Effects of Drainage Water Management in a Corn–Soy Rotation on Soil N2O and CH4 Fluxes
Abstract
:1. Introduction
2. Materials and Methods
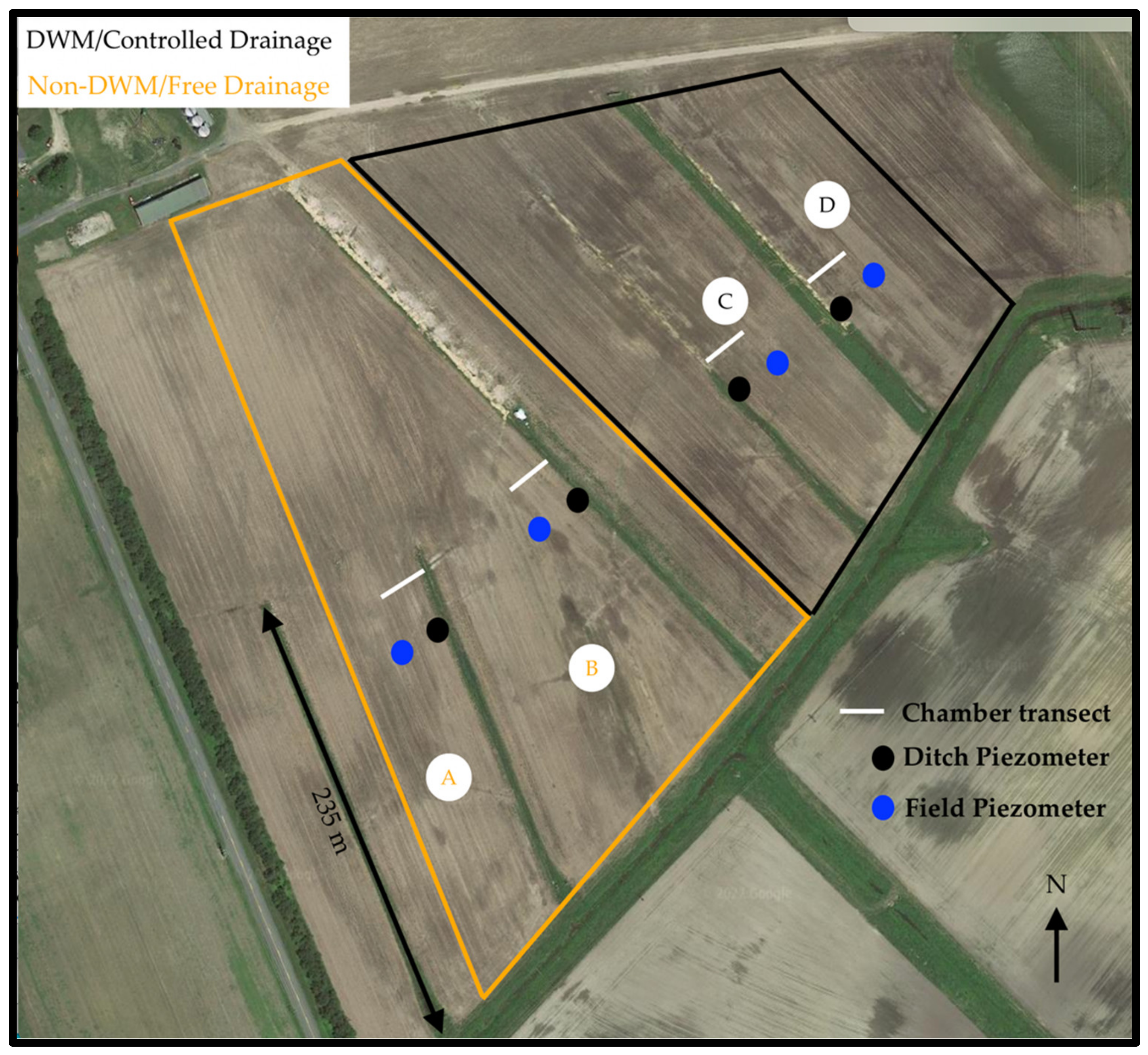
2.1. Study Site Location
2.2. Farm Management
2.3. DWM Management
2.4. Experimental Design
2.5. Flux Measurements
2.6. Soil Chemical and Physical Characteristics
2.7. Non-Parametric DWM Impact Model
2.8. Mixed-Effects Model
2.9. Regression Statistics
2.10. Annual Flux Calculations
2.10.1. Seasonally-Based Flux Interpolation
2.10.2. Mixed-Effects Model Extrapolation
2.10.3. Regression Model N2O Flux Interpolation
3. Results
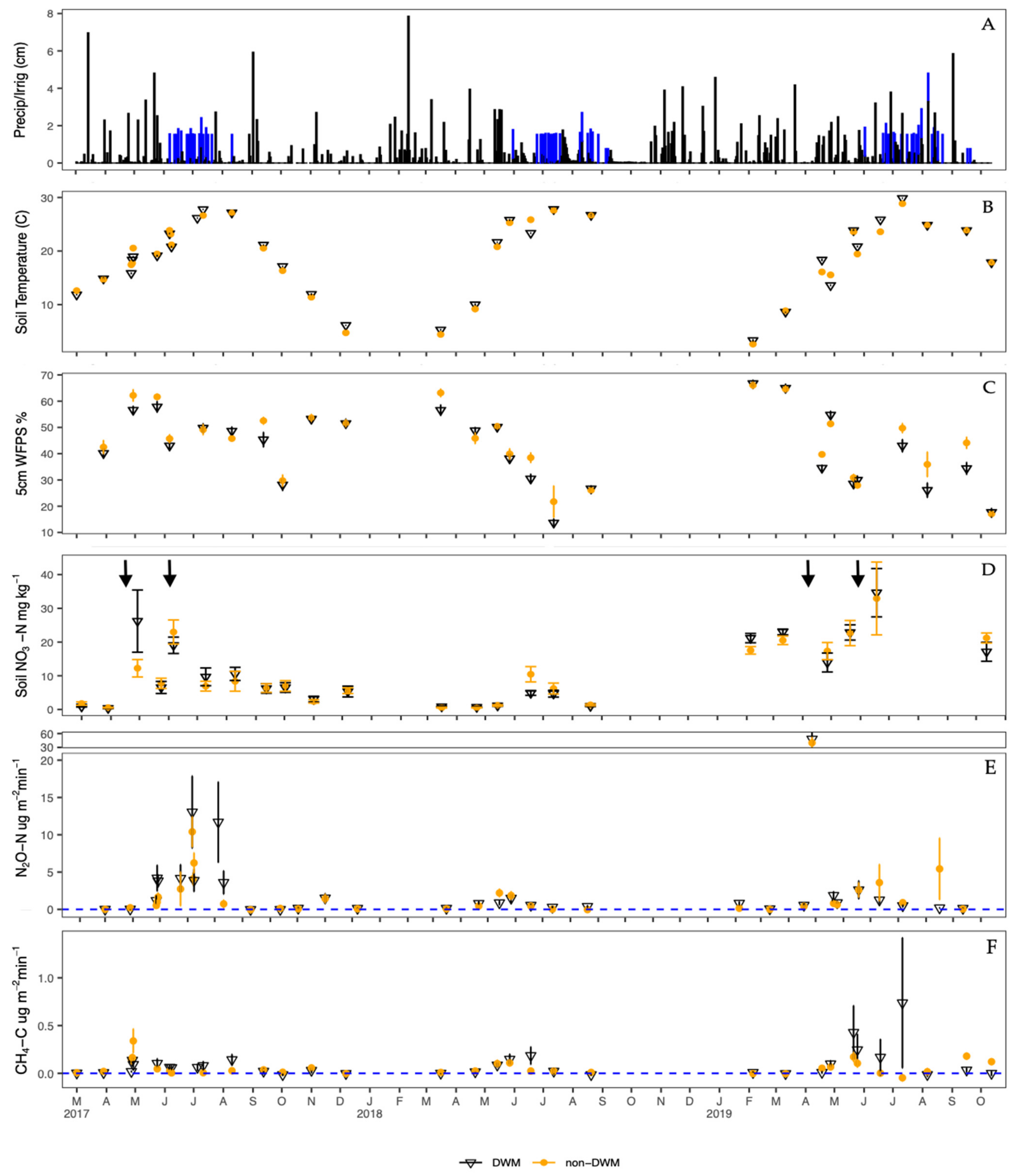
3.1. Environmental Variables
3.1.1. Precipitation and Air Temperature
3.1.2. Soil Temperature and Moisture
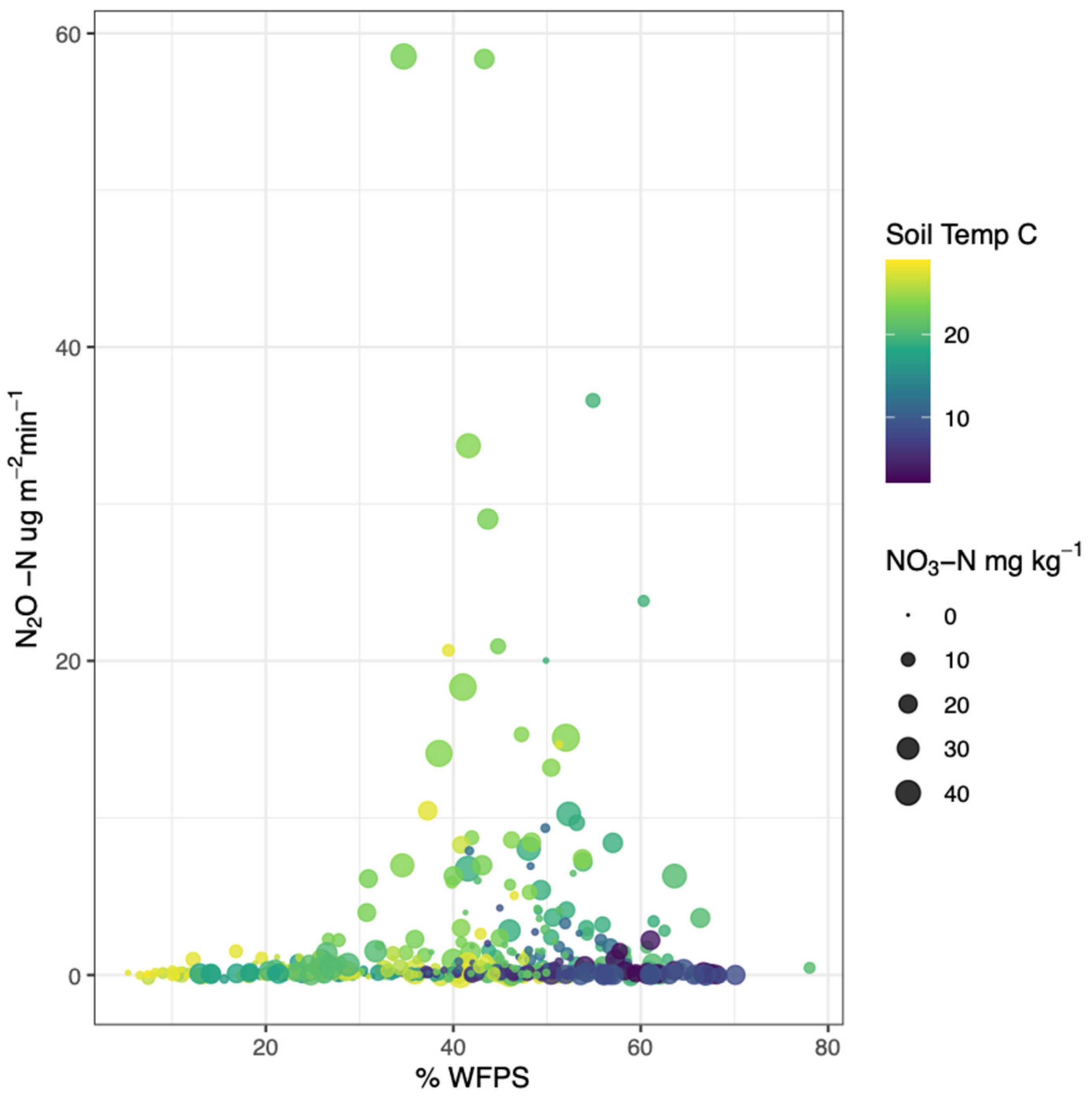
3.1.3. Soil Extractable Nitrogen
3.1.4. Soil Texture and Bulk Density
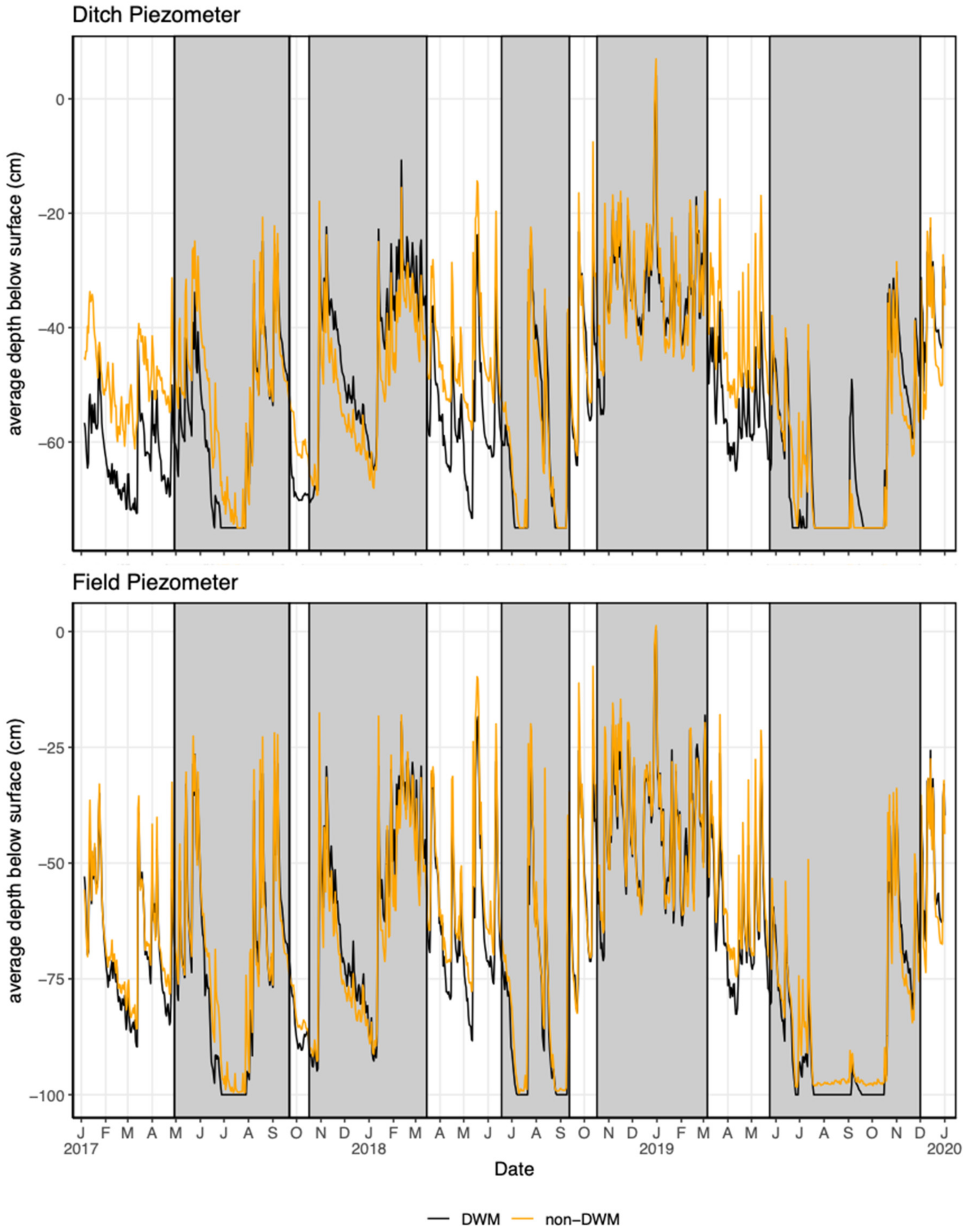
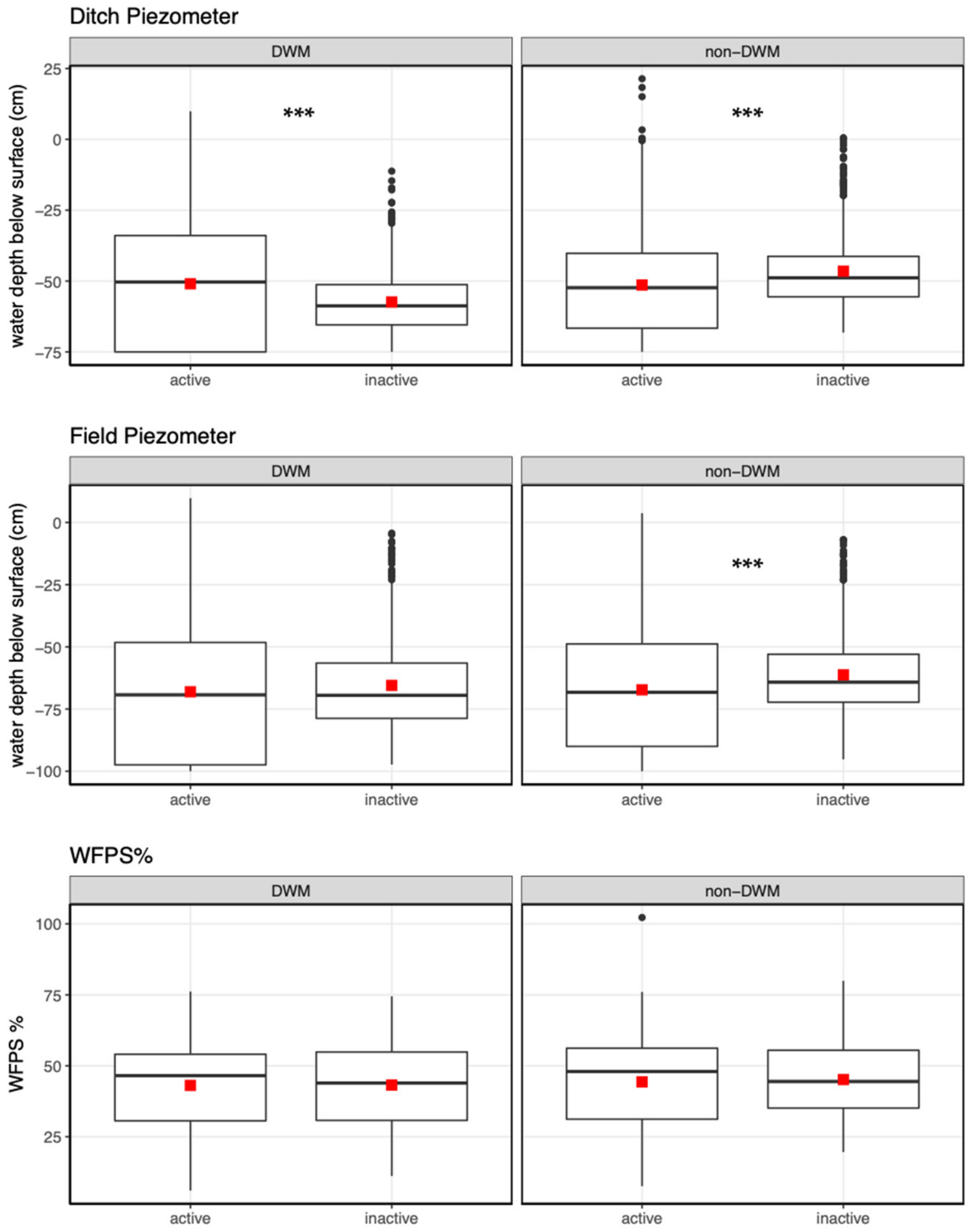
3.2. Piezometers
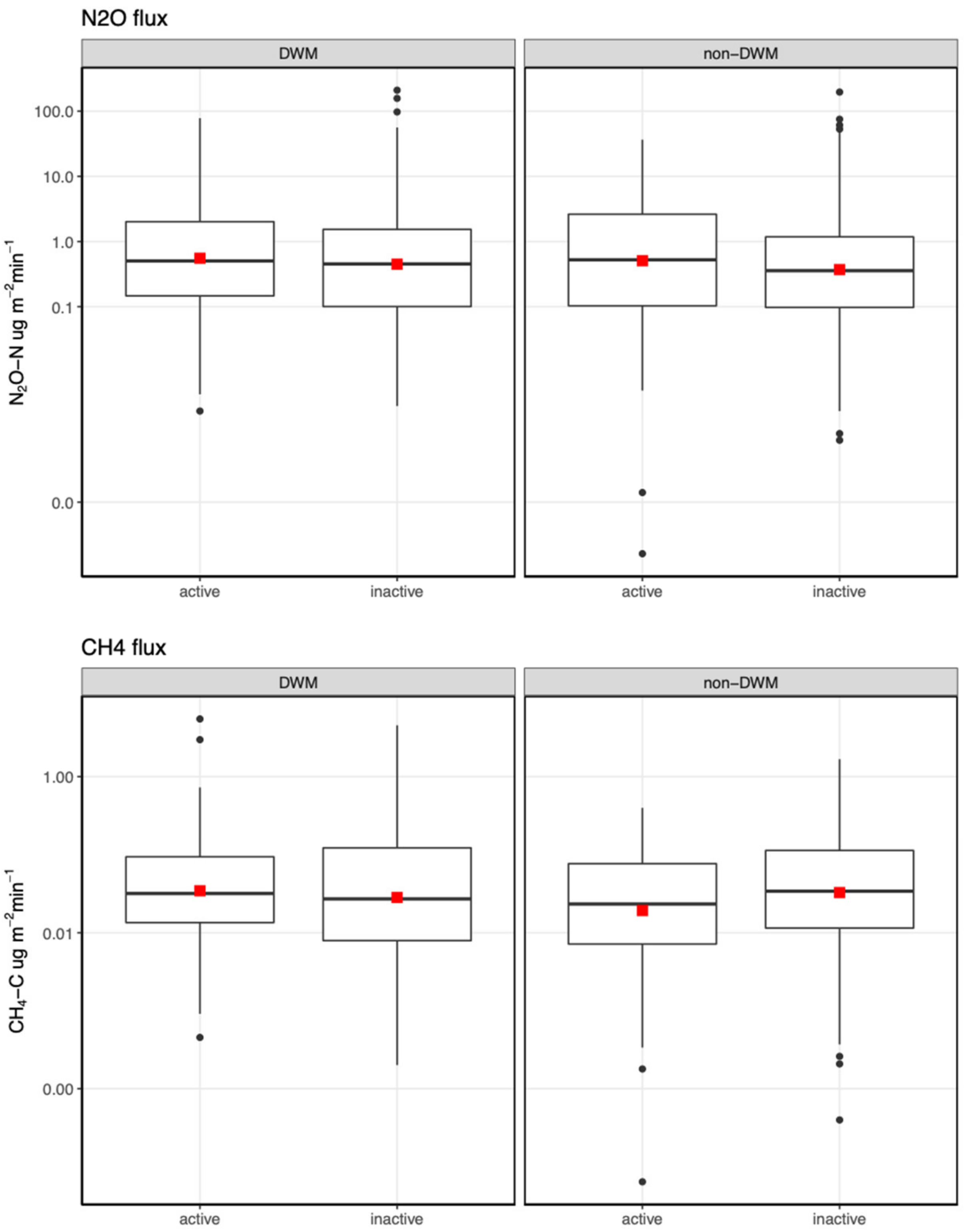
3.3. Chamber Flux Summary
3.4. Non-Parametric DWM Model for Effects on Piezometers, WFPS, and Gas Fluxes
3.5. Mixed-Effects Model Results for N2O and CH4 Fluxes
3.6. N2O Multiple Regression
3.7. Annual Soil Emission Flux Estimates
4. Discussion
4.1. Impact of Drainage Water Management
4.1.1. Groundwater Level
4.1.2. N2O and CH4 Fluxes
4.2. Farm Management Impact on GHGs
4.3. Comparing Annual Soil Emission Estimates
4.4. Comparison to Regional and Global Nitrogen Budget Measures
4.4.1. N2O Emissions Factor
4.4.2. Nitrogen Balance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gilbert, P.M. From hogs to HABs: Impacts of industrial farming in the US on nitrogen and phosphorus and greenhouse gas pollution. Biogeochemistry 2020, 150, 139–180. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.E.; LaMotte, A.E. Attributes for NHDPlus Catchments (Version 1.1) for the Conterminous United States: Nutrient Application (Phosphorus and Nitrogen) for Fertilizer and Manure Applied to Crops (Cropsplit), 2002; Data Series DS-490-08; U.S. Geological Survey Digital: Reston, VA, USA, 2010. [Google Scholar]
