N use efficiency (NUE) is low. N loss represents a significant economic loss to the farmer. NUE is difficult to quantify in real time because of the multiple chemical–biological–physical factors interacting. While there is much scientific understanding of N interactions in the plant–soil system, there is little formal expression of scientific knowledge in farm practice. The objective of this study was to clearly define the factors controlling NUE in wheat production, focusing on N inputs, flows, transformations, and outputs from the plant–soil system. A series of focus groups were conducted with professional agronomists and industry experts, and their technical information was considered alongside a structured literature review. To express this understanding, clear graphical representations are provided in the text. The analysis of the NUE processes revealed 16 management interventions which could be prioritized to increase farm nitrogen use efficiency. These management interventions were grouped into three categories—inputs, flow between pools, and outputs—and include management options through the range of application errors, fertilizer input choice, root development, pests and disease, soil structure, harvesting and storage errors, and soil resources of water, micronutrients, carbon, nitrogen, and pH. It was noted that technical solutions such as fertilizer formulation and managing organic matter require significant supply chain upgrades. It was also noted that farm-scale decision support would be best managed using a risk/probability-based recommender system rather than generic guidelines.
1. Introduction
Nitrogen (N) is an essential input to large-scale crop production. Globally, the demand for N fertilizers was just under 120 million tonnes in 2020 [1], and much of this was produced by the fossil-fuel-reliant Haber–Bosch process [2]. N fertilizer applications for agricultural crops are excessive [3], and N losses are a major environmental issue [4]. In addition, it is estimated that biological fixation of N in soils is between 50–70 million tonnes N year−1 [5]. This means that the biogeochemical cycle of N has been radically changed by agriculture, and N is now regarded as a major environmental pollutant [6].
N management enabled dramatic crop yield increases in the 20th century [4], but wheat yields have plateaued in the 21st century, with only modest increases being attributed to improved nitrogen use efficiency (NUE) [7]. A proportion of the N applied to crops is not assimilated by the crop and therefore is lost to the wider environment [6]. In some countries, the amount that can be legally applied is restricted by legislation [8]. Efficiency is defined as “achieving maximum productivity with minimum wasted effort or expense” (Oxford University Press), so for wheat production, this means achieving the maximum output (sellable yield) for the minimum use of inputs (time, equipment, energy, N, other crop nutrients, and agrochemicals). In practice, the basis for calculating NUE is poorly defined yet assumed to be understood.
NUE can be defined in multiple ways: a ratio per unit N added to the soil of yield (kg grain/kg N) [9], total crop biomass (kg biomass/kg N) [9], or quality of grain harvested (kg grain N/kg N) [9]; the sellable yield value per unit N cost (€ grain/€ N) [10]; the anthropogenic N input per unit annual available N (kg fertilizer N/kg mineralizable N) [11]; or the N removed per unit N added (kg biomass N/kg N) [12]. For the purposes of this work, we define NUE as the kg grain of target N content (i.e., a defined quality) per kg N added (whether mineral N or organic N). Godinot et al. [13] suggest a more refined approach (called system nitrogen efficiency) for assessing NUE, instead of a simple ratio of N in and N out. Their approach considered (i) N emissions from the production of inputs outside the farm; (ii) net flow of N in the farming system; (iii) annual changes in soil N; and (iv) removal by crop and animal products (but not by manure). They demonstrated the approach with a wide variety of farming systems; however, it is difficult to implement as it requires much data, including life cycle assessment data and values for N inputs and outputs. Quemada et al. [14] used NUE (N outputs to inputs at farm scale), N surplus, and N product output over multiple fields and found the largest variation in NUE was attributed to farm management, climate, and soil conditions. EUNEP [15] suggested the ideal NUE occurs when no loss of soil nitrogen occurs, and that farms that cannot achieve this condition should adjust their management practices.
Wheat N management is an open system with inputs to soil mainly from mineral and organic fertilizers [7], and small contributions from N deposition and biological fixation [16]. Fixation can be important in a rotation with a legume crop [17]. N is stored in pools as it flows through the system, as plant available and unavailable forms in the soil [18], as different N species in the plant [19], and as a range of storage proteins in the grain [20]. Various microbial transformations alter the N speciation in the soil, which may make the N unavailable to the crop. N outputs occur through four major pathways: (i) crop N uptake and crop removal at harvest; (ii) gaseous N emissions; (iii) aqueous N losses by leaching and runoff; and (iv) erosion losses when N is bound to soil particles [21,22]. Increasing NUE minimizes N loss to the wider environment and reduces both the economic cost [23] and environmental impacts [6]. Furthermore, increasing NUE reduces the N inputs, optimizes the transfer of N between the pools, and maximizes N in the sellable grain.
Multiple interacting chemical, biological, and physical factors affect NUE, and each of these are spatially and temporally variable over different scales [24,25]. Raun et al. [25] show that significant differences can occur in concentrations of both mobile and immobile nutrients at spatial scales under 1 m. Soil properties other than texture are also subject to temporal variation, and this is especially the case with temperature and moisture content that have important effects on soil N cycling. Sharma and Bali [26] reviewed methods and tools available for N management and found no single method was sufficient to counter N loss. Peralta et al. [27] compared techniques and properties to measure within-field variations in N demand but found the properties that explained N demand in one field were unimportant in the next. They attributed this to the complex, non-deterministic interrelationships between the crop and soil characteristics. Sharma and Bali [26] suggest that optical sensor data would be more useful in NUE decisions than conventional soil and plant tissue testing, but further research about the use of such sensors is required. A soil–weather–time combination can influence N dynamics, so understanding which factors control and regulate transformation and loss processes and how to manage these factors at a given time and location is required. Quemada et al. [14] found the conceptual graphical diagram proposed by the European N expert panel [15] was useful to analyze farm NUE, but soil N dynamics should also be included. This research feedback from the focus groups addressed widely accessible NUE guidance rather than advanced technology and data methods with limited uptake by farmers.
The work presented here is an important step in codifying understanding of N management, which can help ensure NUE does not limit yield or cause unnecessary environmental impacts. Understanding the factors, and their interactions, that regulate N supply, N transformation, and N loss mechanisms can help identify routes to achieve improved NUE without a cost/technology barrier to entry. We present graphical diagrams of each of the N loss and transformation mechanisms as tools for communication and summarize practical actions that can improve NUE. The work was based on the integration of scientific knowledge and the practical experience of working agronomists.
2. Methods
Two approaches were combined to collect the data needed to identify the key properties and information about their interactions. Initially, a literature review collated scientific understanding of the factors controlling the N cycle in terms of inputs, pools, transformations, and outputs in cereal crop production. In addition, focus groups with professional agronomists working in the UK were used to identify both the current understanding of NUE and current practices. The information derived from these two activities was structured using causal loop diagrams [28] and translated to a standard graphical language with the assistance of a graphic designer. The new codified understanding was then used by the agronomists to identify how to better improve field-scale NUE.
2.1. Literature Review
A literature search was conducted using Google Scholar and Scopus with the following criteria:
- Search terms: nitrogen use efficiency; nitrogen cycle; nitrogen loss mechanisms; ammonia volatilization; denitrification; nitrification; immobilization; mineralization; leaching; runoff; erosion; root interaction; compaction; uptake; translocation; application; harvest; grain storage.
- Inclusion terms: soil, nitrogen, cereal crop, field, agriculture, and cereals; precision agriculture; wheat, 2010–2019.
- Exclusion terms: crop breeding, Genetically Modified Organisms (GMO), biochar, tree, medicine. Duplications, papers unrelated to the topic (e.g., rice, barley), and those targeted at nutrition were also excluded.
Review of the title and abstract after applying exclusion terms focused the results, and a full text review identified relevant cited studies to include. For full-text review, 10 papers per search term were selected based on weighting recent publications (post 2015) and the most highly cited ones from the search period. Any studies cited in the top 10 lists but not already selected were also reviewed.
2.2. Focus Groups
The role of the focus groups was to convey the range of opinion and common practice of professionals in commercial agronomy with expertise in wheat production. Three focus groups were convened, each consisting of 10–15 experts, with knowledge of agronomy, crop production, and soil science. Participation was voluntary, and everyone agreed in advance to the sessions being recorded and used for this purpose. Working sessions lasted 60 to 90 min. Each focus group had a specific objective. The first group identified the most important factors influencing NUE as understood in practice and included discussion of NUE, N inputs, soil factors, plant factors, the harvest process, grain storage, and the influence of weather and the environment. The second focused on nitrogen losses including a discussion of N loss mechanisms and transformations and practical N loss reduction strategies. The third group ranked properties that influence N loss risks, N loss mechanisms, and other outputs. Discussions were recorded and transcribed to identify insights related to practical and social factors not commonly found in journal papers. This information was used to enhance the literature knowledge and to place it in the real-world context of commercial practice.
2.3. Structuring of the Information
The key mechanisms and most influential physical, chemical, and biological factors were identified from the literature, and these were used as the starting point for diagramming the processes. Infrastructure and social aspects were included where they had been identified as influential by the focus groups. The final graphic outputs focused on factors that are controlled by the farmer or those imposed on the farmer by external forces, such as market price and weather. The key factors were considered temporally independent during the diagram development, but subsequent analysis considered how the information would interact with spatial and temporal variability.
2.4. Causal Loop Diagrams
The findings were organized hierarchically by inputs, transformations, and outputs, and then by mechanism. The relationships associated with the important mechanisms were expressed as causal loop diagrams (CLD) [28], where each factor (a node in the diagram) was analyzed in terms of its relationship (the edges in the diagram) with other factors as being either a positive or negative correlation, such that factors interact through loops that can be limiting, amplifying, or regulating. This process structured the complex interactions of N in the agricultural system.
2.5. Graphic Design
A common graphic language was created to capture the most influential factors affecting each N mechanism and to depict these factors in diagrams arranged by input, transformation, and output. The style of iconography in the diagrams was designed to be easily understood, requiring only limited technical knowledge and few words. The diagrams were evaluated using separate focus groups, including academic researchers and agriculture practitioners.
3. Results
The initial literature search returned more than 15,000 results for each search term, which was reduced to the 500 most relevant after incorporating exclusion criteria. Preliminary review of the title and abstract further focused the result to the 200 most relevant papers; from those papers, 152 research papers, 2 dissertations, 12 reports/policies, 8 books, and 4 websites were reviewed to represent the body of knowledge.
The contributions from the focus groups further refined the information and provided context by considering practical, social, and infrastructure factors. For example, in deciding what metric to use for NUE, it was agreed to use “harvested offtake as the metric for nitrogen use efficiency” because “that’s the driving factor…that is what we sell.” The preliminary CLD are not presented as they were an intermediate step and were not made with rigorous model development in mind. The final graphics are presented in the discussion so that they can be related to the explanations and implications.
A summary of the most important properties (Table 1) was used as the basis for CLD and graphical representation of N inputs, pools, transformations, and outputs from the soil–crop system. The priority properties were evaluated in terms of the ability of the farmer to exert management control, the importance of spatial and temporal variation, and the ease with which data might be collected. The reasons for this classification are outlined in the following sub-sections: “Management control”, “Spatial and temporal variation”, and “Data acquisition (method and ease)”.

Table 1.
Summary of the key literature identifying priority properties by category, importance of spatial and temporal variability at field scale, the possibility of management control (yes, no, low, partially), and the method and ease of data collection (1 = easy, 4 = difficult).
3.1. Management Control
Godwin and Miller [41] presented factors influencing yield variation and categorized them into those with little or possible control, e.g., factors subject to little if any control include soil texture, climate, and topography [57]. Factors for possible control include soil structure, available water, waterlogging, nutrient levels, pH levels, trace elements, weeds, pests, and diseases. Following this approach, factors important for N loss mechanisms (Table 1) were classified as manageable (Yes), under limited control (Partially), and not controllable (No). NUE management must work within the limits exerted by the uncontrollable factors (e.g., weather factors), and dynamic responses are only possible using those factors which are controllable. Other uncontrollable soil and land resource factors still require attention as they will define management zones.
3.2. Spatial and Temporal Variation
Zhang et al. [58] defined six categories for classifying NUE factors: (i) yield (historical and present), (ii) field (elevation, aspect, and slope), (iii) soil variability (fertility, physical texture, depth and compaction, pH, organic matter, moisture), (iv) crop variability (density, height, nutrient status, chlorophyll content, quality), (v) anomalous factors (competition from weeds and damage from insects and disease), and (vi) management variability (tillage system, seeding rate, choice of rotation, chemical applications), each of which has a spatial and temporal component. Variability was classified as frequent and large (Yes), infrequent or small (Low), and of little importance (No). While weather and soil moisture content can contribute to soil variability, the climate and prevailing weather are also a key contributing factor in field operation decisions [45] and need to be considered as a further layer. For example, O’Neal et al. [56] highlighted the importance of precipitation monitoring in decision making for fertilizer applications.
3.3. Data Acquisition (Method and Ease)
Ease of data collection for the factors highlighted from the literature was categorized from 1 (easy) to 4 (difficult) (Table 1). For the management category, it is easy to collect information on all factors through a query with the farmer, a review of farm records, and publications. Data collection for factors in the weather and climate section can be acquired easily with the use of simple onsite sensors or by accessing information from a local weather station. There are many options for acquiring field data, including installed instruments such as Time Domain Reflectometry (TDR) to measure soil moisture [57] and one-off observations of physical properties, Visual Evaluation of Soil Structure [59] soil sampling for laboratory analysis [60], in situ chemical tests (however, these often have limitations and reduced accuracy [60,61]), and pedo-transfer functions [49,62]. The topography of the field and surrounding area is easily observed for broad classifications, or sensors can be used for more detailed understanding [63]. Crop properties, disease, weeds, and pests can be determined using skilled observations or remote sensing.
4. Discussion
Worldwide, NUE for cereal production is estimated to be between 33 and 40% [64,65,66], while Zhou et al. [22] found a similar NUE for wheat of ~30–42%. The literature review identified the limitations of NUE, while the focus group discussions provided greater understanding of practical and supply chain issues pertinent to NUE. Combining information sources helped to identify where adjustments in management practice can offer improved NUE.
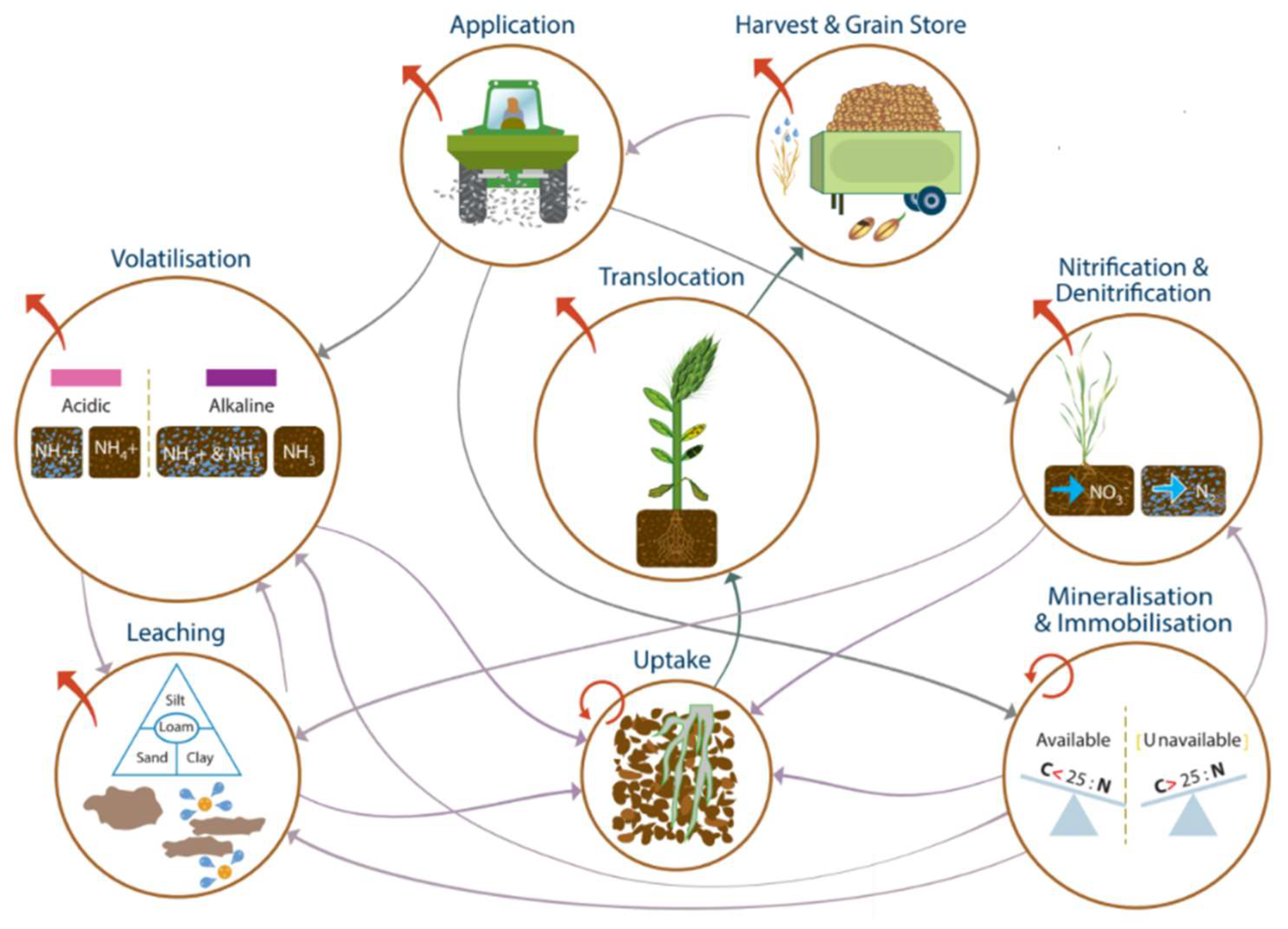
The focus group discussion was structured by flows between pools as well as inputs to and outputs from the system. The interconnected nature of the N pools and flows means there are multiple interactions between properties and processes at each stage, and these were used to help to identify the priority factors for NUE management (Figure 1). This summary view of the information was analyzed in subsequent figures to identify the main opportunities for management intervention.
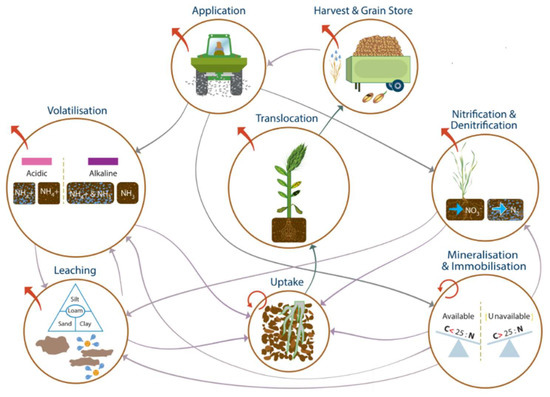
Figure 1.
The N loss mechanism web showing interactions between N pools and the processes that regulate inputs, flows, and outputs.