- Ator, S.W.; Denver, J.M. Understanding the Nutrients in the Chesapeake Bay Watershed and Implications for Management and Restoration: The Eastern Shore; Circular Series 1406; U.S. Geological Survey Digital: Reston, VA, USA, 2015; p. 84. [Google Scholar]
- Sabo, R.D.; Clark, C.M.; Bash, J.; Sobota, D.; Cooter, E.; Dobrowolski, J.P.; Houlton, B.Z.; Rea, A.; Schwede, D.; Morford, S.L.; et al. Decadal shift in nitrogen inputs and fluxes across the contiguous United States: 2002–2012. J. Geophys. Res. Biogeosciences 2019, 124, 3104–3124. [Google Scholar] [CrossRef]
- Smil, V. Nitrogen in crop production: An account of global flows. Glob. Biogeochem. Cycles 1999, 13, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, T.; Lassaletta, L.; Mueller, N.D.; Tubiello, F.N.; Lisk, M.D.; Lu, C.; Conant, R.T.; Dorich, C.D.; Gerber, J.; et al. Quantification of global and national nitrogen budgets for crop production. Nat. Food 2021, 2, 529–540. [Google Scholar] [CrossRef]
- Skaggs, R.W.; Breve, M.A.; Gilliam, J.W. Hydrologic and water quality impacts of agricultural drainage. Crit. Rev. Environ. Sci. Technol. 1994, 24, 1–32. [Google Scholar] [CrossRef]
- Evans, R.O.; Skaggs, R.W.; Gilliam, J.W. Controlled versus conventional drainage effects on water quality. J. Irrig. Drain. Eng. 1995, 121, 271–276. [Google Scholar] [CrossRef]
- Skaggs, R.W.; Fausey, N.R.; Evans, R.O. Drainage water management. J. Soil Water Conserv. 2012, 67, 167A–172A. [Google Scholar] [CrossRef] [Green Version]
- Firestone, M.K.; Davidson, E.A. Microbiological basis of NO and N2O production and consumption in soil. Exch. Trace Gases Terr. Ecosyst. Atmos. 1989, 47, 7–21. [Google Scholar]
- Nömmik, H. Investigations on denitrification in soil. Acta Agric. Scand. 1956, 6, 195–228. [Google Scholar] [CrossRef]