4.1. N Inputs to the System
Application
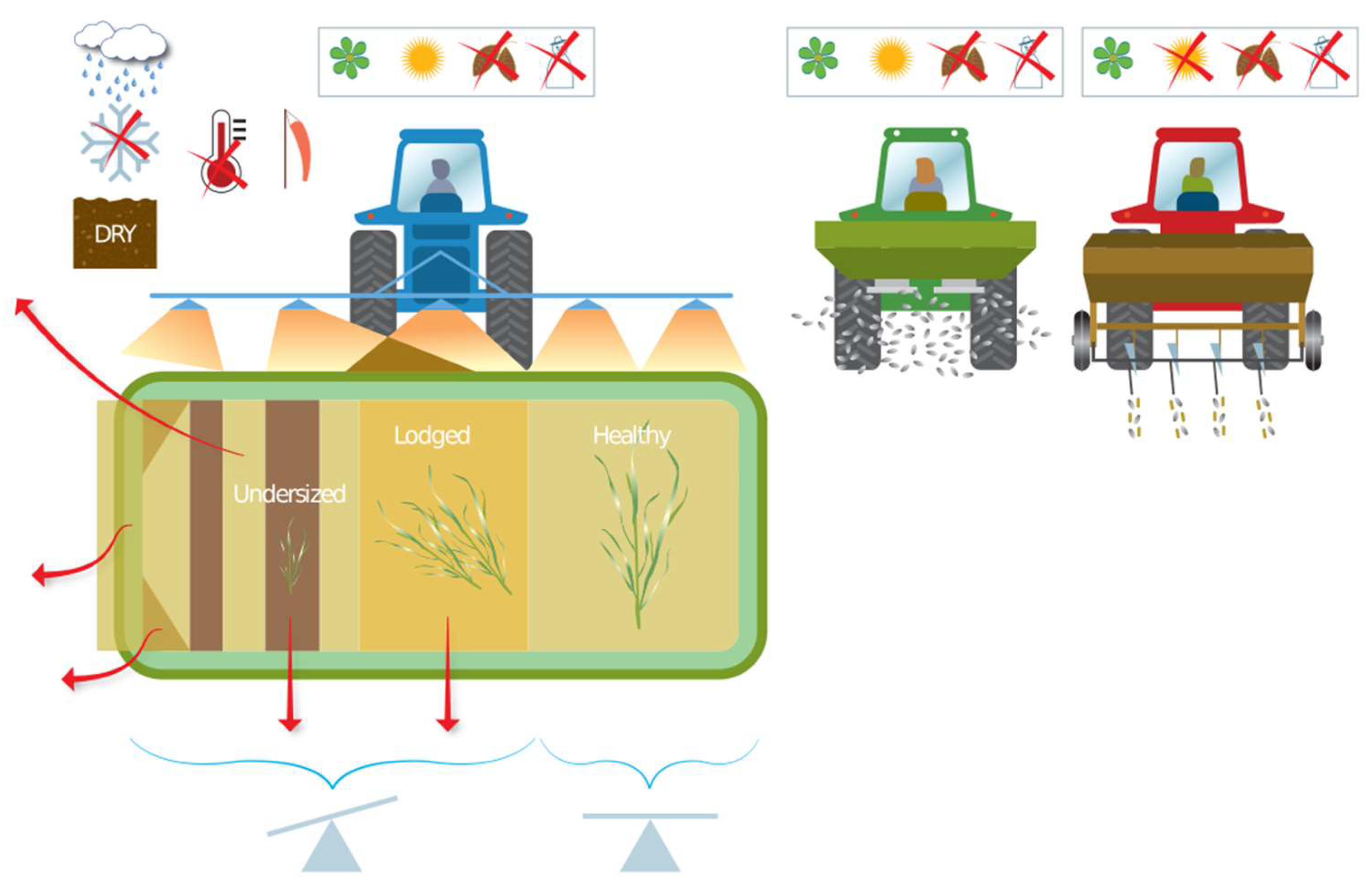
The potential causes of reduced NUE that can arise at the N application stage are presented in Figure 2. The application, in liquid or solid form, can be made directly to the soil surface, above the canopy in spring and summer, or by drilling along with spring sown crops. The focus groups highlighted that solid fertilizer is currently the most common mineral N input, but use of liquid fertilizer is increasing, “with around 10% of farmers we consult … currently using liquid fertilizer, however, it’s expected that more farmers will start using liquid”. Both over- and underlap are important for sprayed input and spinning disk spreading of solid pellets [23]. There are two major concerns for NUE improvement during application: timing and method (including product choice). Timing is both weather and crop growth stage related, which impacts the number and rate of applications. Method relates to human choices and machine type (uniform vs. variable-rate, spread, spray, inject). The application strategy (nutrient type and quantity of nutrients) may be decided months in advance, as noted by the focus group: “nitrogen is often ordered six months in advance, because it is the best price, and there isn’t infrastructure to get it delivered at short notice”. This means farmers are not choosing the N fertilizer best suited to their fields, but the cheapest among what is available at the time of ordering. In practice, the application tactics are also adapted through the season. Application of N is often timed with predicted rainfall, as this can effectively reduce N volatilization. Forrestal et al. [67], for example, observed NH3 losses of 25.3% from urea when followed by heavy rain compared to 39% when followed by only light rain. The focus group noted that “poor weather can ultimately alter the seasons plans, and result in differences between the quantity of N, which is needed, additionally the soil moisture along with soil texture, affects the fields accessibility …. the total amount of nitrogen isn’t decided until you put the last application on, which is generally how much you’ve got left in the shed.” This means operational considerations override the tactical plan, and the management strategy can be regarded as aspirational.
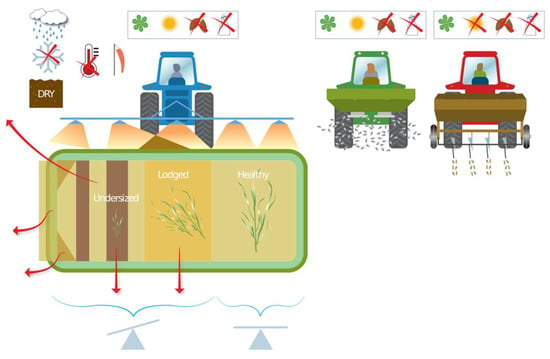
Figure 2.
Causes of reduced NUE at the inputs stage due to application management. Icons show rain, frozen ground, dry soil, high temperatures, low wind, relevant to temperate climate (spring, summer, autumn, and winter), and balance (calibration) of application.
Organic inputs (manures, slurries, crop residues) affect both soil carbon and nitrogen levels, which together will partially regulate subsequent immobilization and mineralization processes [48]. Ichir and Ismaili [68] observed that lower quality crop residue incorporation to the soil resulted in an increased N immobilization process, impacting the following yield by reducing the response to fertilizer N. The benefits from application of organic matter are well recognized, with the focus group noting, “you can never have too much organic matter, and it’s not just the nitrogen that the organic matter is giving, it’s the help it gives to the drainage and mineralization…the biggest limiting factor to applying organic matter, is being able to source it.” Application timing [65,66,67,68,69,70] and application method [69,70,71,72,73,74] are critical to achieving maximum NUE.
N applications are regulated in Europe by the Nitrates Directive (91/676/EEC), which is implemented differently by each member state. Irish regulations (S.I. 605 of 2017) aim to prevent application in the autumn and winter when land is likely to be waterlogged, frozen, or snow covered and there is little plant uptake, leading to greater losses via water. In the growing season, mistimed application can also drive losses, particularly by volatilization in dry, windy, and warm conditions. High wind can also cause spray drift, resulting in unintended variability in application and contamination of the wider environment [75]; the focus group noted, “you get nitrogen loss through the boundaries of the field, under-dosing the crop and not realizing.” This can also be related to the product choice and application method: “certain products are like sugar, and it’ll just blow around. If it’s windy you just can’t spread.” Even if the application timing is right and weather conditions are good, N inefficiency can be caused by poor choice and calibration of equipment [74,76], with the focus group noting, “when considering accuracy of spreading, calibration of spreaders is crucial … it’s only when the calibrations are more than 20% off that you start to see.” Underlap causes reduced N for the crop while overlap causes excess N, perhaps leading to crop lodging and disease. Both result in decreased yield, and overlap results in increased input costs [73]. Corners, turning zones and headlands can also lead to over- and under-application of N: “If you’re spreading solid fertilizer then you’re under-dosing the edges of the field because you don’t want to put any granules outside of the crop area. Liquids are more efficient … you get full application right to the edge of the field.” Modern guidance technologies [77] and auto-steering can prevent misapplications, overlaps, and gaps occurring [78]. While it is difficult to estimate the impact of misapplication on NUE because of the natural variation of the soil, Shockley et al. [79] reported the same yield with a 2.2% fertilizer reduction by using guidance technology. A lack of information on crop N demand when making strategic and tactical decisions can lead to poor choices at the application stage, which have impacts throughout the soil–plant N system and on other N loss mechanisms [67]. While there is theoretical understanding of N management strategy, in practice [80], there are important social, economic, and infrastructure factors to be considered. Despite application being a critical opportunity for the farmer to influence NUE [81], the focus groups highlighted the following three points: (1) “the agronomist has to work with what nitrogen the farmers [have] bought, rather than the other way around”; (2) “[agronomists] can have an influence on many farm decisions; however, when it comes to nitrogen, the farmer’s say is final”; (3) “quite a lot of farmers stick to what they’ve historically done”. Perhaps the greatest opportunity for improved NUE at the farm scale is to account for soil temporal and spatial variability during application [72] so that suitable management information [82] is focused on improving or avoiding poor areas. It will be easier for farmers to make better decisions if they are less influenced by prices in the fertilizer market and have more flexible access to the right equipment at the right time. The focus groups attributed the relatively low cost of N as the main reason for not prioritizing the input of N as a means of improving overall NUE. The economics of synthetic N fertilization has changed drastically with current record fertilizer prices. It is important to mention for arid or semi-arid regions that drought leads to poor fertilizer granule dissolution and thus low uptake and consequently low NUE [83]. If the farmers have fertilized for the highest possible yield, which has not been achieved due to drought, the loss in excess N is not as great as the potential loss in yield, had there not been drought. Under this scenario, the most valuable tool could be a decision tool based on a more accurate, long-term weather forecast. An advanced weather forecast may be more possible in more stable climate zones than in maritime climates which are well known to be very changeable.
Volatilization losses from broadcast slurry can be as high as 80% of N content, while other approaches such as using trailing shoe or dribble bar can reduce those emissions by 70–80% [74]. Incorporation of urea (to 25 cm depth) and manure has been shown to reduce NH3 emissions to as little as 1% [71,84]. However, incorporation of N in wheat fields is most likely to happen during the sowing of spring crops. While Bouwmeester et al. [85] verified conservation of N through banding of urea (at 2.5 cm depths), N can also be conserved by doubling the pellet size. Watson and Kilpatrick [86] increased the size of pellets from 3–4 mm to 6.3–8 mm and reported a 16% reduction in NH3 losses.
4.2. N Flows between Pools
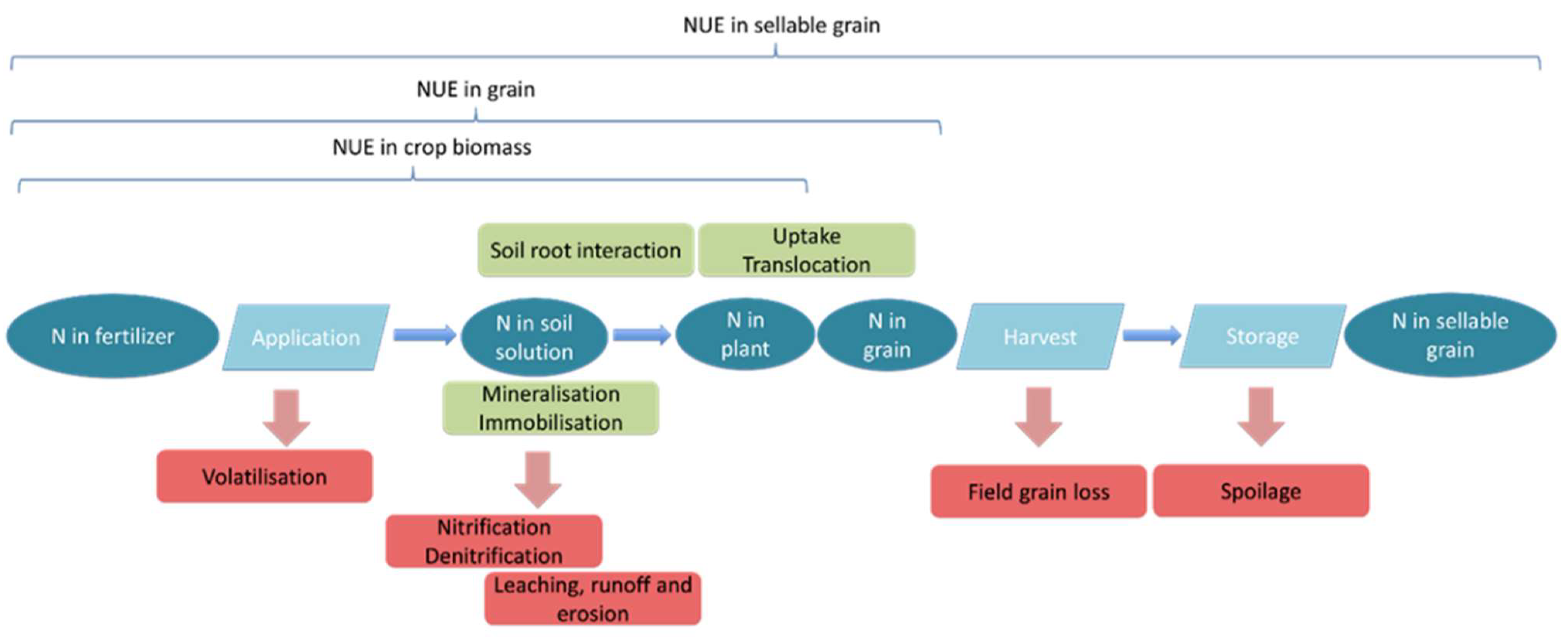
The important pools of N in the soil–plant system (represented as ovals in Figure 3) are N in the fertilizer, N in the soil solution, N in the plant, and N in the grain. The latter distinction is important as some definitions of NUE consider only to the point of N uptake by the plant [9], while others consider N in the sellable yield as the output measure for NUE [9]. Flows between these pools and transformations are mediated by several mechanisms (represented as rectangles in Figure 3). This analysis is focused on the mechanisms that drive supply of plant available N and N loss from the system. Furthermore, the transformation mechanisms are discussed, along with the impact soil N has on the efficiency of N fertilizer additions. These mechanisms regulate whether N can flow to the plant or whether it moves out of the system as a polluting loss.
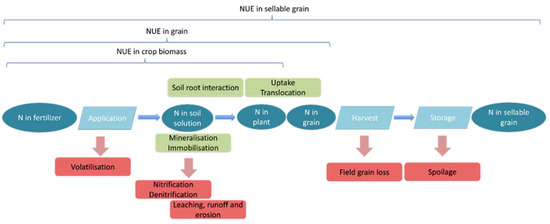
Figure 3.
N flow through the soil plant system. Note: Soil pools are oval, and mechanisms are rectangular. Red rectangles indicate losses; green rectangles indicate transformation.
4.2.1. Mineralization and Immobilization
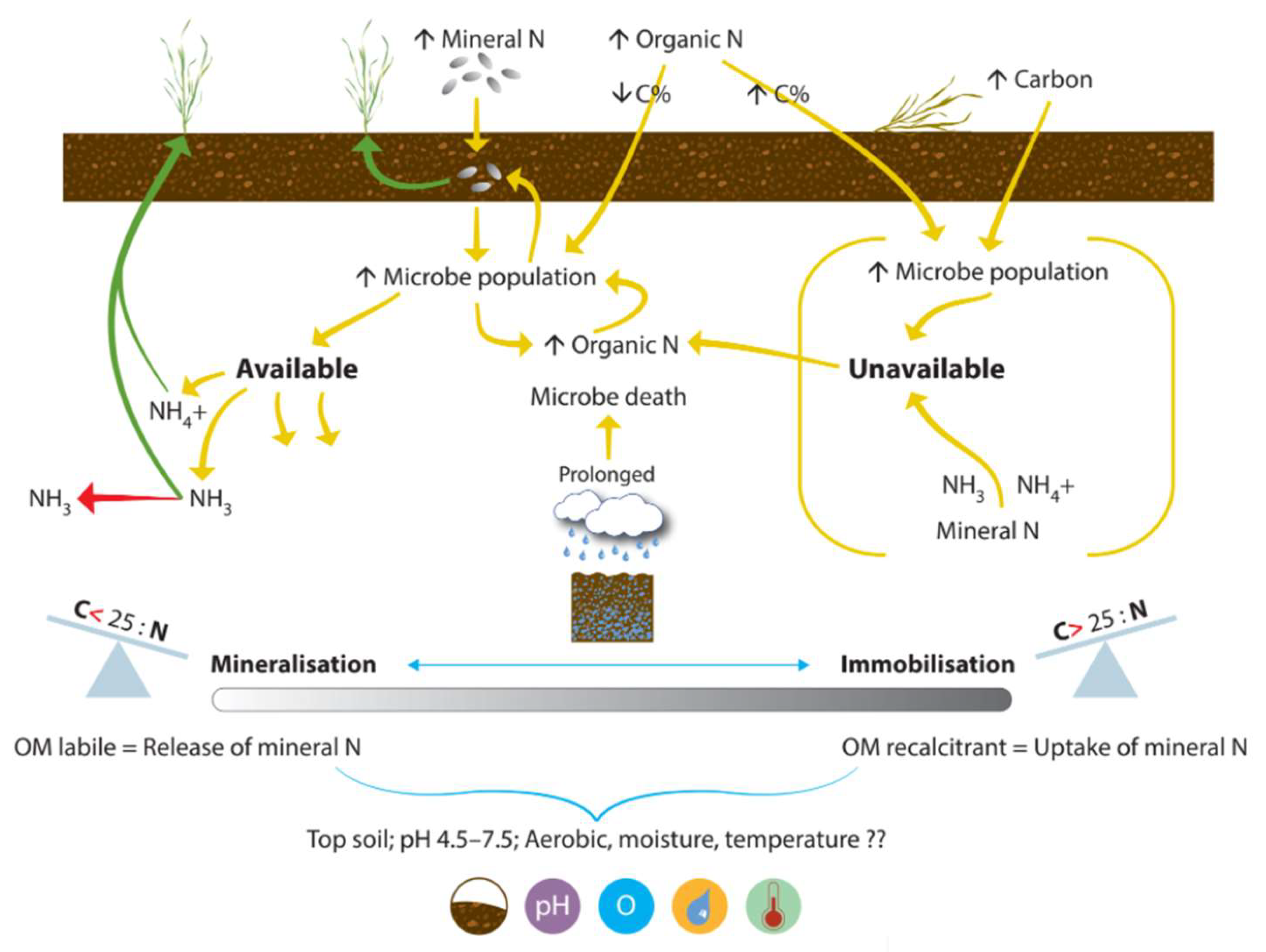
N mineralization is the process by which unavailable crop N in the soil becomes available for crop uptake. Soil microbes compete with crops for available N in the soil. N immobilization occurs due to the uptake of plant available N by soil microbes. The immobilization (N lockup) and mineralization (N release) processes (Figure 4) are continuous, simultaneous, interacting processes in the soil, moving N between the soil solution pool (which is not available for plant uptake) and the plant available N pool [16]. Microbial activity is optimal between pH 6.5 and 7.8 [87], so lime addition to acidic soil can result in a spike in N mineralization [88].