- Carstensen, M.V.; Hashemi, F.; Hoffmann, C.C.; Zak, D.; Audet, J.; Kronvang, B. Efficiency of mitigation measures targeting nutrient losses from agricultural drainage systems: A review. Ambio 2020, 49, 1820–1837. [Google Scholar] [CrossRef]
- Drury, C.F.; Tan, C.S.; Reynolds, W.D.; Welacky, T.W.; Oloya, T.O.; Gaynor, J.D. Managing tile drainage, subirrigation, and nitrogen fertilization to enhance crop yields and reduce nitrate loss. J. Environ. Qual. 2009, 38, 1193–1204. [Google Scholar] [CrossRef]
- Sunohara, M.D.; Craiovan, E.; Topp, E.; Gottschall, N.; Drury, C.F.; Lapen, D.R. Comprehensive nitrogen budgets for controlled tile drainage fields in eastern Ontario, Canada. J. Environ. Qual. 2014, 43, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, J.; Davidson, E.; Fox, R.; Koontz, E.; Fisher, T.; Castro, M.; Zhu, Q.; Gustafson, A. Pollution Swapping of N2O and CH4 Emissions with Dissolved Nitrogen and Phosphorus Export in Drainage Water Managed Agricultural Field. In Proceedings of the AGU 2019 Fall Meeting, San Francisco, CA, USA, 9–13 December 2019. [Google Scholar] [CrossRef]
- Kanter, D.; Wagner-Riddle, C.; Groffman, P.M.; Davidson, E.A.; Galloway, J.N.; Gourevitch, J.D.; van Grinsven, H.J.M.; Houlton, B.Z.; Keeler, B.L.; Ogle, S.M.; et al. Improving the social cost of nitrous oxide. Nat. Clim. Change 2021, 11, 1008–1010. [Google Scholar] [CrossRef]
- Prather, M.J.; Hsu, J.; DeLuca, N.M.; Jackman, C.H.; Oman, L.; Douglass, A.R.; Fleming, E.L.; Strahan, S.E.; Steenrod, S.D.; Søvde, O.A.; et al. Measuring and modeling the lifetime of nitrous oxide including its variability. J. Geophys. Res. Atmos. 2015, 120, 5693–5705. [Google Scholar] [CrossRef]
- US-EPA. In Inventory of US Greenhouse Gas Emissions and Sinks, 1990–2019; US Environmental Protection Agency, 2021. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks (accessed on 13 November 2021).
- Xu, R.; Tian, H.; Pan, N.; Thompson, R.L.; Canadell, J.G.; Davidson, E.A.; Nevison, C.; Winiwarter, W.; Shi, H.; Pan, S.; et al. Magnitude and uncertainty of nitrous oxide emissions from North America based on bottom-up and top-down approaches: Informing future research and national inventories. Geophys. Res. Lett. 2021, 48, e2021GL095264. [Google Scholar] [CrossRef]
- Knowles, R. Methane: Processes of production and consumption. Agric. Ecosyst. Eff. Trace Gases Glob. Clim. Change 1993, 55, 145–156. [Google Scholar]
- Pachauri, R.K.; Meyers, L.A. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Fisher, T.R.; Fox, R.J.; Gustafson, A.B.; Lewis, J.; Millar, N.; Winstene, J.R. Fluxes of nitrous oxide and nitrate from agricultural fields on the Delmarva Peninsula: N biogeochemistry and economics of field management. Agric. Ecosyst. Environ. 2018, 254, 162–178. [Google Scholar] [CrossRef]
- Dobbie, K.E.; McTaggart, I.P.; Smith, K.A. Nitrous oxide emissions from intensive agricultural systems: Variations between crops and seasons, key driving variables, and mean emission factors. J. Geophys. Res. Atmos. 1999, 104, 26891–26899. [Google Scholar] [CrossRef]
- Kumar, S.; Nakajima, T.; Kadono, A.; Lal, R.; Fausey, N. Long-term tillage and drainage influences on greenhouse gas fluxes from a poorly drained soil of central Ohio. J. Soil Water Conserv. 2014, 69, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Fernández, F.G.; Venterea, R.T.; Fabrizzi, K.P. Corn nitrogen management influences nitrous oxide emissions in drained and undrained soils. J. Environ. Qual. 2016, 45, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, B.A.; Gilliam, J.W. Water table management effects on denitrification and nitrous oxide evolution. Soil Sci. Soc. Am. J. 1995, 59, 1694–1701. [Google Scholar] [CrossRef]
- Elmi, A.; Burton, D.; Gordon, R.; Madramootoo, C. Impacts of water table management on N2O and N2 from a sandy loam soil in southwestern Quebec, Canada. Nutr. Cycl. Agroecosystems 2005, 72, 229–240. [Google Scholar] [CrossRef]