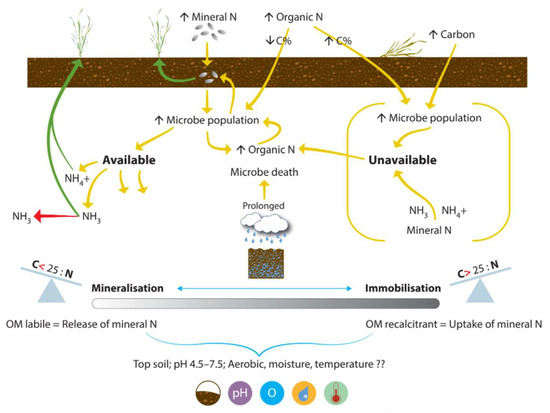
Figure 4.
The mineralization and immobilization processes that regulate the balance of N between soil solution and plant available N pools. Icons show: crop, crop residue, rainfall, wet ground, balance of CNR ratio, and pH, oxygen, moisture, and temperature.
The balance between these pools is regulated by the carbon-to-nitrogen ratio (CNR), and the type of organic carbon (OC) available. Rainfall, air temperature, microbial population, soil moisture, soil temperature, and soil texture are critical factors influencing the processes [89]. The focus groups drew attention to the benefit of increasing farmer understanding: “mineralizable N is not regularly considered, and soil N to grain is not really looked at … we regularly assume that every year is a blank piece of paper, apart from when beans, legumes, or a cover crop have been grown.“ In practice, the mineralization process is exploited for benefit: “we apply organic matter (such as farmyard manure or vegetable waste) early to get more out of the nitrogen later in the season.” There is already trust in this approach, so improving communication may help refine the practice.
The literature shows the importance of N mineralization in crop growth; there are numerous 15N studies that show more than half of the N found in crops (straw and ear) was derived from soil N and not from recently applied fertilizer [90,91,92,93]. Carranca [90] reported that a significant amount of the applied N (15 to 66%) was found in the soil solution after harvest. However, Stevens et al. [93] reported that fertilizer N accounted for an increasing proportion of crop N uptake as the N rate was increased, while Olson et al. [94] showed that more fertilizer N and less soil N were taken up by the plant following spring applications. The latter finding was attributed to immobilization as most of the applied fertilizer remaining in the soil was found in the top 10 cm, with only 9.6–11.5% occurring in mineral form. Schindler and Knighton [95] reported that between 27 and 39% of applied N remained in the rooting zone and would potentially be available for subsequent cropping. They also provided evidence that climatic conditions affect the movement of N between the available and unavailable soil N pools, as well as the efficiency of the plant to utilize fertilizer N, ultimately impacting fertilizer NUE.
Khan et al. [61] reported a substantial reduction in fertilizer N use achieved by accounting for the soil capacity to supply plant-available N through mineralization. Mulvaney et al. [3] showed fertilizer requirements and crop N response are reduced by more extensive mineralization. Furthermore, Mulvaney et al. [3] defends the need to apply N in response to soil N availability, showing that without soil N tests, fields are often over-fertilized. Sanchez et al. [96] observed a dilution effect on mineralizable N by the addition of compost with a low mineralization rate. Furthermore, they documented that more N was mineralized when multiple organic amendments were combined, and they concluded that a diverse cropping system may increase the soil capacity to supply N to a growing crop while maintaining desirable levels of soil organic matter. The same conclusion was reached by Maltas et al. [97].
In a soil with a CNR of less than 25–30:1, N will be mineralized, releasing plant-available N, as microbes decompose OM. When soil has a CNR greater than 25–30:1, N will be immobilized as microbes will assimilate the plant-available N, making it unavailable for plant uptake [88,98]. The microbes involved in this process can be symbiotic with the plant (i.e., living in root nodules of legume plants, or in the rhizospheres of wheat and grasses) or free-living in the soil. While symbiotic microbes can utilize root exudates, free-living microbes rely on decomposing plant material from above- and belowground for their C energy source [99]. An addition of a C source, such as crop residue, will increase the availability of a C energy source; however, few N2-fixing microbe species are able to use straw directly for fixation, as most species rely on C source first decomposed to smaller components by other organisms [100,101].
The mineralization and immobilization processes are reduced at low temperature, and in both low water availability and saturated soils. At low temperatures, OM decomposes more slowly, but each 10 °C increase in temperature doubles the rate of OM decomposition [42]. Andersen and Jensen [44] found recalcitrant breakdown was slower than the breakdown of labile C at low temperature, indicating that gross N immobilization is more sensitive to low temperatures than gross N mineralization. Mineralization increases in the labile OM component of soils [102]. Mikha et al. [103] showed that wet and dry cycles stimulate microbial activity, increasing mineralization, microbe death, and exposure to organic residues. The stimulated activity reduces significantly with repeated wetting and drying.
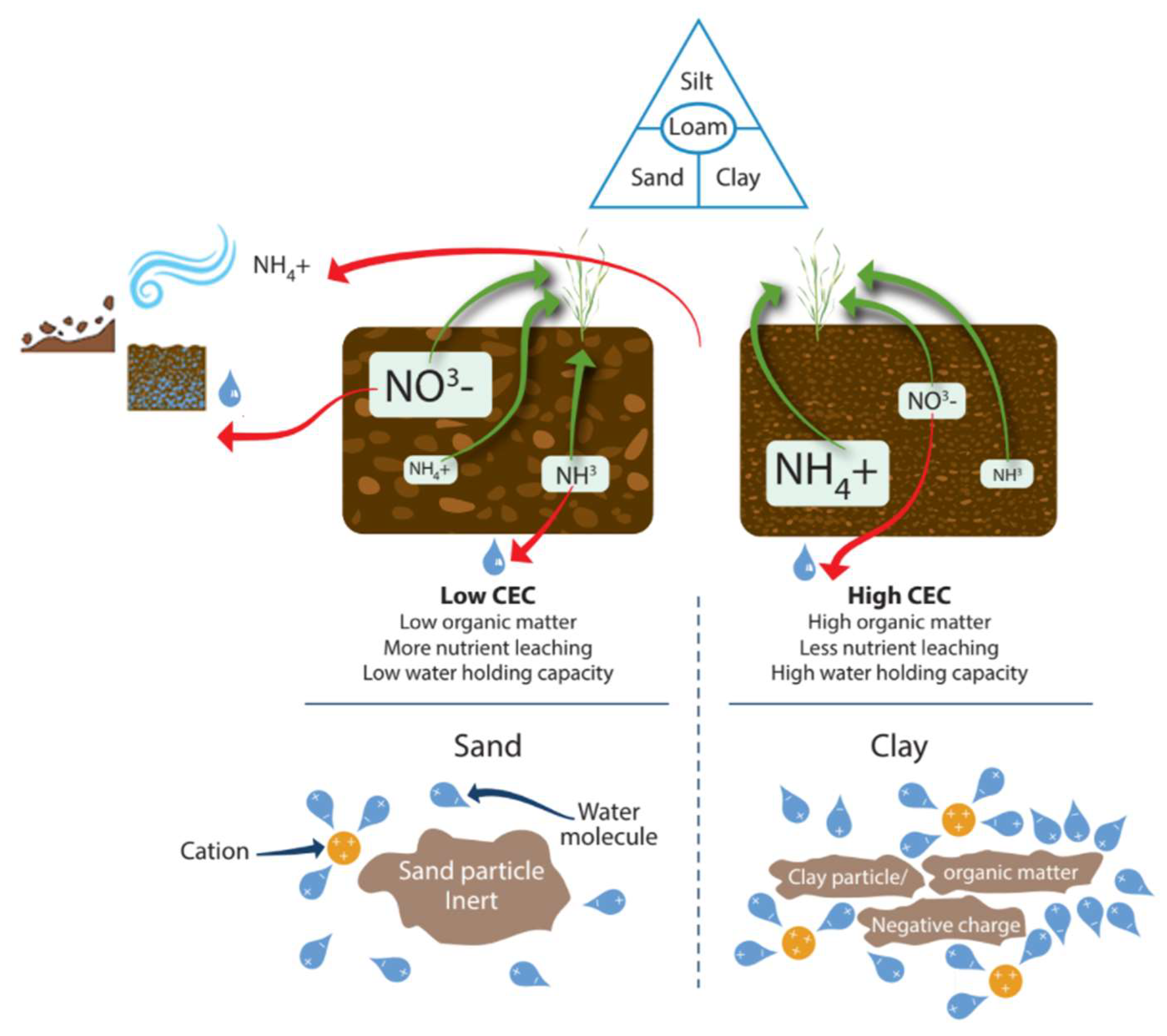
N-mineralization varies widely among soils, ranging from <100 to >400 mg kg−1 NH4+, with lower values associated with very well drained loamy sand soils and the highest values associated with moderately drained clay loam soils [104]. The spatial and temporal variation in N mineralization impacts crop response to N fertilizers and NUE [105]. Burke et al. [106] reported that the annual spatial variability of net N mineralization was between 2 g N/m2 and 10 g N/m2 in different regions under grass vegetation in Central USA. In Northern France, maximum net immobilization was calculated to be around 51 kg N ha−1 with a rate constant of 0.031 per day, with a strong correlation to C decomposition [107]. Slurry N is rapidly immobilized by microbes in the first week after application [108] but leads to enhanced mineralization and plant availability of N [109] with the mineralization of native soil N increasing at higher N application rates [110].
4.2.2. Root–Soil Interaction
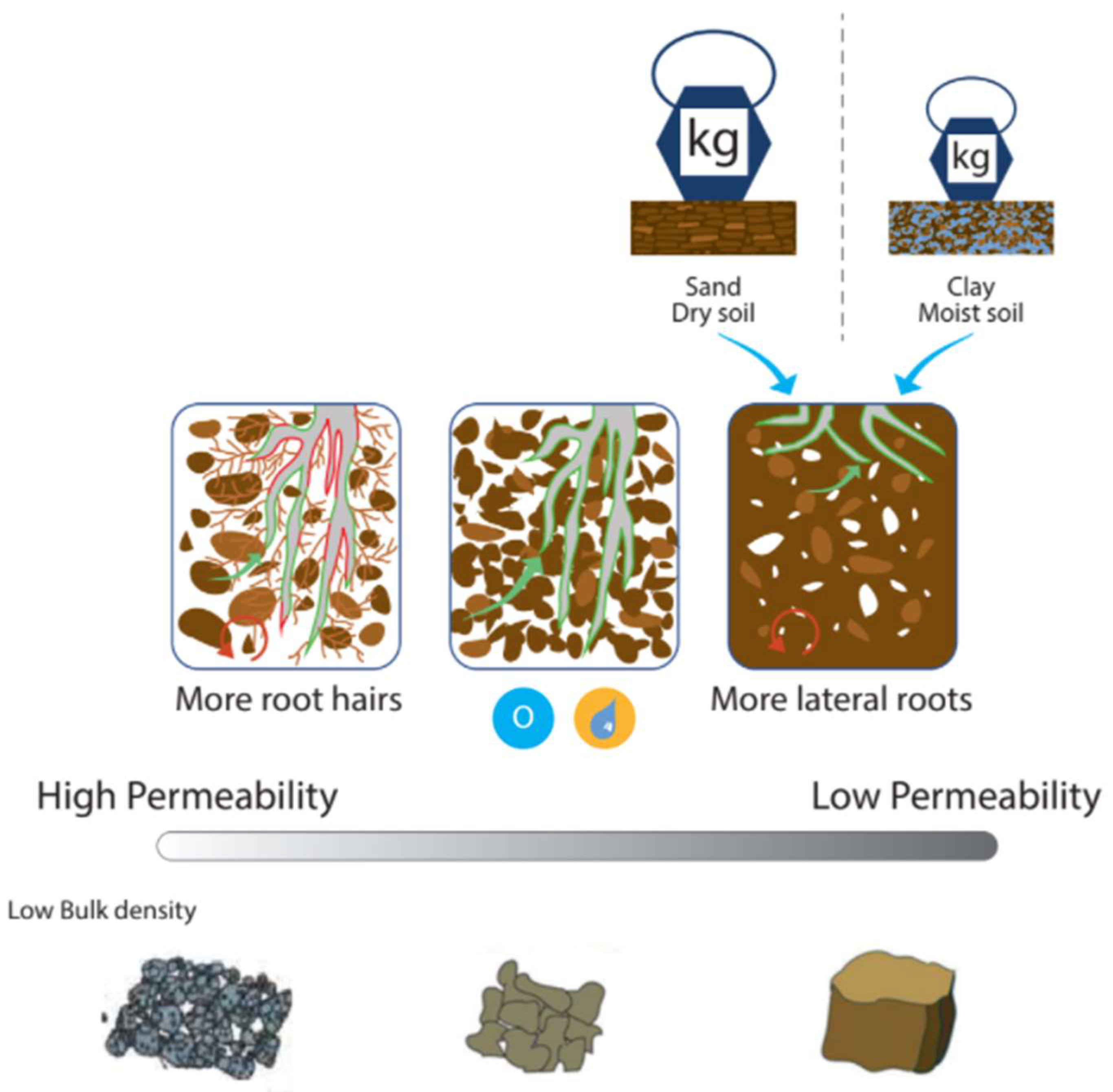
An important flow between pools is the uptake of N by plant roots, which is regulated by the soil–root environment (Figure 5). There is little opportunity for management to regulate root–soil interaction once the crop is sown and established as the famer can no longer change the physical properties of the soil environment in which the crop is growing. The magnitude of the flow depends on the amount of available N and root interception [54], which depends on the distribution of roots in the soil [111] and the contact between soil and root [112]. The best approach is for farmers to maintain optimum soil structure, which will facilitate optimum NUE. Poor structure will limit pore space, root growth, water infiltration rate, and drainage, which causes temporary anaerobic waterlogged environments [39]. Soils with high sand and low OM content are particularly susceptible to limitations in root–soil interaction [113]. Hamilton et al. [114] suggest that increasing soil OM and reducing trafficking will help improve soil structure. The focus group discussed encouraging early plant growth and having a deep rooting system to help resist drought stress and maximize the root–soil interface. Ali et al. [115] found greater yields were correlated with earlier sowing. Shahgholi and Janatkhah [116] found the application of OM reduced compaction to a greater extent in clay loam soils, and an application of 8% OM decreased soil compaction by 9.25%.
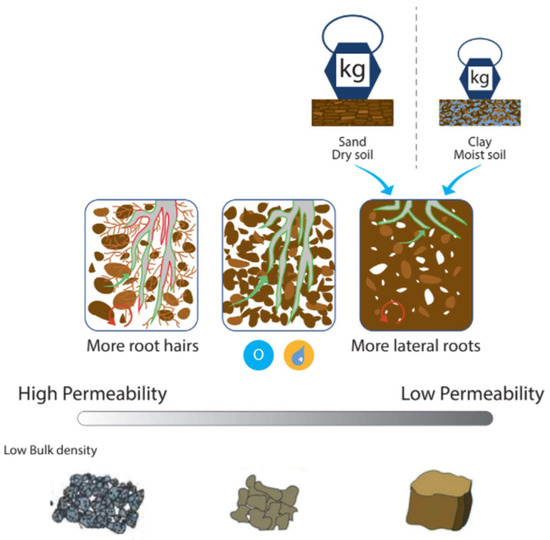
Figure 5.
Soil–root interaction as a regulation of N uptake by the plant. Icons show: weight/pressure applied to dry and wet ground, and oxygen and water availability.
Repeated cultivation of soil to the same depth each year can result in the formation of a hard pan layer. The pan can be impenetrable to roots and can restrict water infiltration, movement, and plant nutrient availability [117]. Soil compaction alters root formation and root distribution, reducing root length and causing the roots to grow thicker. Consequently, both root–soil exploration and root–soil interactions are reduced [118], requiring water and nutrients to travel greater distances to reach the nearest root [119]. The effect of compaction on root growth varies with weather conditions. High bulk density in dry years is detrimental to crop growth, while in wet years, compaction increases denitrification and the risk of root diseases [120,121]. A large root system in the soil profile is beneficial for efficient N acquisition [9,70]. Kulmatiski et al. [70] found a good correlation between uptake and resource availability. While consolidation can improve structure, benefit crop growth, and reduce erosion [122], high levels of compaction adversely affect soil root growth, decrease nutrient uptake, and increase emissions of N and runoff nutrients [123]. Compaction is particularly important for NUE because it will reduce N uptake as roots cannot explore sufficient soil volume [124], an issue also highlighted by the focus group. Resistance to nutrient transfer also depends on the size and shape of the paths along which nutrients must travel [125]. A soil bulk density of 1.3 to 1.6 g/cm3 (dependent on soil texture) is ideal because the soil is consolidated and thus can support the plant but also offers little resistance to root penetration [50] and facilitates water movement. Atwell [126] found roots from loosened soil had higher NO3− concentrations than those from compact soil. The total concentration of solutes was 19–31% smaller in roots growing in compact soil than in roots from loosened soil.
Soil compaction affects soil’s physical [127], chemical [128], and biological [129] properties. The main factors that increase compaction levels include soil moisture, size of external load, size of contact area, tire inflation pressure, number of passes, and type of soil [30]. The most damage is caused during the first pass, but damage increases with each pass, and a few heavy loads are less damaging than frequent light loads [130,131]. Kumar et al. [132] found compacting and drying increased bulk density by 37% for a clay loam soil and by 17% for a loam, and that sand was almost fully compacted in its natural state (94%). Soil–root interaction is related to physical limitations, which a farmer can manage during cultivation [133] and can maintain through field traffic management [134]. The majority of soil compaction in modern agriculture is from indiscriminate heavy vehicle traffic [130], particularly on wet soil with higher clay content [135]. This has significant implications for the timing of field activities to minimize restriction of crop development. It is unlikely that N fertilization can be used to manage root biomass, so cultivation and management of deep compaction are critical prior to the growing season [121]. The focus group highlighted the need for easy, visual assessment and communication of soil structure to help long- and short-term root–soil management.
4.2.3. Uptake and Translocation
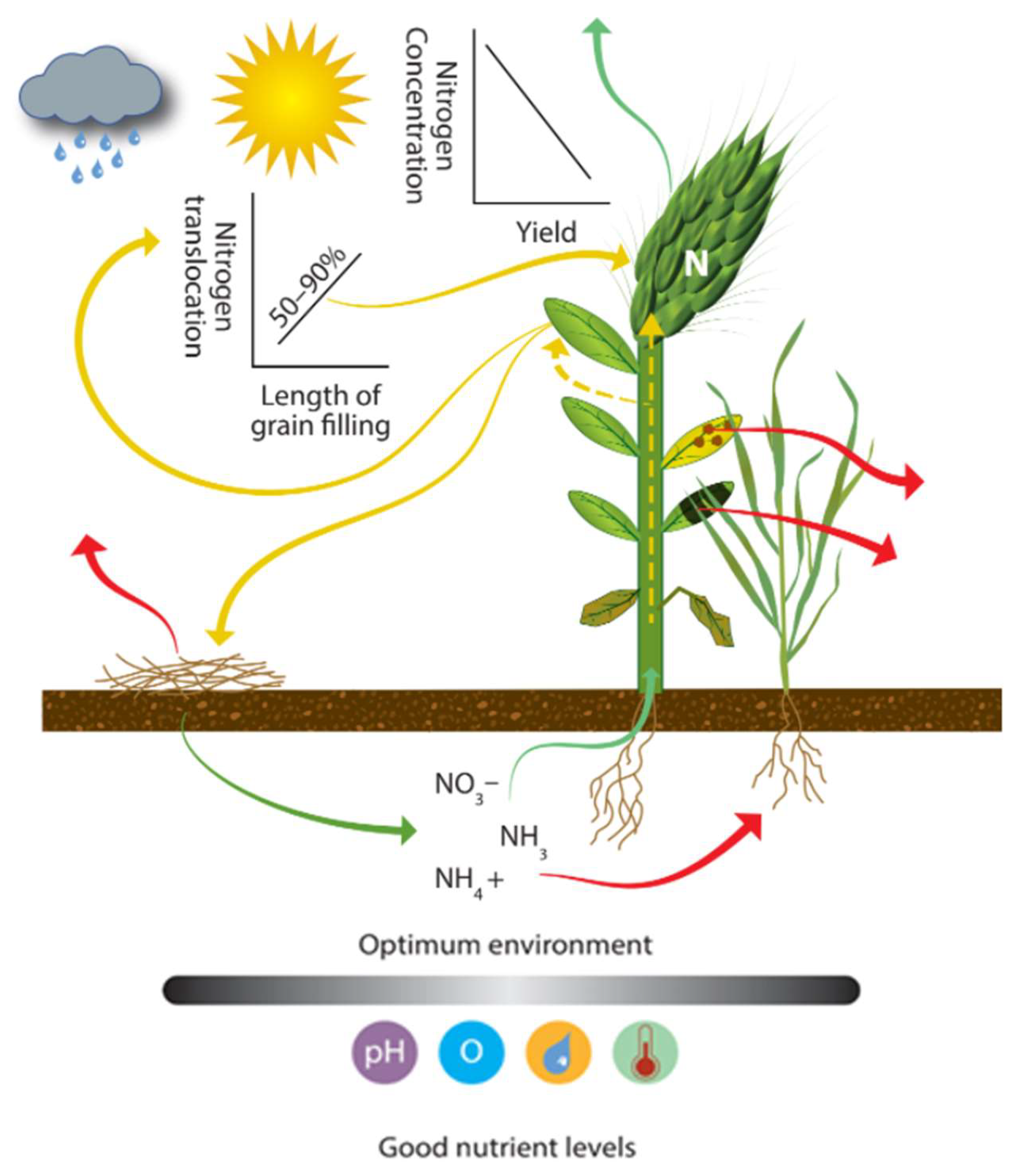
The movement of N into the growing plant depends on physical, chemical, and biological processes (Figure 6). Assuming good soil–root interaction (Figure 5), the important regulating factors are pH, temperature, water content, trace elements, pests, and crop N demand [136]. Without proper husbandry, it is not uncommon for wheat yields to be suppressed by as much as 50% [81]. Plant N uptake and translocation to the grain are impacted by N availability, which is also regulated by all the loss mechanisms discussed in this paper. Many factors affecting crop uptake and translocation require planning and consideration before drilling and need to be incorporated into the strategic planning, not just dealt with as a tactical adaptation. The focus group noted the practice of applying extra N to mask the limiting effect of compaction on uptake and overall NUE. Crop N demand depends on crop physiology, crop density, size, vigor, weather (light, temperature), and water availability. In temperate regions, the amount of solar radiation received varies with aspect and topography [41]. Dufour et al. [34] investigated the impact of shade on cereal crops and found that yield was decreased by 50% when light was reduced by 31%. The observed yield reduction was in both yield and grain weight. Dufour et al. [34] noted that under the reduced lighting, the concentration of protein in the grain increased by up to 38%.
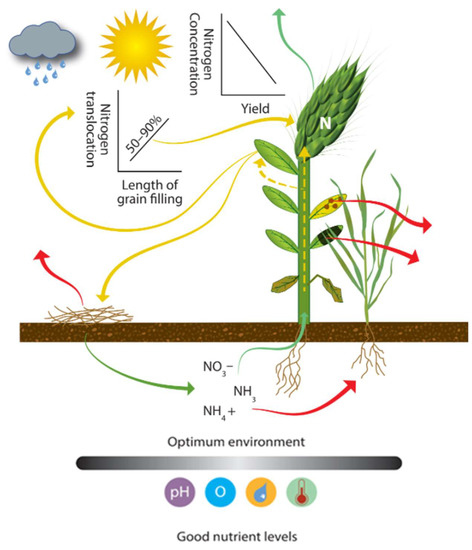
Figure 6.
Uptake and translocation of N by the plant pool. Icons show: rainfall, light intensity, pH, oxygen, plant available water, and temperature.
Plants can uptake both available organic N and inorganic N, with organic N requiring less effort to assimilate, most notably in small plants with high N concentration such as cereal crops [137]. Gioseffi et al. [138] showed that the uptake of organic N can be around half the rate of NO3− and NH4+ in wheat. In a study by Ma et al. [139], the potential competition for nutrient uptake between the wheat crop and soil microbes was highlighted. Their study was based on 13C, 15N labeling and involved two field sites where a series of fertilizer amendments were applied (a combination of NPK fertilizers, pig manure, and cereal straw residue). They found that in two different trial sites, 6–21% and 6–11% of the added 13C-15N was assimilated by wheat, while 18–35% and 8–20% was captured by soil microorganisms. The uptake of N by the plant pool can be the last important N flow regulating NUE if calculated relative to plant uptake as a proportion of N applied. However, translocation from plant to grain determines NUE if calculated based on grain N as a proportion of N applied (Figure 2). The length of grain filling is important because as little as 50% or as much as 90% of N can be remobilized from degraded stem and leaf proteins to the grain [140,141,142]. If crop senescence is induced due to limited water availability, less N is translocated [141,142]; therefore, soil infiltration and hydraulic conductivity rates can affect yield through soil water availability [143]. Haynes and Naidu [144] noted that yield increases from added manure were likely a result of greater soil water-holding capacity (WHC). Plant-available water and timing of rainfall in the pre- or post-anthesis growth period for dryland and irrigated crops are critical [32]. Delayed senescence can lead to higher yield, but when N is deficient, it can cause a decrease in N and protein concentration in the grain [35]. Crop N demand changes with crop growth stages, but this is predictable [69], so it can be used as the basis of N management. N uptake by plant roots can be reduced or stopped during the grain ripening growth stage [145]. If translocation is limited, it is likely that N remaining in the plant will be greater, which, if incorporated into the soil, will lower the CNR of the OM, encouraging mineralization (Figure 4).
A pH range of 6.5–7.5 is optimum for availability of plant nutrients while being generally highly compatible to plant root growth [146]. In addition, most nutrients (particularly micronutrients) tend to be less available at pH > 7.5 and are more available at slightly acidic pH ~6.5–6.8 [146]. However, molybdenum is more available at moderately alkaline pH values [146]. The optimal pH may vary slightly for the uptake of different nutrients depending on soil type and OM content [147], as reported in the Illinois Agronomy Handbook [148], where liming is recommended to maintain the optimum pH for cereal crops between 6.2 and 7.3. Another factor is soil depth relative to the water table, since water and nutrient availability can be limited by salt accumulation within the root zone of soils with poor drainage [149].
Temperature affects the growth and metabolism of plants and can impact crop resistance to disease [136]. The ideal growing temperature range is 12 °C to 25 °C [136]. At temperatures ~10 °C outside of this range, uptake will still occur, but at a reduced rate. In extreme temperatures, the crop will not be able to uptake N, even if the farmer has ideal management [150]. Managing this uncertainty is a major issue for optimum NUE. If NUE is calculated for grain rather than the whole plant, translocation is also important, and it is known that temperature can affect the starch and protein content of the grain [151]. Another factor affecting N uptake is the soil water content, which of course is impacted by weather. If the soil is too wet, it prevents N uptake because the plant is unable to respire, a limitation that begins to occur when the soil is wetter than field capacity [152]. If the soil is too dry, it will reach a point where plant growth is also limited (referred to as the critical soil moisture deficit) [152], and nutrients will not be available in a soluble form for root uptake [153]. At the permanent wilting point, all plant growth stops. The plant’s general vigor is also related to availability of trace elements and other nutrients [154]. In Figure 6, trace elements and other macro and micronutrients are referred to collectively as “good nutrient levels”, but they are a key factor in achieving optimum NUE [155]. However, trace elements and other macro and micronutrients introduce further complex interactions influencing soil NUE which are outside the scope of this paper. The focus groups identified that the other soil nutrient properties are regularly checked to make sure they are not limiting. Another factor that regulates crop vigor and uptake is pests and diseases, including competition from weeds [55]. Susceptibility to pests and diseases is related to nutrient deficiency (and soil root interaction, Figure 5), which will increase crop vulnerability to specific diseases [156,157].
4.3. N Outputs from the System
The output of N from the soil–plant system is ideally through the harvested crop (removing N from the field). However, N is also lost to the environment through pathways associated with pollution. The impact of losses can be local, such as surface water eutrophication caused by leaching losses [158], regional such as atmospheric ammonia deposition [159], or global such as increasing the concentration of atmospheric N2O driving climate change [160]. Globally, crop N grain recovery averages around 40% for cereal systems [64], while Zhou et al. [22] reported recovery of just under 34% in wheat grain. Raun and Johnson [67] found that worldwide, NUE for cereal production is approximately 33%, while Raun and Schepers [161] found that splitting the N rate is one of the best ways to increase NUE.
These global data indicate that over half of the N added is lost to a polluting pathway. Velthof et al. [21] reported the largest N loss pathway in European agriculture to be denitrification (44 kg N ha−1) followed by NH3 volatilization (17 kg N per ha), leaching (16 kg N per ha), and emissions of N2O and NOX. Zhou et al. [22] reported that nitrate (NO3−) leaching accounted for 78% of water-borne losses, and NH3 volatilization accounted for 93% of the annual gaseous N losses. Zhou et al. [22] reported 30 to 75 kg N ha−1 lost over winter, with leaching being 10 times more important than erosion and runoff combined. These figures indicate the magnitude of the problem associated with NUE. Zhou et al.’s [22] study was conducted on weathered soils in the subtropics, which are well drained, accentuating N loss by leaching rather than denitrification. To optimize NUE, it is necessary to maximize recovery in the harvested plant and to ensure soil pools are at the optimum size to maintain crop growth and in the optimum conditions to minimize loss pathways. N losses vary depending on the form of fertilizer N applied, and through the growing season. Volatilization is most significant shortly after N application, and leaching and runoff are more important over the winter (for temperate latitude agriculture). The focus group noted the “uncertainty about what we’re losing out the bottom” and recognized that to have N loss information would be very useful for the decision-making process.
4.3.1. Volatilization
Volatilization (Figure 7) involves gaseous NH3 loss from the system. Depending on conditions, up to 80% of applied N can be lost through this pathway [68]. Choice of fertilizer, fertilizer placement, and application timing (with regard to weather) can drastically reduce volatilization loss. The majority of volatilization occurs within a week of application [68], and these early losses are enhanced by light rainfall (<0.3 mm) [66]. However, volatilization after application is greatly reduced if there is sufficient rain to move the N down into the soil profile (more than 2.5 cm) [90,104]. Incorporation of urea and manures to 25 cm depths also reduces the volatilization loss of NH3 [71,84]. Soil with a greater buffering capacity (because of CEC, clay content, SOM, or a combination of these) has a reduced rate of NH3 loss because rapid pH change is restricted or due to increased adsorption of NH4+ to clay particles [46]. Losses are greater if the soil is wet prior to N application, and then proceeds to dry (particularly if it is windy, with high temperatures) [35,84], which is more likely to be a problem with sandy soils [162]. Work by Sommer et al. [163] found the NH3 loss rate increased when wind speeds increased up to 2·5 m/s. However, Bouwmeester et al. [85] found that increasing the wind velocity from 1.7 to 3.4 ms−1 reduced the comparative NH3 loss from wet soil from 19% to 7.5%, likely due to more rapid drying of the soil surface from greater wind velocity, rather than prolonged drying. Harty et al. [164] reported increased volatilization of NH3 on sustained low-water-filled pore space. Volatilization losses are greatest when moisture in the topsoil is maintained but is not high enough to promote leaching either from high humidity (80–95%) or with <8 mm of rain every 3 days. This means the timing of N application prior to heavy rainfall, will minimize volatilization losses, linking again to the importance of accurate N application. It is desirable that N is moved into the soil through water solubility, as this will provide protection against volatilization losses, and it is more likely the N will be in the rooting zone, but if there is too much leaching, the NO3− will pass the root zone and be lost to the plant. There is substantially more NO3− leaching in temperate soils, which have limited capacity for anion exchange, compared to tropical soils, which have a much greater potential for anion retention [165].
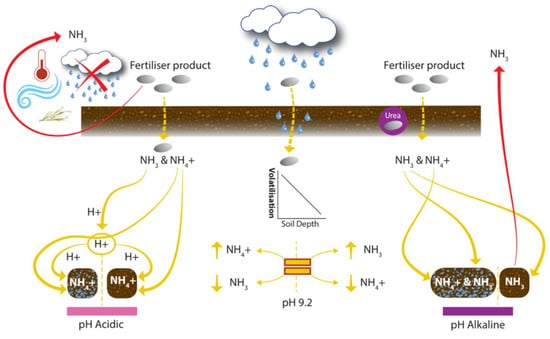
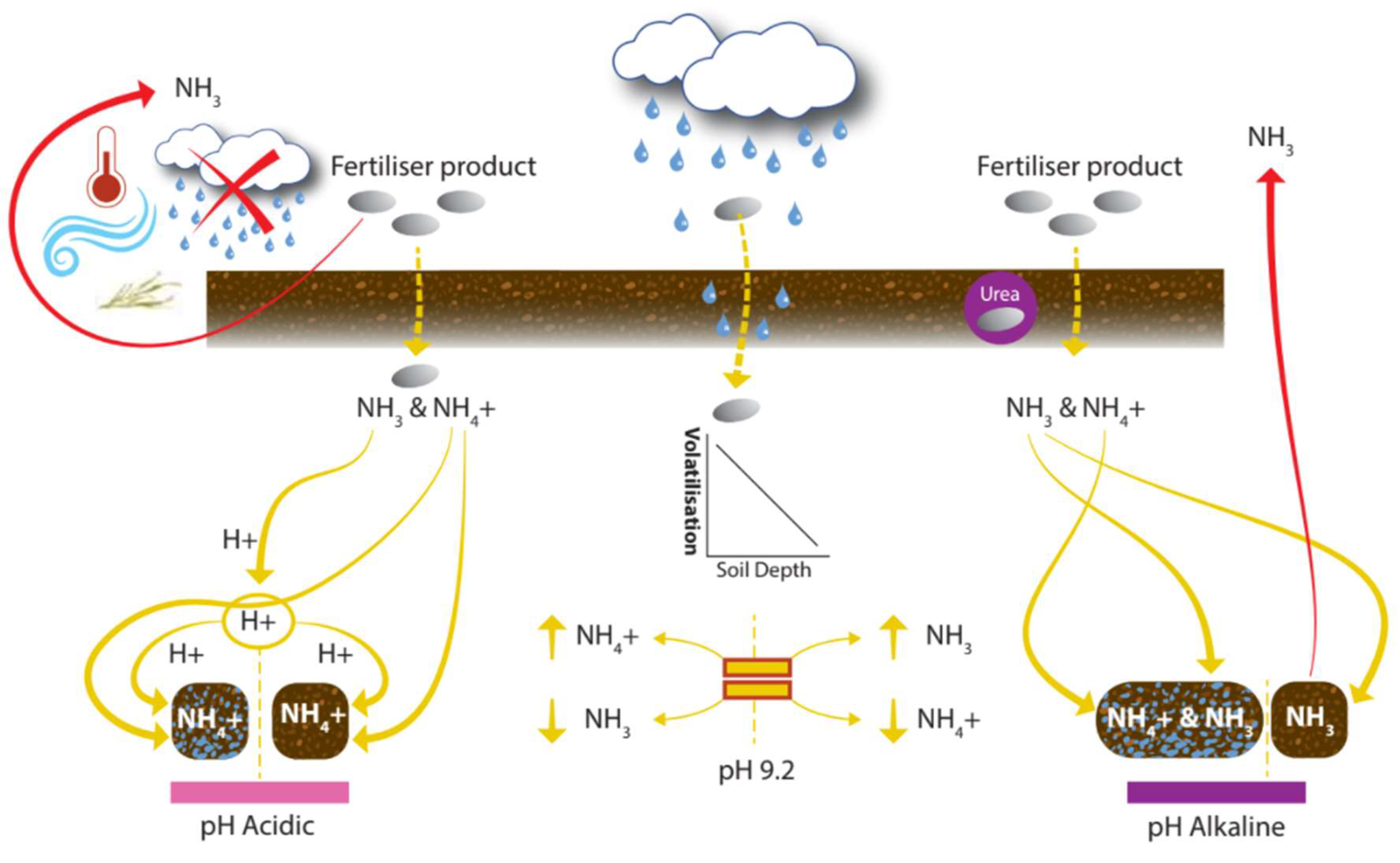
Figure 7.
Control of volatilization losses from the soil–plant system. Icons show: temperature, wind, rainfall, and crop residue.
Acidity is an important regulator of volatilization; pH affects the balance of NH3 and NH4+ in the soil. At pH > 9.2, the quantity of NH3 in the soil is greater than NH4+. Raising pH by one unit can cause the NH3 concentration to increase nearly tenfold; at pH 6, 7, 8, and 9, NH3 dissolved in soil solution accounts for 0.1, 1, 10, and 50% of the ammoniacal pool, respectively [37]. Due to the importance of pH on nutrient availability, amendments with lime are made every few years. Tian et al. [166] reported significant linear relationships between air temperature and NH3 volatilization rates. The focus group noted the impact of soil temperature at application and commented that perhaps more attention should be given to it. Choice of fertilizer product is part of the application stage, but product choice can drastically reduce volatilization. Urea can be particularly problematic due to its alkaline hydrolysis, where the soil pH around the urea fertilizer granule spikes (temporarily), which enhances volatilization loss [52]. SOM may buffer the impact of urease (an enzyme which hydrolyzes urea, forming ammonia and carbon dioxide) on soil pH [167]. Crop residues impact soil moisture and temperature and increase the activity of urease enzyme [52]. Additionally, there are N products with urease inhibiting enzyme coating, which is a decision at the application stage. The amount of N which gets into the soil solution will impact other N loss mechanisms, while factors such as soil moisture, pH, OM, and clay content which impact volatilization have additional impacts on the other N loss mechanisms. Volatilization can be reduced by maintaining a suitable pH~7 [37], and by tactical decisions made at the application stage around timing and the type of fertilizer applied.