- Nangia, V.; Sunohara, M.; Topp, E.; Gregorich, E.; Drury, C.; Gottschall, N.; Lapen, D. Measuring and modeling the effects of drainage water management on soil greenhouse gas fluxes from corn and soybean fields. J. Environ. Manag. 2013, 129, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Crézé, C.M.; Madramootoo, C.A. Water table management and fertilizer application impacts on CO2, N2O and CH4 fluxes in a corn agro-ecosystem. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Datta, A.; Smith, P.; Lal, R. Effects of long-term tillage and drainage treatments on greenhouse gas fluxes from a corn field during the fallow period. Agric. Ecosyst. Environ. 2013, 171, 112–123. [Google Scholar] [CrossRef]
- Van Zandvoort, A.; Lapen, D.; Clark, I.; Flemming, C.; Craiovan, E.; Sunohara, M.; Boutz, R.; Gottschall, N. Soil CO2, CH4, and N2O fluxes over and between tile drains on corn, soybean, and forage fields under tile drainage management. Nutr. Cycl. Agroecosystems 2017, 109, 115–132. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Robertson, G.P. Statistical challenges in analyses of chamber-based soil CO2 and N2O emissions data. Soil Sci. Soc. Am. J. 2015, 79, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Levy, P.E.; Gray, A.; Leeson, S.R.; Gaiawyn, J.; Kelly, M.P.C.; Cooper, M.D.A.; Dinsmore, K.J.; Jones, S.K.; Sheppard, L.J. Quantification of uncertainty in trace gas fluxes measured by the static chamber method. Eur. J. Soil Sci. 2011, 62, 811–821. [Google Scholar] [CrossRef]
- Topp, G.C.; Davis, J.L.; Annan, A.P. Electromagnetic determination of soil water content: Measurements in coaxial transmission lines. Water Resour. Res. 1980, 16, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Maynard, D.G.; Kalra, Y.P.; Crumbaugh, J.A. Nitrate and exchangeable ammonium nitrogen. In Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 1993; Volume 1. [Google Scholar]
- Robertson, G.P.; Coleman, D.C.; Sollins, P.; Bledsoe, C.S. Standard Soil Methods for Long-Term Ecological Research; Oxford University Press: Oxford, UK, 1999; Volume 2. [Google Scholar]
- Gavlak, R.; Horneck, D.; Miller, R.O. Soil, Plant and Water Reference Methods for the Western Region; WREP-125; Western Region Extension Publication: Logan, UT, USA, 2005. [Google Scholar]
- Van der Weerden, T.J.; Noble, A.N.; Luo, J.; de Klein, C.A.M.; Saggar, S.; Giltrap, D.; Gibbs, J.; Rys, G. Meta-analysis of New Zealand’s nitrous oxide emission factors for ruminant excreta supports disaggregation based on excreta form, livestock type and slope class. Sci. Total Environ. 2020, 732, 139235. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Jankowski, K.; Neill, C.; Davidson, E.A.; Macedo, M.N.; Costa, C.; Galford, G.L.; Santos, L.M.; Lefebvre, P.; Nunes, D.; Cerri, C.E.P.; et al. Deep soils modify environmental consequences of increased nitrogen fertilizer use in intensifying Amazon agriculture. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Matson, P.A.; Billow, C.; Hall, S.; Zachariassen, J. Fertilization practices and soil variations control nitrogen oxide emissions from tropical sugar cane. J. Geophys. Res. Atmos. 1996, 101, 18533–18545. [Google Scholar] [CrossRef]
- Fisher, T.R.; Jordan, T.E.; Staver, K.W.; Gustafson, A.B.; Koskelo, A.I.; Fox, R.J.; Sutton, A.J.; Kana, T.; Beckert, K.A.; Stone, J.P.; et al. The Choptank Basin in transition: Intensifying agriculture, slow urbanization, and estuarine eutrophication. In Coastal Lagoons: Systems of Natural and Anthropogenic Change; Kennish, M.J., Paerl, H.W., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 135–165. [Google Scholar]
- Williams, M.R.; King, K.W.; Fausey, N.R. Drainage water management effects on tile discharge and water quality. Agric. Water Manag. 2015, 148, 43–51. [Google Scholar] [CrossRef]
- Lavaire, T.; Gentry, L.E.; David, M.B.; Cooke, R.A. Fate of water and nitrate using drainage water management on tile systems in east-central Illinois. Agric. Water Manag. 2017, 191, 218–228. [Google Scholar] [CrossRef]