4.3.2. Nitrification/Denitrification
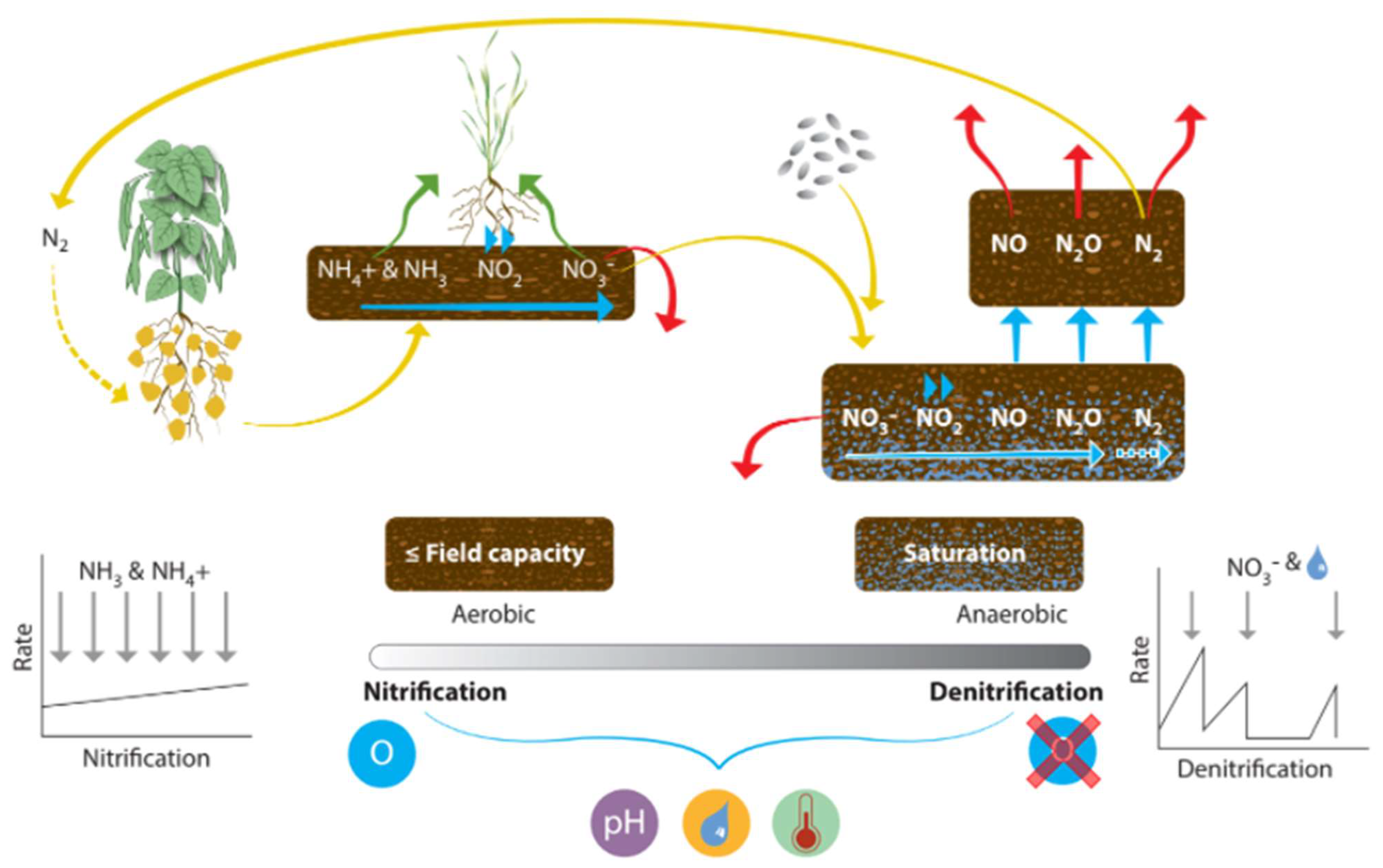
Worldwide denitrification losses for N2 and N2O are estimated at 28% and 3.5% of N inputs, respectively [168]. The denitrification and nitrification processes are outlined in Figure 8. Nitrification is the process by which NH3 is converted into nitrite (NO2−) and then to NO3− by nitrifying bacteria. Denitrification is the process of reducing NO3− and NO2− to N2O and dinitrogen gas (N2). The processes are driven by microbial activity, particularly ammonia oxidizing bacteria and archaea [168]. Additionally, legumes can fix atmospheric N2 into plant-available N [169], but in typical wheat and cereal production systems, this is not a pathway during the growing season. Denitrification, the reduction of N species, is the most important N loss pathway in European agriculture [21]. Mulvaney [3] explored N emissions from the addition of different fertilizer compositions, and they found nitrite was denitrified much more readily than nitrate; however, they showed that all fertilizers promoted denitrification of 15NO3− compared to the untreated soil. The emissions from fertilizers decreased in the order of ammonia (anhydrous NH3 creating the highest emissions), urea (CO(NH2)2), and diammonium phosphate ((NH4)2HPO4), with ammonium sulfate ((NH4)2SO4), ammonium nitrate (NH4NO3), and ammonium dihydrogen phosphate (NH4H2PO4) creating the least emissions, being roughly equal. The water-filled pore space (WFPS) of the soil determines whether nitrification or denitrification will be the dominant process because denitrification occurs in anaerobic conditions (often associated with heavy rainfall, poor drainage, and compaction). Nitrification from ammonia oxidizers (NH3 to NO2−) and nitrite oxidizers (NO2− to NO3−) requires O2 and is thus optimal in aerobic conditions. Complete denitrification to N2 occurs when water-filled pore space (WFPS) is >80% of the total pore space [168], and while not directly damaging, it does represent a large energy loss. Incomplete denitrification at <80% WFPS releases environmentally damaging gases, particularly N2O, a highly potent greenhouse gas [170]. At 70% WFPS, most N2O emitted is produced by denitrification, but at 35–60% WFPS, nitrification is the dominant producer of N2O [129]. Nitrification was limited at 20% WFPS due to low water availability. Sawyer [171] estimated denitrification loss of NO3− in waterlogged soil to be around ~2% per day [171], while Jordan [172] explored denitrification losses from grassland in Northern Ireland and found that from clay loam and silty clay soil, denitrification exceeded 0.1 kg N ha−1 per day when WFPS > 70%, when temperatures were > 4 °C, and when soil nitrate was > 2 mg N kg−1 in the top 10cm of soil. Rates of loss are impacted by fertilizer type. Over 12 months, a maximum of 79 kg N ha−1 loss from a 300 kg N ha−1 CAN application on the silty clay soil was reported [172]. Jordan and Smith [172] applied modeling techniques and calculated an average agricultural total N loss from denitrification of 19.5 kg N ha−1 yr−1 for Northern Ireland. They also applied calculations to previous studies to estimate denitrification losses of 15–20 kg N ha−1 yr−1 for Northern Ireland and 21–32 kg N ha−1 yr−1 for the Ayrshire Basin in Scotland. Rates also depend on soluble and mineralizable C, the energy sources for the heterotrophic biomass [121]. Denitrification rates are subject to extreme temporal and spatial variability caused by N fertilization rates, and soil–weather interaction [173,174]. High denitrification rates can occur in anaerobic microsites in well-aerated soils [174]. The concentration of NH3 and the availability of free O2 determines the rate of nitrification [175]. Denitrification is limited by the availability of an easily accessible C source. While nitrifying bacteria compete for ammonium with heterotrophic microbes [176], nitrification is a relatively constant process across ecosystems compared to denitrification.
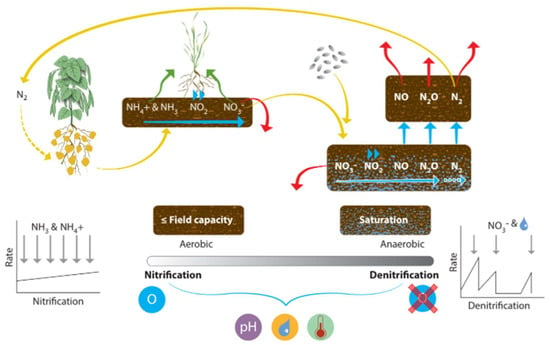
Figure 8.
The nitrification-denitrification process that regulates change in the soil solution and plant-available N pools. Icons show: crop uptake, mineral and legume plants (nitrogen fixing), oxygen availability, pH, water availability and temperature.
Rainfall after an extended dry period can cause an increase in soluble C, causing a spike in denitrification. Nitrification in soil is generally limited by the initial oxidation of NH3 to NO2−, in which archaeal ammonia oxidizers play a significant role [177,178,179]. The reported optimal pH range of nitrifying bacteria is conflicting. Nitrification was originally thought to occur only under neutral and slightly alkaline conditions; however, it is now widely accepted that nitrification can occur in a wider range including acid soils, but the rate of nitrification will vary in different environments [179]. Alleman [180] suggested the optimum pH would be in the range of 7.2 to 8.2 and that nitrification declined below pH 7.0 and nearly ceased around pH 4.5 [88]. However, acid-adapted bacteria can have a growth range of pH 5–7.5 [181]. Furthermore, N oxidation in low-pH soils (<5.5) soils is dominated by archaea rather than bacteria [182].
Nitrifying bacteria are optimized at soil moisture around field capacity and temperatures between 25 and 30 °C. Das et al. [183] investigated nitrification rates and found at 40 °C that it took 11 days for the added NH4±N to be completely nitrified, 15 days at a constant 30 °C, and at 30 days at 20 °C, 20% of the added NH4+-N was immobilized. Campbell and Biederbeck [184] found that nitrification was less under fluctuating temperatures (3 to 14 °C) than at a corresponding constant mean temperature, stating that low temperatures (3 °C) had a biostatic effect on the nitrifiers in their soil. Chandra [185] noted that significantly less nitrification occurred when the incubation temperature changed from sub-optimal to optimal (27 °C) than when the temperature was optimal initially and shifted to lower temperatures afterwards.
Good soil structure is seen as a way of reducing denitrification losses. The focus group noted that farmers tend to treat each growing season as a “blank piece of paper” because N losses over the winter, particularly in waterlogged and heavy clay soil, makes predicting the remaining soil N very uncertain. Tile drainage can also be added to the fields to help increase drainage and aeration in the field. The benefit of increasing drainage is dependent on the field’s climate and soil [186]. Husk et al. [186] recommend pairing tile drainage with denitrification bioreactors to significantly reduce N emitted through subsurface drainage systems.
4.3.3. Leaching, Runoff, and Erosion
Estimates for N leaching from agricultural soils range from 19% [187] to 30% [188] of N inputs. Aqueous N loss from the soil occurs mainly by leaching, but closely related runoff and erosion pathways are also possible in wheat and cereal production scenarios (Figure 9). Leaching is the movement of chemicals downwards through the soil profile due to mass movement in water [189]. Runoff loss is the mass movement of chemicals in water that has little to no infiltration into the soil [190]. Erosion can be caused by wind moving dry soil particles with N attached, or by water during which times, erosion and runoff losses will be caused by the same moving water [182]. During and following rainfall, water moves more freely through wet soils due to greater infiltration rates and hydraulic conductivity [152]. When the soil saturates, runoff will commence, but it can also occur when rainfall rate exceeds infiltration rate [152]. N added in wet conditions in well-drained soil is at high risk of becoming a leaching loss (whereas in poorly drained soil, denitrification is more likely). The focus group noted that field drainage is always a concern, because there always seems to be either too much or too little drainage. Greater N loss by leaching is expected on sandy and light soils. Olson et al. [94] argued that they observed little leaching in their experiment due to immobilization. Askegaard et al. [53] found greatest leaching after a field had stubble incorporation (~55 kg N ha−1) and lowest leaching when there was a soil cover from a catch crop (avg. 20 kg N ha−1). Soil type is also an important factor influencing leaching, and coarse-textured soils have a greater risk of N leaching than soils with finer texture [191,192]. When Gaines and Gaines [193] compared different sandy soil textures, they showed that (i) soil texture impacts NO3− retention, (ii) leaching losses were significantly higher at sandy loam sites compared to clay loam sites, and (iii) high N application rates on sandy loam sites lead to high leaching losses. If there is little N in pools near the soil surface, runoff and erosion will cause little N loss. The easiest way to minimize erosion and runoff losses is to maintain crop cover and soil consolidation [112,194], which also helps maintain SOM content [194].
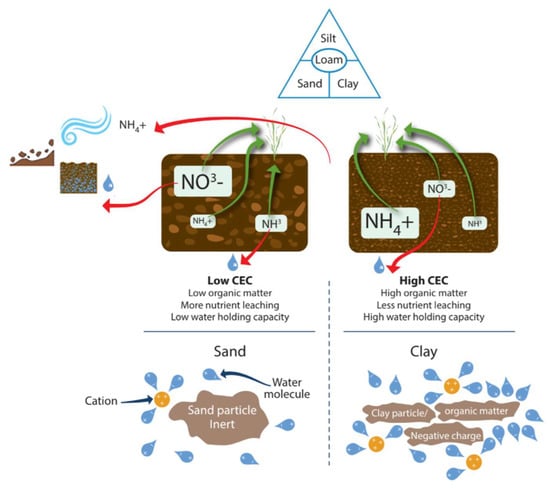
Figure 9.
Factors controlling leaching, runoff, and erosion loss pathway from the soil-plant system. Icons show: wind, slope and wet ground, and water bonding to cation charge.
The important factors in the field to understand the likelihood of aqueous losses are topography, texture, structure, WHC, current soil water content, OM, and CEC. The CEC of SOM and some clay minerals is impacted by pH, with low CEC being found in more acidic soils. The occurrence of isomorphous substitution is where a cation in the clay is replaced by a cation of higher or lower charge, which leads to the development of a permanent positive or negative charge [195,196]. Furthermore, pH affects the balance of NH3 and NH4+ in the soil. Leaching can move N away from the surface, reducing N volatilization, but it can also result in N flowing away from the plant soil system. High clay soils hold more water and bind NH4+. Compared to sandy soil, 83% more N was retained in clay-amended soil [197]. CEC reflects nutrient (K+, Ca2+, Mg2+, and NH4+) retention capacity in the soil and thus NH4+ retention. High CEC (clay or OM rich) soils are also less susceptible to leaching losses than low CEC soils (sand rich soils). Greater CEC helps reduce leaching losses. The positive charge in soils affects the retention of nitrate in soils and is a pH-dependent variable. This nitrate retention occurs especially in Oxisols, Ultisols, Alfisols, Spodosols, and Andisols [198]. Nitrate retention in these soils is attributed to the positive charge retaining the anion and preventing its downward leaching [199]. Verhagen and Laanbroek [176] report that this occurs more readily in allophanic Andisols than in Ultisols and Oxisols.
Bigelow et al. [51] looked at potential leaching losses in high-sand-content soils and OM amendments to increase CEC and concluded that with a CEC lower than 2 cmolc kg−1, 95% leaching of NH4+ occurs, and with a CEC closer to 10 cmolc kg−1, <8% leaching of NH4+ occurs. Soils with a clay mineral interlayer can bind NH4+ [200]. NH+4 ions bound to the clay mineral interlayers are protected against leaching and nitrification [201]. Topography plays an important role in water availability for crop production and the balance between infiltration (leading to leaching) and runoff (leading to erosion) [202,203,204]. The focus group noted that a well-managed field with good structure can be negatively impacted and be subject to ponding due to poorly structured soils in upslope fields, meaning NUE can be compromised by the effect of a neighboring field. Compaction will limit both infiltration rate and hydraulic conductivity, typically associated with anaerobic waterlogged environments (leading to excess runoff) [39]. OM is the most important regulator of soil structure and soil strength to prevent excessive erosion and runoff [205]. The focus group noted that OM is recognized by farmers as a great benefit, allowing farmers to “get away with more”; however, “it is very difficult to raise the OM of the soil”, particularly for farmers who do not have an available source, such as livestock or compost. Both Khan et al. [206] and Mulvaney et al. [207] report that applications of fertilizer N depletes SOM, as it promotes C utilization and N mineralization of microbes. Some movement of N through the mechanisms of runoff and leaching are essential to proper soil functioning [68], but it is necessary for the farmer to recognize when poor structure and low OM content interact with CEC to make a field more vulnerable to needless aqueous losses that cause reduced NUE.