- Smith, E.L.; Kellman, L.M. Nitrate loading and isotopic signatures in subsurface agricultural drainage systems. J. Environ. Qual. 2011, 40, 1257–1265. [Google Scholar] [CrossRef]
- Lalonde, V.; Madramootoo, C.A.; Trenholm, L.; Broughton, R.S. Effects of controlled drainage on nitrate concentrations in subsurface drain discharge. Agric. Water Manag. 1996, 29, 187–199. [Google Scholar] [CrossRef]
- Parkin, T.B.; Kaspar, T.C. Nitrous oxide emissions from corn–soybean systems in the Midwest. J. Environ. Qual. 2006, 35, 1496–1506. [Google Scholar] [CrossRef] [Green Version]
- Baggs, E.M.; Stevenson, M.; Pihlatie, M.; Regar, A.; Cook, H.; Cadisch, G. Nitrous oxide emissions following application of residues and fertiliser under zero and conventional tillage. Plant Soil 2003, 254, 361–370. [Google Scholar] [CrossRef]
- Bremner, J.M.; Breitenbeck, G.A.; Blackmer, A.M. Effect of nitrapyrin on emission of nitrous oxide from soil fertilized with anhydrous ammonia. Geophys. Res. Lett. 1981, 8, 353–356. [Google Scholar] [CrossRef]
- Ullah, S.; Moore, T.R. Biogeochemical controls on methane, nitrous oxide, and carbon dioxide fluxes from deciduous forest soils in eastern Canada. J. Geophys. Res. Biogeosciences 2011, 116. [Google Scholar] [CrossRef] [Green Version]
- Venterea, R.T.; Coulter, J.A. Split application of urea does not decrease and may increase nitrous oxide emissions in rainfed corn. Agron. J. 2015, 107, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Cowan, N.; Maire, J.; Krol, D.; Cloy, J.M.; Hargreaves, P.; Murphy, R.; Carswell, A.; Jones, S.K.; Hinton, N.; Anderson, M.; et al. Agricultural soils: A sink or source of methane across the British Isles? Eur. J. Soil Sci. 2021, 72, 1842–1862. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Volume 4—Agriculture, Forestry and Other Land Use, Chapter 3. Consistent Representation of Lands. 2019. Available online: https://www.ipcc-nggip.iges.or.jp/public/2019rf/vol4.html (accessed on 13 November 2021).