4.3.4. Harvest and Grain Storage
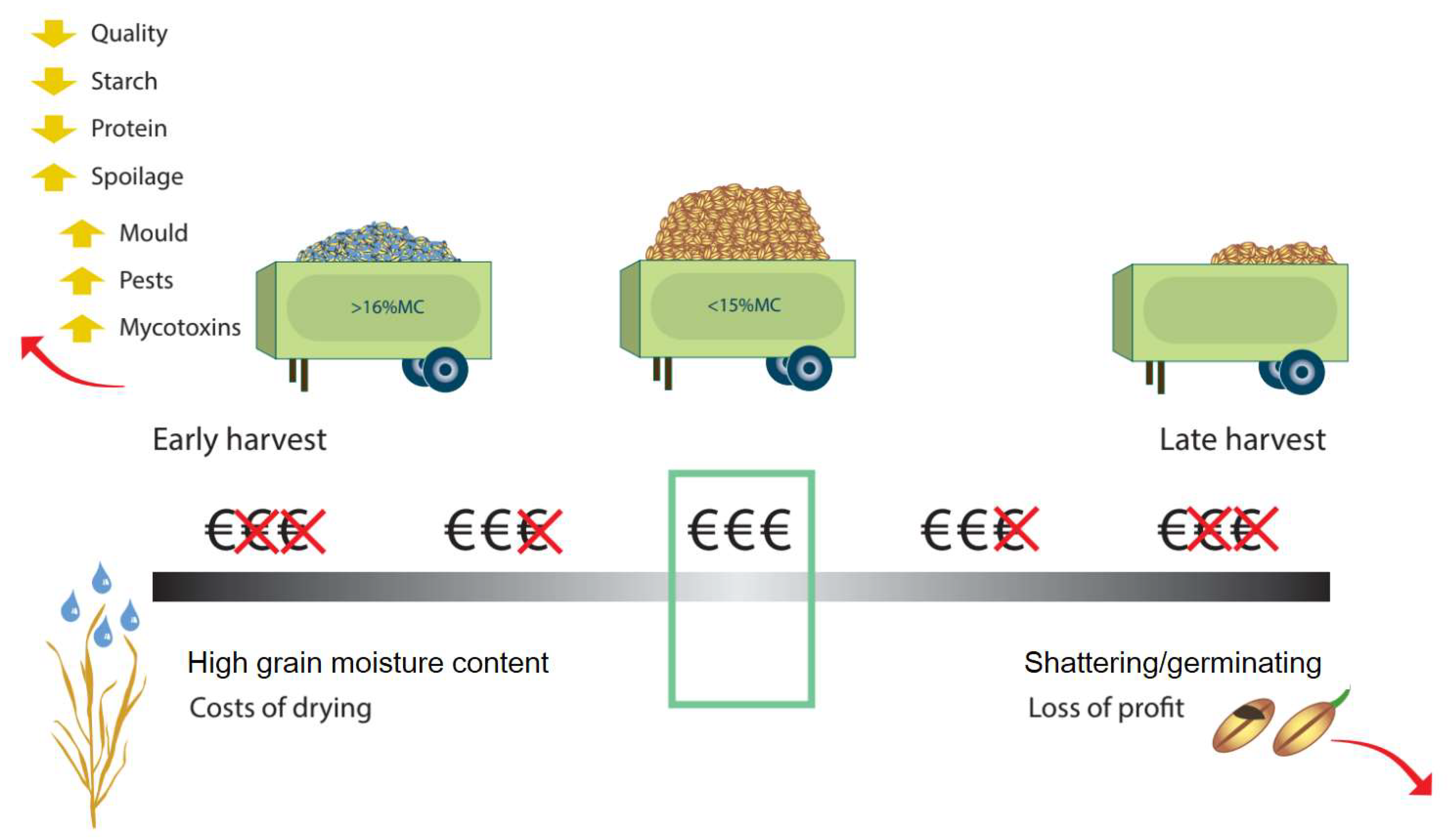
The removal of crop from the field during harvest (Figure 10) will determine the NUE calculation (Figure 3), as will losses due to storage conditions prior to sale or distribution (Figure 10). The most important factors are grain moisture content at the time of harvest, which carries over to storage, the timing of harvest relative to growth stage and moisture content, machine availability to achieve ideal timing, harvesting method, drying facilities (to bring the grain to the ideal moisture content for storage), the storage facility, and the perceived value of the crop. If the crop is harvested too early, it will tend to have poorer quality, with lower starch and protein content and higher moisture content [140]. Harvesting shortly after rain will result in the grain moisture content being above the critical 15% moisture content threshold for safe storage [132]. Harvesting too late risks yield losses from grain being dropped from the ear, early germination, or shattering of grain [132]. Different harvesting and threshing techniques can have an impact on grain losses [208], but mechanization has drastically reduced these losses [132].
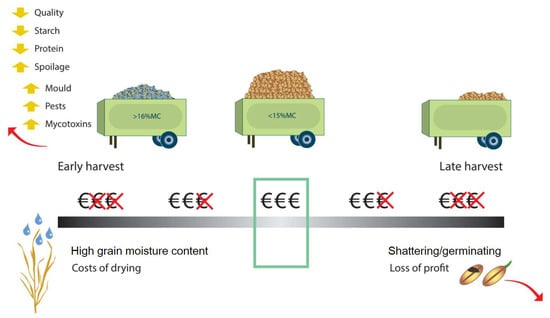
Figure 10.
Factors controlling harvest and grain store losses.
Safe storage sometimes requires forced drying to prevent spoilage. Prior to the development of scientific understanding of post-harvest conditions, poor storage could result in 80% loss of wheat and other cereal grains [132]. However, with scientific storage, loss is typically reduced to 1–2% [132]. The focus group noted that post-harvest loss was generally not thought of as important by farmers in the UK and Ireland, and for most purposes, NUE should be considered in terms of N offtake at the time of harvest. The losses that arise during storage can be categorized as weight, quality, seed viability, commercial and spoilage [209] all of which reduce the economic return [210]. These are driven by conditions conducive to pests and disease infestation, which are mainly regulated by water content.
4.4. Identifying Priority Factors for NUE Management
Quemada et al. [14] derived an NUE “modest target” for arable farms of 61% and suggest that farms not achieving this target should adjust farming practices. The analysis of processes relating to NUE revealed 16 manageable causes of NUE reduction (Table 2). While they can be classified in terms of how they interact with inputs, flows, and outputs from the soil–plant system, there are a number of recurrent themes related to equipment, timing, organic matter additions, and pH regulation. The general opinion of the focus group discussions is also summarized in Table 2, regarding both importance (for perceived impact on yield and NUE) and concern (representing if the subject is currently worth improving, regarding its current control and the practical, social, and economical aspects of making improvements). These values are ranked as “high”, “mid”, “low”, and in one case “variable”. The variable classification is given due to the range of opinions provided during the focus groups.

Table 2.
Manageable causes of nitrogen use efficiency (NUE) reduction, the associated risk, and management solutions.
Equipment choice will influence the quality of N application, whether as an organic manure, a liquid spray, or a pelleted fertilizer [23,71,84,86]. Correct calibration, training, proper route planning, and modern control will all ensure that the input of N is as intended and accurately placed. For farmers relying on contractors, the quality of application could be a criterion in the contract as well as the amount and approximate timing. In general, current practice is conducive to NUE, but more attention needs to be paid to application timing. Equipment choice for harvesting is routinely adequate or good. One effect of equipment choice is soil structure damage, particularly deep compaction and surface deformation and crusting. Equipment should be chosen to cause the least damage possible if the timing of operations is not ideal [211]. While it is understood that there are ideal soil conditions for field traffic, the stochastic nature of weather means it is not possible to complete all operations under ideal soil water conditions [56,211]. This means equipment selection must focus on the probability of having to operate in less-than-ideal conditions. A move to low ground pressure and lighter equipment may offer advantages for NUE by reducing damage to the soil pore network [30,211]. The focus group pointed out that in practice, farmers do not have free choice of equipment at the times they want it, but that overall, equipment is probably not causing a great deterioration in NUE in their estimation.
The timing of operations is also important. The key points are the sowing date for strong establishment and maximum root development, and fertilizer spreading to match additional input to plant demand, which will be governed by critical growth stages and harvest date to maximize off-take from the field governed by crop moisture and growth stage [69,115,135,140,141,142]. The focus group indicated that timing is usually limited by machine and labor availability to the farmer. For instance, during March and April, a farm tractor might be required for spring sowing, crop protection, and fertilizer management. Likewise, for contracted work, perfect timing for all fields may not be possible because of logistical constraints. Integrated, regional planning of machinery resources through farmer cooperation and coordination of contractors has some potential to improve NUE on farms by ensuring the right machines are used for the most efficient input and output while minimizing soil damage.
The addition of OM to the soil is perhaps the most important intervention not commonly achieved by farmers [48,114,116,198]. The focus group indicated that if there is no livestock production nearby and no other source of OM, such as compost or food processing residues, then the cost and logistics involved in transporting OM become prohibitive. Where a manageable supply of OM is available, it should be utilized, provided the N content is high enough to maintain a CNR in the soil, which does not cause excessive immobilization leading to limited crop N uptake [88,99]. Khan et al. [61] showed that excessive N applications deteriorate SOC, which, along with environmental damage, reduces the long-term economic profitability of the field. To maintain the SOC and maximize the potential benefits of N fertilizers, Khan et al. [61] suggested that both a reduced N input and the incorporation of highly carbonaceous residue (the research suggested maize residue) into the soil should be considered. Regional logistics need to be planned to maximize the location of such OM sources. Increasing OM will have many benefits, but will improve NUE [48,115,117,198]. The management of pH has long been part of farm practice, but the focus group noted that interest in liming had fallen away in recent years. It is clear that maintaining ideal pH for N uptake, micronutrient uptake, and minimum volatilization (due to alkaline soils impacting gaseous losses of NH3) will improve NUE. There is perhaps scope for integrating pH management with OM management, fertilizer application, and other field activities to reduce the overhead and adverse environmental consequences from multiple tractor-passes [115,122].
Assessment of the literature in conjunction with the focus group revealed there is plenty of scope for improving NUE to reduce environmental consequences and maximize financial return for the farm. There are perhaps three main reasons why NUE management has not receive close attention on farm: (1) historically, N has been relatively cheap compared to other inputs (but 2020/2021 saw a considerable increase in the price of N fertilizers); (2) the disruption caused by uncertain weather each year makes it difficult to quantify the benefit of detailed management; and (3) regulation and legislation governing N management is quite general, and there is little perceived advantage of investing effort into NUE improvement compared to cultivation, pest management, and harvest. The focus group noted, “…farmers tend to put more nitrogen on because it’s cheaper to put a bit more on than it is to lose out on that yield….”. This “insurance philosophy” approach that many farmers and growers take has long exacerbated intensive N fertilization for wheat and other cereal production. This approach often ignores the environmental consequences of N pollution and is currently being called into question by the current crisis in fertilizer pricing. Additionally, N policies such as the recent EU Farm to Fork Strategy have been introduced to address excessive N applications. The EU Farm to Fork strategy aims to reduce fertilizer use by at least 20% by 2030 (and nutrient losses by at least 50%).
There are fertilizer formulations that can help improve NUE, but these are restricted by the supply chain and price pattern for such bulk products. The focus group noted that “…there is very little … way to buy N at short notice; it’s often bought … in advance, when N has the lowest price.” Farmers are encouraged to buy in advance with cheap prices, regardless of which fertilizer formulation will be best in each circumstance, meaning they will have to use what they have already bought. This is driven by the needs of the manufacturers, not the needs of the farmer.
The synoptic situation and the pattern of local weather conditions can cause quite different trajectories in crop growth and soil state that cause a very wide uncertainty band around any N management decision. The development of risk/probability-based recommendation systems might offer some comfort to the farmer and help drive awareness of NUE management. A stark statement about regulation from the focus group revealed the situation in practice: “I can honestly say the amount of times I’ve spoken to a farmer about NUE, I can count on one hand….”. Combining this with the philosophy that “…a pound spent on N is a pound well spent…” and “…certain factors are given a lower prioritization because you’re not penalized for them...” leads to the strong opinion in the focus group that “legislation would drive farmers to use N more efficiently”. With the development of greater mechanistic understanding of N dynamics on the farm, and the ability to monitor, measure, and record activity, it should be possible to move from general N regulations to site-specific methods that encourage optimum NUE. However, there must be trust in the advice and approach, and to do that, the focus group noted, “…the results have to be specific to the farmer for them to be believed.” This means management advice needs to be simple and cost-effective to be successful [82]. It is clear from the analysis summarized in Table 2 that targeted advice on equipment selection (for cultivation, compaction control, timing, and accuracy); timing of activities based on risk/probability; safe, high N OM additions; and regular pH management have scope to greatly improve NUE on farms. Furthermore, consideration should be given to adjusting fertilizer N rates according to the climate of the area and the spatial variation of soil N supply.
5. Conclusions
The objective of this work was to clearly define the factors that control NUE for wheat production, to express this understanding graphically, and to identify management interventions most likely to offer NUE improvement. The factors identified were accurate application, fertilizer formulation, application timing, increasing SOM while maintaining a suitable CNR, pH management, maximizing the root network, controlling pests, ensuring micronutrient availability, maintaining good soil structure for root development, aeration and water movement, optimum harvest timing, maximizing plant N uptake, and minimizing losses by denitrification, volatilization, leaching, and runoff. The control of each of these factors was represented in diagrams, which were validated by working agronomists to convey information about controlling factors in a simple manner. The analysis indicated that the most important management interventions for improving NUE will be using the best machinery possible (including variable-rate options), timing (including site specific methods), increasing SOM content, and managing pH. These could be supported with a risk/probability-based recommendation system to help farmers without being confounded by the stochastic nature of unpredictable weather.
If improvements to NUE are to be achieved, monitoring the factors controlling N losses and soil N availability needs to be simple and accessible. Moreover, N fertilizer amendments should be made in response to N already in the soil solution, as greater soil N availability limits crop response to N fertilization. Determining the available mineral N in spring could be the basis for N application, while in autumn, it could indicate the quality of N management. While data for some properties are simple to acquire, others require skills and/or equipment. The interpretation presented has focused on properties and interventions that can be built on existing technical innovations. It is clear that economic return is the main driver for agricultural decisions, and there are some common practices that are believed to provide greater economic return but reduce NUE. This reflects the historically low price of N, uncertainty about N pools and flows at any time, and the unpredictable nature of the weather. While some actions may reduce N loss in the short term, significant uptake of NUE practices will require change in legislation, economic incentives, risk/probability-based recommender systems, and the development of supply chains to enable faster, flexible N supply to the farm.
Author Contributions
All authors contributed to the Conceptualization, methodology was R.L.W. and N.M.H., and Visualization was R.L.W. with the help of UCD graphics department. Resources and data curation, from published papers was provided by all authors, whilst information from the agricultural focus groups were collected by R.L.W. and N.M.H. Writing—original draft preparation, was R.L.W. and M.A.H.; writing—review and editing, was through N.M.H. and R.L.W. supervision, project administration, and funding acquisition, was N.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Foundation Ireland Strategic Partnerships Program (16/SPP/3296) co-funded by Origin Enterprises Plc.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
At the beginning of each focus group, consent was obtained from everyone for the recording and use of the discussion.
Acknowledgments
We would like to thank Seán Ó Domhnaill from the UCD graphics design department, whose work improved the diagrams in the manuscript. We would also like to thank the agricultural focus groups from industry and the UCD focus group for the discussion and input on the clarity of the graphics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO—Food and Agriculture Organization of the United Nations. World Fertilizer Trends and Outlook to 2020; FAO: Rome, Italy, 2017. [Google Scholar]
- Jenkinson, D.S. The impact of humans on the nitrogen cycle, with focus on temperate arable agriculture. Plant Soil 2001, 228, 3–15. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Need for a soil-based approach in managing nitrogen fertilizers for profitable corn production. Soil Sci. Soc. Am. J. 2006, 70, 172–182. [Google Scholar] [CrossRef]
- Gervois, S.; Ciais, P.; de Noblet-Ducoudré, N.; Brisson, N.; Vuichard, N.; Viovy, N. Carbon and water balance of European croplands throughout the 20th century. Glob. Biogeochem. Cycles 2008, 22, GB2022. [Google Scholar] [CrossRef]
- Herridge, F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Nykvis, B.; Persson, A.; Moberg, F.; Persson, L.; Cornell, S.; Rockström, J. National Environmental Performance on Planetary Boundaries; Swedish Environmental Protection Agency: Bromma, Sweden, 2013.
- Brisson, N.; Gate, P.; Gouache, D.; Charmet, G.; Oury, F.X.; Huard, F. Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crop. Res. 2010, 119, 201–212. [Google Scholar] [CrossRef]
- Oenema, O.; Bleeker, A.; Braathen, N.A.; Budňakova, M.; Bull, K.; Čermak, P.; Geupel, M.; Hicks, K.; Hoft, R.; Kozlova, N.; et al. Nitrogen in current European policies. In The European Nitrogen Assessment; Cambridge University Press: Cambridge, UK, 2011; pp. 62–81. [Google Scholar]
- Peng, S.; Buresh, R.J.; Huang, J.; Yang, J.; Zou, Y.; Zhong, X.; Wang, G.; Zhang, F. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop. Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Collins, C.; Phelan, S. CROPS COSTS AND RETURNS 2019 Crops, Environment and Land-Use Programme. Oak Park teagasc.ie. Available online: https://www.teagasc.ie/media/website/publications/2019/Crops-Costs-and-Returns-2019.pdf (accessed on 1 November 2020).
- Omara, P.; Macnack, N.; Aula, L.; Raun, B. Effect of long-term beef manure application on soil test phosphorus, organic carbon, and winter wheat yield. J. Plant Nutr. 2017, 40, 1143–1151. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Mullen, R.W.; Freeman, K.W.; Thomason, W.E.; Lukina, E.V. Improving nitrogen use efficiency in cereal grain production with optical sensing and variable rate application. Agron. J. 2002, 94, 815–820. [Google Scholar] [CrossRef]
- Godinot, O.; Carof, M.; Vertès, F.; Leterme, P. SyNE: An improved indicator to assess nitrogen efficiency of farming systems. Agric. Syst. 2014, 127, 41–52. [Google Scholar] [CrossRef]
- Quemada, M.; Lassaletta, L.; Jensen, L.S.; Godinot, O.; Brentrup, F.; Buckley, C.; Foray, S.; Hvid, S.K.; Oenema, J.; Richards, K.G.; et al. Exploring nitrogen indicators of farm performance among farm types across several European case studies. Agric. Syst. 2020, 177, 102689. [Google Scholar] [CrossRef]
- EUNEP (European Union, Nitrogen Expert Panel). Nitrogen Use Efficiency (NUE) an Indicator for the Utilization of Nitrogen in Food Systems; Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar]
- Winsor, G.W. Mineralisation and immobilisation of nitrogen in soil. J. Sci. Food Agric. 1958, 9, 792–801. [Google Scholar] [CrossRef]
- Chalk, P.M. Dynamics of biologically fixed N in legume-cereal rotations: A review. Aust. J. Agric. Res. 1998, 49, 303–316. [Google Scholar] [CrossRef]
- Deng, S.; Moore, J.; Tabatabai, M. Characterization of active nitrogen pools in soils under different cropping systems. Biol. Fertil. Soils 2000, 32, 302–309. [Google Scholar] [CrossRef]
- Pate, J.S. Uptake, assimilation and transport of nitrogen compounds by plants. Soil Biol. Biochem. 1973, 5, 109–119. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Velthof, G.L.; Oudendag, D.; Witzke, H.P.; Asman, W.A.H.; Klimont, Z.; Oenema, O. Integrated assessment of nitrogen losses from agriculture in EU-27 using MITERRA-EUROPE. J. Environ. Qual. 2009, 38, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, B.; Brüggemann, N.; Dannenmann, M.; Wang, Y.; Butterbach-Bahl, K. Sustaining crop productivity while reducing environmental nitrogen losses in the subtropical wheat-maize cropping systems: A comprehensive case study of nitrogen cycling and balance. Agric. Ecosyst. Environ. 2016, 231, 1–14. [Google Scholar] [CrossRef]
- Scharf, P.C.; Lory, J.A. Calibrating corn color from aerial photographs to predict sidedress nitrogen need. Agron. J. 2002, 94, 397–404. [Google Scholar] [CrossRef]
- Dhillon, N.S.; Samra, J.S.; Sadana, U.S.; Nielsen, D.R. Spatial variability of soil test values in a Typic Ustochrept. Soil Technol. 1994, 7, 163–171. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V.; Phillips, S.B.; Westerman, R.L. Effect of long-term N fertilization on soil organic C and total N in continuous wheat under conventional tillage in Oklahoma. Soil Tillage Res. 1998, 47, 323–330. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2017, 10, 51. [Google Scholar] [CrossRef]
- Peralta, N.R.; Costa, J.L.; Balzarini, M.; Franco, M.C.; Córdoba, M.; Bullock, D. Delineation of management zones to improve nitrogen management of wheat. Comput. Electron. Agric. 2015, 110, 103–113. [Google Scholar] [CrossRef]
- Haraldsson, H.V. Introduction to System Thinking and Causal Loop Diagrams; Lund University: Lund, Sweden, 2004. [Google Scholar]
- Rasmussen, P.E.; Parton, W.J. Long-term effects of residue management in wheat-fallow: I. Inputs, yield, and soil organic matter. Soil Sci. Soc. Am. J. 1994, 58, 523–530. [Google Scholar] [CrossRef]
- Elaoud, A.; Chehaibi, S. Soil compaction due to tractor traffic. J. Fail. Anal. Prev. 2011, 11, 539–545. [Google Scholar] [CrossRef]
- Do the Figures Add Up on Your Farm Machinery Investments? Farming Independent. Available online: https://www.independent.ie/business/farming/machinery/do-the-figures-add-up-on-your-farm-machinery-investments-38838101.html (accessed on 12 November 2020).
- Angus, J.F.; Van Herwaarden, A.F. Increasing water use and water use efficiency in dryland wheat. Agron. J. 2001, 93, 290–298. [Google Scholar] [CrossRef]
- McBratney, A.; Whelan, B.; Ancev, T.; Bouma, J. Future directions of precision agriculture. Precis. Agric. 2005, 6, 7–23. [Google Scholar] [CrossRef]
- Dufour, L.; Metay, A.; Talbot, G.; Dupraz, C. Assessing light competition for cereal production in temperate agroforestry systems using experimentation and crop modelling. J. Agron. Crop Sci. 2013, 199, 217–227. [Google Scholar] [CrossRef]
- Jones, C.; Olson-Rutz, K. Practices to Increase Wheat Grain Protein; Montana State University Extension; EBO206; Montana State University: Bozeman, MT, USA, 2012. [Google Scholar]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef]
- Schmidt, E.L. Nitrification in soil. Nitrogen Agric. Soils 1982, 22, 253–288. [Google Scholar]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Needham, P.; Moore, G.; Scholz, G. Soil structure decline. In Soil Guide: A Handbook for Understanding and Managing Agricultural Soils; Moore, G., Ed.; Agriculture Western Australia: South Perth, Australia, 1998; pp. 64–79. [Google Scholar]
- Angulo-Jaramillo, R.; Vandervaere, J.P.; Roulier, S.; Thony, J.L.; Gaudet, J.P.; Vauclin, M. Field measurement of soil surface hydraulic properties by disc and ring infiltrometers: A review and recent developments. Soil Tillage Res. 2000, 55, 1–29. [Google Scholar] [CrossRef]
- Godwin, R.J.; Miller, P.C.H. A review of the technologies for mapping within-field variability. Biosyst. Eng. 2003, 84, 393–407. [Google Scholar] [CrossRef]
- Hoyle, F.C. The Effect of Soluble Organic Carbon Substrates, and Environmental Modulators on Soil Microbial Function and Diversity. Ph.D. Dissertation, University of Western Australia, Perth, Australia, 2006. [Google Scholar]
- Palosuo, T.; Kersebaum, K.C.; Angulo, C.; Hlavinka, P.; Moriondo, M.; Olesen, J.E.; Patil, R.H.; Ruget, F.; Rumbaur, C.; Takáč, J.; et al. Simulation of winter wheat yield and its variability in different climates of Europe: A comparison of eight crop growth models. Eur. J. Agron. 2011, 35, 103–114. [Google Scholar] [CrossRef]
- Andersen, M.K.; Jensen, L.S. Low soil temperature effects on short-term gross N mineralisation–immobilisation turnover after incorporation of a green manure. Soil Biol. Biochem. 2001, 33, 511–521. [Google Scholar] [CrossRef]
- Mosier, A.; Syers, J.K.; Freney, J.R. (Eds.) Agriculture and the Nitrogen Cycle: Assessing the Impacts of Fertilizer Use on Food Production and the Environment; Island Press: Washington, DC, USA, 2013; Volume 65. [Google Scholar]
- Dari, B.; Rogers, C.W.; Walsh, O.S. Understanding factors controlling ammonia volatilization from fertilizer nitrogen applications. Univ. Ida. Ext. Bul 2019, 926, 1–4. [Google Scholar]
- Kroulík, M.; Kumhála, F.; Hůla, J.; Honzík, I. The evaluation of agricultural machines field trafficking intensity for different soil tillage technologies. Soil Tillage Res. 2009, 105, 171–175. [Google Scholar] [CrossRef]
- Van Veen, J.A.; Ladd, J.N.; Amato, M. Turnover of carbon and nitrogen through the microbial biomass in a sandy loam and a clay soil incubated with [14C(U)] glucose and [15N](NH4)2SO4 under different moisture regimes. Soil Biol. Biochem. 1985, 17, 747–756. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, C.M. Soil evaporation and its affecting factors under crop canopy. Commun. Soil Sci. Plant analysis 2007, 38, 259–271. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Mooney, S.J. Exploring the interacting effect of soil texture and bulk density on root system development in tomato (Solanum lycopersicum L.). Environ. Exp. Bot. 2013, 91, 38–47. [Google Scholar] [CrossRef]
- Bigelow, C.A.; Bowman, D.C.; Cassel, D.K. Nitrogen leaching in sand-based rootzones amended with inorganic soil amendments and sphagnum peat. J. Am. Soc. Hortic. Sci. 2001, 126, 151–156. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; MacDonald, J.D.; Bissonnette, N.; Bertrand, N. Ammonia volatilization following surface application of urea to tilled and no-till soils: A laboratory comparison. Soil Tillage Res. 2009, 103, 310–315. [Google Scholar] [CrossRef]
- Askegaard, M.; Olesen, J.E.; Rasmussen, I.A.; Kristensen, K. Nitrate leaching from organic arable crop rotations is mostly determined by autumn field management. Agric. Ecosyst. Environ. 2011, 142, 149–160. [Google Scholar] [CrossRef]
- Reichardt, K.; Timm, L.C. How plants absorb nutrients from the soil. In Soil, Plant and Atmosphere; Springer: Cham, Switzerland, 2020; pp. 313–330. [Google Scholar]
- Delin, S.; Nyberg, A.; Lindén, B.; Ferm, M.; Torstensson, G.; Lerenius, C.; Gruvaeus, I. Impact of crop protection on nitrogen utilisation and losses in winter wheat production. Eur. J. Agron. 2008, 28, 361–370. [Google Scholar] [CrossRef]
- O’neal, M.; Frankenberger, J.R.; Ess, D.R.; Lowenberg-Deboer, J.M. Profitability of on-farm precipitation data for nitrogen management based on crop simulation. Precis. Agric. 2004, 5, 153–178. [Google Scholar] [CrossRef]
- Curioni, G.; Chapman, D.N.; Pring, L.J.; Royal, A.C.; Metje, N. Extending TDR capability for measuring soil density and water content for field condition monitoring. J. Geotech. Geoenviron. Eng. 2018, 144, 04017111. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, M.; Wang, N. Precision agriculture—A worldwide overview. Comput. Electron. Agric. 2002, 36, 113–132. [Google Scholar] [CrossRef]
- Visual Soil Examination and Evaluation (VSEE). Teagasc. Available online: https://www.teagasc.ie/environment/soil/research/square/visual-soil-examination-and-evaluation/ (accessed on 5 September 2019).
- McDonald, N.T.; Watson, C.J.; Lalor, S.T.; Laughlin, R.J.; Wall, D.P. Evaluation of soil tests for predicting nitrogen mineralization in temperate grassland soils. Soil Sci. Soc. Am. J. 2014, 78, 1051–1064. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R. The potassium paradox: Implications for soil fertility, crop production and human health. Renew. Agric. Food Syst. 2014, 29, 3–27. [Google Scholar] [CrossRef]
- Cholpankulov, E.D.; Inchenkova, O.P.; Paredes, P.; Pereira, L.S. Cotton irrigation scheduling in Central Asia: Model calibration and validation with consideration of groundwater contribution. Irrig. Drain. J. Int. Comm. Irrig. Drain. 2008, 57, 516–532. [Google Scholar] [CrossRef]
- Ibáñez-Asensio, S.; Marques-Mateu, A.; Moreno-Ramón, H.; Balasch, S. Statistical relationships between soil colour and soil attributes in semiarid areas. Biosyst. Eng. 2013, 116, 120–129. [Google Scholar] [CrossRef]
- Gardner, J.B.; Drinkwater, L.E. The fate of nitrogen in grain cropping systems: A meta-analysis of 15N field experiments. Ecol. Appl. 2009, 19, 2167–2184. [Google Scholar] [CrossRef] [PubMed]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Godwin, R.J. Agricultural Engineering in Development: Tillage for Crop Production in Areas of Low Rainfall; FAO: Rome, Italy, 1990. [Google Scholar]
- Forrestal, P.J.; Harty, M.; Carolan, R.; Lanigan, G.J.; Watson, C.J.; Laughlin, R.J.; McNeill, G.; Chambers, B.J.; Richards, K.G. Ammonia emissions from urea, stabilized urea and calcium ammonium nitrate: Insights into loss abatement in temperate grassland. Soil Use Manag. 2016, 32, 92–100. [Google Scholar] [CrossRef]
- Ichir, L.L.; Ismaili, M. Decomposition and nitrogen dynamics of wheat residues and impact on wheat growth stages. Comptes Rendus Biol. 2002, 325, 597–604. [Google Scholar] [CrossRef]
- Miola, E.C.; Aita, C.; Rochette, P.; Chantigny, M.H.; Angers, D.A.; Bertrand, N.; Gasser, M.O. Static chamber measurements of ammonia volatilization from manured soils: Impact of deployment duration and manure characteristics. Soil Sci. Soc. Am. J. 2015, 79, 305–313. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Adler, P.B.; Stark, J.M.; Tredennick, A.T. Water and nitrogen uptake are better associated with resource availability than root biomass. Ecosphere 2017, 8, e01738. [Google Scholar] [CrossRef]
- Smith, K.A.; Jackson, D.R.; Misselbrook, T.H.; Pain, B.F.; Johnson, R.A. PA—Precision agriculture: Reduction of ammonia emission by slurry application techniques. J. Agric. Eng. Res. 2000, 77, 277–287. [Google Scholar] [CrossRef]
- Mamo, M.; Malzer, G.L.; Mulla, D.J.; Huggins, D.R.; Strock, J. Spatial and temporal variation in economically optimum nitrogen rate for corn. Agron. J. 2003, 95, 958–964. [Google Scholar] [CrossRef]
- Alley, M.M.; Scharf, P.C.; Brann, D.E.; Baethgen, W.E.; Hammons, J.L. Nitrogen Management for Winter Wheat: Principles and Recommendations; Virginia Cooperative Extension: Petersburg, VA, USA, 2009. [Google Scholar]
- Lalor, S.T.J. Cattle Slurry on Grassland-Application Methods and Nitrogen Use Efficiency. Ph.D. Dissertation, Wageningen University and Research, Wageningen, The Netherlands, 2014. [Google Scholar]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Calibration and Spreaders. CF Fertelisers. Available online: http://www.cffertilisers.co.uk/advice-services/advice/calibration-and-spreaders/ (accessed on 10 December 2020).
- Griffin, T.; Lambert, D.; Lowenberg-DeBoer, J. Economics of lightbar and auto-guidance GPS navigation technologies. Precis. Agric. 2005, 5, 581–587. [Google Scholar]
- Schwarz, J.; Herold, L.; Pölling, B. Typology of PF Technologies; FP7 Project Future Farm. Available online: http://www.futurefarm.eu/ (accessed on 2 February 2018).
- Shockley, J.M.; Dillon, C.R.; Stombaugh, T.S. A whole farm analysis of the influence of auto-steer navigation on net returns, risk, and production practices. J. Agric. Appl. Econ. 2011, 43, 57–75. [Google Scholar] [CrossRef]
- Blaylock, A.D.; Kaufmann, J.; Dowbenko, R.D. Nitrogen fertilizer technologies. In Proceedings of the Western Nutrient Management Conference, Salt Lake City, UT, USA, 3–4 March 2005; Volume 6, pp. 8–13. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Colaço, A.F.; Bramley, R.G. Do crop sensors promote improved nitrogen management in grain crops? Field Crop. Res. 2018, 218, 126–140. [Google Scholar] [CrossRef]
- Baral, B.R.; Pande, K.R.; Gaihre, Y.K.; Baral, K.R.; Sah, S.K.; Thapa, Y.B.; Singh, U. Increasing nitrogen use efficiency in rice through fertilizer application method under rainfed drought conditions in Nepal. Nutr. Cycl. Agroecosyst. 2020, 118, 103–114. [Google Scholar] [CrossRef]
- Black, A.S.; Sherlock, R.R.; Smith, N.P. Effect of timing of simulated rainfall on ammonia volatilization from urea, applied to soil of varyingmoisture content. J. Soil Sci. 1987, 38, 679–687. [Google Scholar] [CrossRef]
- Bouwmeester, R.J.B.; Vlek, P.L.G.; Stumpe, J.M. Effect of environmental factors on ammonia volatilization from a urea-fertilized soil. Soil Sci. Soc. Am. J. 1985, 49, 376–381. [Google Scholar] [CrossRef]
- Watson, C.J.; Kilpatrick, D.J. The effect of urea pellet size and rate of application on ammonia volatilization and soil nitrogen dynamics. Fertil. Res. 1991, 28, 163–172. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Haynes, R.J. The decomposition process: Mineralization, immobilization, humus formation. In Mineral Nitrogen in the Plant-Soil Systems; Academic Press: Orlando, FL, USA, 1986; pp. 52–126. [Google Scholar]
- Nannipieri, P.; Eldor, P. The chemical and functional characterization of soil N and its biotic components. Soil Biol. Biochem. 2009, 41, 2357–2369. [Google Scholar] [CrossRef]
- Carranca, C.; De Varennes, A.; Rolston, D.E. Variation in N-recovery of winter wheat under Mediterranean conditions studied with 15N-labelled fertilizers. Eur. J. Agron. 1999, 11, 145–155. [Google Scholar] [CrossRef]
- Sanaa, M.; Mhiri, A.; Cleemput, O.V.; Baert, L. Field study of the fate of labelled fertilizer nitrogen applied to wheat on calcareous Tunisian soils. Bull. Soc. Belg. Pedol. 1992, 42, 245–255. [Google Scholar]
- Isfan, D.; D’Avignon, A.; Lamarre, M. Nitrogen-15 fertilizer recovery in spring wheat and soil as related to the rate and time of application. In Nuclear and Related Techniques in Soil-Plant Studies for Sustainable Agriculture and Environmental Preservation, Proceedings of the Symposium of Nuclear and Related Techniques in Soil-Plant Studies on Sustainable Agriculture and Environmental Preservation, Vienna, Austria, 17–21 October 1994; International Atomic Energy Agency: Vienna, Austria, 1995. [Google Scholar]
- Stevens, W.B.; Hoeft, R.G.; Mulvaney, R.L. Fate of nitrogen-15 in a long-term nitrogen rate study: II. Nitrogen uptake efficiency. Agron. J. 2005, 97, 1046–1053. [Google Scholar] [CrossRef]
- Olson, R.V.; Murphy, L.S.; Moser, H.C.; Swallow, C.W. Fate of tagged fertilizer nitrogen applied to winter wheat. Soil Sci. Soc. Am. J. 1979, 43, 973–975. [Google Scholar] [CrossRef]
- Schindler, F.V.; Knighton, R.E. Fate of fertilizer nitrogen applied to corn as estimated by the isotopic and difference methods. Soil Sci. Soc. Am. J. 1999, 63, 1734–1740. [Google Scholar] [CrossRef]
- Sanchez, J.E.; Willson, T.C.; Kizilkaya, K.; Parker, E.; Harwood, R.R. Enhancing the mineralizable nitrogen pool through substrate diversity in long term cropping systems. Soil Sci. Soc. Am. J. 2001, 65, 1442–1447. [Google Scholar] [CrossRef]
- Maltas, A.; Charles, R.; Jeangros, B.; Sinaj, S. Effect of organic fertilizers and reduced-tillage on soil properties, crop nitrogen response and crop yield: Results of a 12-year experiment in Changins, Switzerland. Soil Tillage Res. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Prescott, C.E.; Chappell, H.N.; Vesterdal, L. Nitrogen turnover in forest floors of coastal Douglas-fir at sites differing in soil nitrogen capital. Ecology 2000, 81, 1878–1886. [Google Scholar] [CrossRef]
- Roper, M.M.; Gupta, V.V.S.R. Enhancing Non-symbiotic N Fixation in Agriculture. Open Agric. J. 2016, 10, 7–27. [Google Scholar] [CrossRef]
- Halsall, D.M.; Turner, G.L.; Gibson, A.H. Straw and xylan utilization by pure cultures of nitrogen-fixing Azospirillum spp. Appl. Environ. Microbiol. 1985, 49, 423–428. [Google Scholar] [CrossRef]
- Adl, S.M. The Ecology of Soil Decomposition; CABI: Wallingford UK, 2003. [Google Scholar]
- Herlihy, M. Nitrogen mineralisation in soils of varying texture, moisture and organic matter. Plant Soil 1979, 53, 255–267. [Google Scholar] [CrossRef]
- Mikha, M.M.; Rice, C.W.; Milliken, G.A. Carbon and nitrogen mineralization as affected by drying and wetting cycles. Soil Biol. Biochem. 2005, 37, 339–347. [Google Scholar] [CrossRef]
- Wilson, J.P.; Gallant, J.C. Terrain Analysis; Principles and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Fiez, T.E.; Miller, B.C.; Pan, W.L. Assessment of spatially variable nitrogen fertilizer management in winter wheat. J. Prod. Agric. 1994, 7, 86–93. [Google Scholar] [CrossRef]
- Burke, I.C.; Lauenroth, W.K.; Parton, W.J. Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecology 1997, 78, 1330–1340. [Google Scholar] [CrossRef]
- Mary, B.; Recous, S.; Darwis, D.; Robin, D. Interactions between decomposition of plant residues and nitrogen cycling in soil. Plant Soil 1996, 181, 71–82. [Google Scholar] [CrossRef]
- Sørensen, P.; Jensen, E.S. Mineralization-immobilization and plant uptake of nitrogen as influenced by the spatial distribution of cattle slurry in soils of different texture. Plant Soil 1995, 173, 283–291. [Google Scholar] [CrossRef]
- Wickramasinghe, K.N.; Rodgers, G.A.; Jenkinson, D.S. Transformations of nitrogen fertilizers in soil. Soil Biol. Biochem. 1985, 17, 625–630. [Google Scholar] [CrossRef]
- Azam, F. Added nitrogen interaction in the soil-plant system—A review. Pak. J. Agron. 2002, 1, 54–59. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- O’Sullivan, M.F.; Simota, C. Modelling the environmental impacts of soil compaction: A review. Soil Tillage Res. 1995, 35, 69–84. [Google Scholar] [CrossRef]
- Dutartre, P.; Bartoli, F.; Andreux, F.; Portal, J.M.; Ange, A. Influence of content and nature of organic matter on the structure of some sandy soils from West Africa. In Soil Structure/Soil Biota Interrelationships; Elsevier: Amsterdam, The Netherlands, 1993; pp. 459–478. [Google Scholar]
- Hamilton, G.; Fisher, P.; Baimbridge, M.; Bignell, J.; Sheppard, J.; Bowey, R. Managing Grey Clays: To Maximise Production and Sustainability; Department of Primary Industries and Regional Development: Perth, Australia, 2005.
- Ali, M.A.; Ali, M.; Sattar, M.; Ali, L. Sowing date effect on yield of different wheat varieties. J. Agric. Res. 2010, 48, 157–162. [Google Scholar]
- Shahgholi, G.; Janatkhah, J. Investigation of the effects of organic matter application on soil compaction. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Derg. 2018, 28, 175–185. [Google Scholar] [CrossRef]
- Hoad, S.P.; Russell, G.; Lucas, M.E.; Bingham, I.J. The management of wheat, barley, and oat root systems. Adv. Agron. 2001, 74, 193–246. [Google Scholar]
- Grzesiak, S.; Grzesiak, M.T.; Hura, T.; Marcińska, I.; Rzepka, A. Changes in root system structure, leaf water potential and gas exchange of maize and triticale seedlings affected by soil compaction. Environ. Exp. Bot. 2013, 88, 2–10. [Google Scholar] [CrossRef]
- Tardieu, F. Growth and functioning of roots and of root systems subjected to soil compaction. Towards a system with multiple signalling? Soil Tillage Res. 1994, 30, 217–243. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Beusen, A.H.W.; Griffioen, J.; Van Groenigen, J.W.; Hefting, M.M.; Oenema, O.; Van Puijenbroek, P.J.T.M.; Seitzinger, S.; Slomp, C.P.; Stehfest, E. Global trends and uncertainties in terrestrial denitrification and N2O emissions. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130112. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; McNeill, A.; Davidson, R.; Tester, M.; Samec, M.; Korošak, D.; Sturrock, C.; Mooney, S.J. Quantifying the effect of soil compaction on three varieties of wheat (Triticum aestivum L.) using X-ray Micro Computed Tomography (CT). Plant Soil 2012, 353, 195–208. [Google Scholar] [CrossRef]
- Soane, B.D.; Van Ouwerkerk, C. Implications of soil compaction in crop production for the quality of the environment. Soil Tillage Res. 1995, 35, 5–22. [Google Scholar] [CrossRef]
- Nadian, H.; Smith, S.E.; Alston, A.M.; Murray, R.S. The effect of soil compaction on growth and P uptake by Trifolium subterraneum: Interactions with mycorrhizal colonisation. Plant Soil 1996, 182, 39–49. [Google Scholar] [CrossRef]
- Barley, K.P. The configuration of the root system in relation to nutrient uptake. Adv. Agron. 1970, 22, 159–201. [Google Scholar]
- Atwell, B.J. The effect of soil compaction on wheat during early tillering: II. Concentrations of cell constituents. New Phytol. 1990, 115, 37–41. [Google Scholar] [CrossRef]
- Barzegar, A.R.; Asoodar, M.A.; Ansari, M. Effectiveness of sugarcane residue incorporation at different water contents and the Proctor compaction loads in reducing soil compactibility. Soil Tillage Res. 2000, 57, 167–172. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Whalley, W.R.; Dumitru, E.; Dexter, A.R. Biological effects of soil compaction. Soil Tillage Res. 1995, 35, 53–68. [Google Scholar] [CrossRef]
- Chygarev, Y.; Lodyata, S. Research of tyre rigidity in terms of ecological safety of agricultural landscapes. In Proceedings of the VII Międzynarodowe Sympozjum Ekologiczne Aspekty Mechanizacji Nawożenia, Ochrony Roślin Uprawy Gleby i Zbioru Roślin Uprawnych, Warszawa, Poland, 18–19 September 2000; pp. 171–176. [Google Scholar]
- Bakker, D.M.; Davis, R.J. Soil deformation observations in a Vertisol under field traffic. Soil Res. 1995, 33, 817–832. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.W.; Kaspar, T.C. Soil compaction and root growth: A review. Agron. J. 1994, 86, 759–766. [Google Scholar] [CrossRef]
- Bennie, A.T.P.; Botha, F.J.P. Effect of deep tillage and controlled traffic on root growth, water-use efficiency and yield of irrigated maize and wheat. Soil Tillage Res. 1986, 7, 85–95. [Google Scholar] [CrossRef]
- Nyéki, A.; Milics, G.; Kovács, A.J.; Neményi, M. Effects of soil compaction on cereal yield: A review. Cereal Res. Commun. 2017, 45, 1–22. [Google Scholar] [CrossRef]
- Farahbakhsh, F.; Hamzehzarghani, H.; Massah, A.; Tortosa, M.; Yassaie, M.; Rodriguez, V.M. Comparative metabolomics of temperature sensitive resistance to wheat streak mosaic virus (WSMV) in resistant and susceptible wheat cultivars. J. Plant Physiol. 2019, 237, 30–42. [Google Scholar] [CrossRef]
- Cott, G.M.; Jansen, M.A.; Megonigal, J.P. Uptake of organic nitrogen by coastal wetland plants under elevated CO2. Plant Soil 2020, 450, 521–535. [Google Scholar] [CrossRef]
- Gioseffi, E.; de Neergaard, A.; Schjørring, J.K. Interactions between uptake of amino acids and inorganic nitrogen in wheat plants. Biogeosciences 2012, 9, 1509–1518. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, L.; Wang, J.; Ma, J.; Zheng, N.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Fertilizer regime changes the competitive uptake of organic nitrogen by wheat and soil microorganisms: An in-situ uptake test using 13C, 15N labelling, and 13C-PLFA analysis. Soil Biol. Biochem. 2018, 125, 319–327. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol. 2008, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, S.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Use of the stable nitrogen isotope to reveal the source-sink regulation of nitrogen uptake and remobilization during grain filling phase in maize. PLoS ONE 2016, 11, e0162201. [Google Scholar] [CrossRef]
- Basche, A.D.; DeLonge, M.S. Comparing infiltration rates in soils managed with conventional and alternative farming methods: A meta-analysis. PLoS ONE 2019, 14, e0215702. [Google Scholar] [CrossRef]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Johnson, M.G.; Spokas, K. Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 2016, 142, 176–183. [Google Scholar] [CrossRef]
- Roques, S.; Kendall, S.; Smith, K.; Newell Price, P.; Berry, P. A Review of the Non-NPKS Nutrient Requirements of UK Cereals and Oilseed Rape; Research Review No. 78; HGCA: Kenilworth, UK, 2013. [Google Scholar]
- Fernández, F.G.; Hoeft, R.G. Managing soil pH and crop nutrients. In Illinois Agronomy Handbook, 24th ed.; University of Illinois at Urbana-Champaign: Urbana, IL, USA, 2009; pp. 91–112. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Volume 29. [Google Scholar]
- Wheat Section 4 Plant Growth and Physiology. GRDC. Available online: https://grdc.com.au/__data/assets/pdf_file/0026/370673/GrowNote-Wheat-North-04-Physiology.pdf (accessed on 5 September 2019).
- Impa, S.M.; Vennapusa, A.R.; Bheemanahalli, R.; Sabela, D.; Boyle, D.; Walia, H.; Jagadish, S.K. High night temperature induced changes in grain starch metabolism alters starch, protein, and lipid accumulation in winter wheat. Plant Cell Environ. 2020, 43, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.P.O.; Diamond, J.; Finkele, K.; Holden, N.M.; Brereton, A.J. Predicting the soil moisture conditions of Irish grasslands. Ir. J. Agric. Food Res. 2005, 44, 95–110. [Google Scholar]
- Hu, T.; Sørensen, P.; Wahlström, E.M.; Chirinda, N.; Sharif, B.; Li, X.; Olesen, J.E. Root biomass in cereals, catch crops and weeds can be reliably estimated without considering aboveground biomass. Agric. Ecosyst. Environ. 2018, 251, 141–148. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between nutrition, plant diseases and pests. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 283–298. [Google Scholar]
- FAO. The State of Food and Agriculture 2001; No. 33; Food and Agriculture Organization: Rome, Italy, 2001. [Google Scholar]
- Doyle, B.; Cummins, T.; Augustenborg, C.; Aherne, J. Ambient Atmospheric Ammonia in Ireland 2013–2014; Report 193; Environmental Protection Agency of Ireland: Wexford, Irleand, 2014. [Google Scholar]
- De Klein, C.A.; Shepherd, M.A.; van der Weerden, T.J. Nitrous oxide emissions from grazed grasslands: Interactions between the N cycle and climate change—A New Zealand case study. Curr. Opin. Environ. Sustain. 2014, 9, 131–139. [Google Scholar] [CrossRef]
- Raun, W.R.; Schepers, J.S. Nitrogen management for improved use efficiency. Nitrogen Agric. Syst. 2008, 49, 675–693. [Google Scholar]
- Fan, X.H.; Li, Y.C.; Alva, A.K. Effects of temperature and soil type on ammonia volatilization from slow-release nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2011, 42, 1111–1122. [Google Scholar] [CrossRef]
- Sommer, S.G.; Olesen, J.E.; Christensen, B.T. Effects of temperature, wind speed and air humidity on ammonia volatilization from surface applied cattle slurry. J. Agric. Sci. 1991, 117, 91–100. [Google Scholar] [CrossRef]
- Harty, M.A.; McGeough, K.L.; Carolan, R.; Müller, C.; Laughlin, R.J.; Lanigan, G.J.; Richards, K.G.; Watson, C.J. Gross nitrogen transformations in grassland soil react differently to urea stabilisers under laboratory and field conditions. Soil Biol. Biochem. 2017, 109, 23–34. [Google Scholar] [CrossRef]
- Kotlar, A.M.; de Carvalho, H.W.P.; Iversen, B.V.; van Lier, Q.D.J. Nitrate leaching from layered double hydroxides in tropical and temperate soils. Appl. Clay Sci. 2020, 184, 105365. [Google Scholar] [CrossRef]
- Tian, G.; Cai, Z.; Cao, J.; Li, X. Factors affecting ammonia volatilisation from a rice–wheat rotation system. Chemosphere 2001, 42, 123–129. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; No. 80; Food & Agriculture Organization: Rome, Italy, 2005. [Google Scholar]
- Banning, N.C.; Maccarone, L.D.; Fisk, L.M.; Murphy, D.V. Ammonia-oxidising bacteria not archaea dominate nitrification activity in semi-arid agricultural soil. Sci. Rep. 2015, 5, 11146. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Pate, J.S. An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crop. Res. 2000, 65, 211–228. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Sawyer, J.E.; Lundvall, J.; Hawkins, J.S.; Barker, D.W. Sensing Nitrogen Stress in Corn; Iowa State University: Ames, IA, USA, 2011. [Google Scholar]
- Jordan, C. The effect of fertiliser type and application rate on denitrification losses from cut grassland in Northern Ireland. Fertil. Res. 1989, 19, 45–55. [Google Scholar] [CrossRef]
- Hyde, B.P.; Hawkins, M.J.; Fanning, A.F.; Noonan, D.; Ryan, M.; O’toole, P.; Carton, O.T. Nitrous oxide emissions from a fertilized and grazed grassland in the South East of Ireland. Nutr. Cycl. Agroecosyst. 2006, 75, 187–200. [Google Scholar] [CrossRef]
- Stark, C.H.; Richards, K.G. The continuing challenge of agricultural nitrogen loss to the environment in the context of global change and advancing research. Dyn. Soil Dyn. Plant 2008, 2, 1–12. [Google Scholar]
- Firestone, M.K.; Davidson, E.A. Microbiological basis of NO and N2O production and consumption in soil. Exch. Trace Gases Between Terr. Ecosyst. Atmos. 1989, 47, 7–21. [Google Scholar]
- Verhagen, F.J.; Laanbroek, H.J. Competition for ammonium between nitrifying and heterotrophic bacteria in dual energy-limited chemostats. Appl. Environ. Microbiol. 1991, 57, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bottomley, P.J.; Myrold, D.D. Contributions of ammonia-oxidizing archaea and bacteria to nitrification in Oregon forest soils. Soil Biol. Biochem. 2015, 85, 54–62. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Campbell, C.D.; Chapman, S.J.; Freitag, T.E.; Nicol, G.W.; Singh, B.K. Multi-factorial drivers of ammonia oxidizer communities: Evidence from a national soil survey. Environ. Microbiol. 2013, 15, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Alleman, J.E. Elevated nitrite occurrence in biological wastewater treatment systems. Water Sci. Technol. 1985, 17, 409–419. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Uchiyama, I.; Toyoda, A.; Wang, Y.; Shimomura, Y.; Okubo, T.; Kurisu, F.; Hirono, Y.; Nonaka, K.; et al. An acid-tolerant ammonia-oxidizing γ-proteobacterium from soil. ISME J. 2017, 11, 1130–1141. [Google Scholar] [CrossRef]
- Cogle, A.L.; Rao, K.P.C.; Yule, D.F.; Smith, G.D.; George, P.J.; Srinivasan, S.T.; Jangawad, L. Soil management for Alfisols in the semiarid tropics: Erosion, enrichment ratios and runoff. Soil Use Manag. 2002, 18, 10–17. [Google Scholar] [CrossRef][Green Version]
- Das, B.S.; Kluitenberg, G.J.; Pierzynski, G.M. Temperature dependence of nitrogen mineralization rate constant: A theoretical approach. Soil Sci. 1995, 159, 294–300. [Google Scholar] [CrossRef]
- Campbell, C.A.; Biederbeck, V.O. Influence of fluctuating temperatures and constant soil moistures on nitrogen changes in amended and unamended loam. Can. J. Soil Sci. 1972, 52, 323–336. [Google Scholar] [CrossRef]
- Chandra, P. Note on the effect of shifting temperatures on nitrification in a loam soil. Can. J. Soil Sci. 1962, 42, 314–315. [Google Scholar] [CrossRef]
- Husk, B.R.; Anderson, B.C.; Whalen, J.K.; Sanchez, J.S. Reducing nitrogen contamination from agricultural subsurface drainage with denitrification bioreactors and controlled drainage. Biosyst. Eng. 2017, 153, 52–62. [Google Scholar] [CrossRef]
- Lin, B.L.; Sakoda, A.; Shibasaki, R.; Suzuki, M. A modelling approach to global nitrate leaching caused by anthropogenic fertilisation. Water Res. 2001, 35, 1961–1968. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental Panel on Climate Change/Organization for Economic Cooperation and Development. Guidelines for National Greenhouse Gas Inventories; OECD/OCDE: Paris, France, 1997. [Google Scholar]
- Randall, G.W.; Delgado, J.A.; Schepers, J.S. Nitrogen management to protect water resources. Nitrogen Agric. Syst. 2008, 49, 911–945. [Google Scholar]
- Harmel, D.; Qian, S.; Reckhow, K.; Casebolt, P. The MANAGE database: Nutrient load and site characteristic updates and runoff concentration data. J. Environ. Qual. 2008, 37, 2403–2406. [Google Scholar] [CrossRef] [PubMed]
- Van der Werff, P.A.; Baars, A.; Oomen, G.J.M. Nutrient balances and measurement of nitrogen losses on mixed ecological farms on sandy soils in the Netherlands. Biol. Agric. Hortic. 1995, 11, 41–50. [Google Scholar] [CrossRef]
- Lind, A.M.; Debosz, K.K.; Maag, M. N-balance for mineral N on spring barley cropped sandy loam and coarse sandy soil with mineral and organic fertilizers. Acta Agric. Scand. B Plant Soil Sci. 1995, 45, 39–50. [Google Scholar] [CrossRef]
- Gaines, T.P.; Gaines, S.T. Soil texture effect on nitrate leaching in soil percolates. Commun. Soil Sci. Plant Anal. 1994, 25, 2561–2570. [Google Scholar] [CrossRef]
- Ruiz-Colmenero, M.; Bienes, R.; Eldridge, D.J.; Marques, M.J. Vegetation cover reduces erosion and enhances soil organic carbon in a vineyard in the central Spain. Catena 2013, 104, 153–160. [Google Scholar] [CrossRef]
- Meetei, T.T.; Devi, Y.B.; Chanu, T.T. Ion Exchange: The Most Important Chemical Reaction on Earth after Photosynthesis. Int. Res. J. Pure Appl. Chem 2020, 21, 31–42. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. (Eds.) Biochar: A guide to Analytical Methods; Csiro Publishing: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tahir, S.; Marschner, P. Clay addition to sandy soil reduces nutrient leaching—Effect of clay concentration and ped size. Commun. Soil Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Qafoku, N.P.; Van Ranst, E.; Noble, A.; Baert, G. Variable charge soils: Their mineralogy, chemistry and management. Adv. Agron. 2004, 84, 160–217. [Google Scholar]
- Kubotera, H. Factors influencing nitrate retention in 3 Andisol profiles in Kyushu, Japan. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Guo, P.C.; Bogring, J.; Scherer, H.W. Verhalten von Dünger-NH4 in Böden unterschiedlicher tonmineralischer Zusammensetzung im Inkubationsversuch. Z. Pflanz. Bodenkd. 1983, 146, 752–759. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Bullock, D.G. Correlation of corn and soybean grain yield with topography and soil properties. Agron. J. 2000, 92, 75–83. [Google Scholar] [CrossRef]
- Kitchen, N.R.; Drummond, S.T.; Lund, E.D.; Sudduth, K.A.; Buchleiter, G.W. Soil electrical conductivity and topography related to yield for three contrasting soil–crop systems. Agron. J. 2003, 95, 483–495. [Google Scholar] [CrossRef]
- Jiang, P.; Thelen, K.D. Effect of soil and topographic properties on crop yield in a North-Central corn–soybean cropping system. Agron. J. 2004, 96, 252–258. [Google Scholar] [CrossRef]
- Mills, W.C.; Thomas, A.W.; Langdale, G.W. Rainfall retention probabilities computed for different cropping-tillage systems. Agric. Water Manag. 1988, 15, 61–71. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef]
- Ali, M.A.; Khalid, L. Grain losses of wheat as affected by different harvesting and threshing techniques. Int. J. Res. Agric. For. 2015, 2, 20–26. [Google Scholar]
- Boxall, R.A. Post-harvest losses to insects—A world overview. Int. Biodeterior. Biodegrad. 2001, 48, 137–152. [Google Scholar] [CrossRef]
- Hollins, P.D.; Kettlewell, P.S.; Parsons, S.T.; Atkinson, M.D. The impact of supply, demand and grain quality on the UK bread and feed wheat price differential in the UK. J. Agric. Sci. 2006, 144, 411–419. [Google Scholar] [CrossRef]
- Cambi, M.; Certini, G.; Fabiano, F.; Foderi, C.; Laschi, A.; Picchio, R. Impact of wheeled and tracked tractors on soil physical properties in a mixed conifer stand. iForest Biogeosci. For. 2015, 9, 89. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).