- Hergoualc’H, K.; Mueller, N.; Bernoux, M.; Kasimir, Ä.; van der Weerden, T.J.; Ogle, S.M. Improved accuracy and reduced uncertainty in greenhouse gas inventories by refining the IPCC emission factor for direct N2O emissions from nitrogen inputs to managed soils. Glob. Change Biol. 2021, 27, 6536–6550. [Google Scholar] [CrossRef]
- Fisher, T.R.; Fox, R.J.; Gustafson, A.B.; Koontz, E.; Lepori-Bui, M.; Lewis, J. Localized water quality improvement in the choptank estuary, a tributary of Chesapeake Bay. Estuaries Coasts 2021, 44, 1274–1293. [Google Scholar] [CrossRef]
- Conant, R.T.; Berdanier, A.B.; Grace, P.R. Patterns and trends in nitrogen use and nitrogen recovery efficiency in world agriculture. Glob. Biogeochem. Cycles 2013, 27, 558–566. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Basso, B.; Shuai, G.; Zhang, J.; Robertson, G.P. Yield stability analysis reveals sources of large-scale nitrogen loss from the US Midwest. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Roy, E.D.; Wagner, C.R.H.; Niles, M.T. Hot spots of opportunity for improved cropland nitrogen management across the United States. Environ. Res. Lett. 2021, 16, 035004. [Google Scholar] [CrossRef]
- Eagle, A.J.; McLellan, E.L.; Brawner, E.M.; Chantigny, M.H.; Davidson, E.A.; Dickey, J.B.; Linquist, B.A.; Maaz, T.M.; Pelster, D.E.; Pittelkow, C.M.; et al. Quantifying on-farm nitrous oxide emission reductions in food supply chains. Earth’s Future 2020, 8, e2020EF001504. [Google Scholar] [CrossRef]
- Verchot, L.V.; Davidson, E.; Cattanio, J.H.; Ackerman, I.L.; Erickson, H.E.; Keller, M. Land use change and biogeochemical controls of nitrogen oxide emissions from soils in eastern Amazonia. Glob. Biogeochem. Cycles 1999, 13, 31–46. [Google Scholar] [CrossRef]
- Courtois, E.A.; Stahl, C.; Burban, B.; Van den Berge, J.; Berveiller, D.; Bréchet, L.; Soong, J.L.; Arriga, N.; Peñuelas, J.; Janssens, I.A. Automatic high-frequency measurements of full soil greenhouse gas fluxes in a tropical forest. Biogeosciences 2019, 16, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, J.R.; Outhwaite, J.; Smukler, S.M. Comparison of CO2, CH4 and N2O soil-atmosphere exchange measured in static chambers with cavity ring-down spectroscopy and gas chromatography. Agric. For. Meteorol. 2015, 211, 48–57. [Google Scholar] [CrossRef]
- Nickerson, N. Evaluating Gas Emission Measurements using Minimum Detectable Flux (MDF); Eosense Inc.: Dartmouth, Canada, 2016. [Google Scholar]
| N2O-N (μg N m−2 min−1) | CH4-C (μg C m2 min−1) | |||
|---|---|---|---|---|
| Year | DWM (A) | Non-DWM (A) | DWM (A) | Non-DWM (A) |
| 2017 | 0.94 ± 0.38 a | 0.81 ± 0.38 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a |
| 2018 | 0.48 ± 0.40 b | 0.39 ± 0.39 b | 0.03 ± 0.01 a | 0.02 ± 0.01 a |
| 2019 | 1.20 ± 0.39 a | 1.02 ± 0.39 a | 0.04 ± 0.01 a | 0.03 ± 0.01 a |
| N2O-N (μg N m−2 min−1) | CH4-C (μg C m−2 min−1) | ||
|---|---|---|---|
| N Fertilization Period | Mean ± SE | Seasonal Period | Mean ± SE |
| Post-fertilization | 2.15 ± 0.38 a | Spring | 0.04 ± 0.001 a |
| Non-fertilization | 0.21 ± 0.38 b | Non-spring | 0.02 ± 0.001 b |
| N2O-N kg ha−1 yr−1 (Mean ± SE) | ||||||
|---|---|---|---|---|---|---|
| Seasonally-Based Interpolation | Mx Effects Model Mean Extrapolated | Regression-Based Interpolation | ||||
| Year | DWM | Non-DWM | DWM | Non-DWM | DWM | Non-DWM |
| 2017 | 6.4 ± 1.1 | 4.3 ± 0.8 | 4.9 ± 1.9 | 4.3 ± 1.9 | 3.9 ± 1.0 | 3.8 ± 0.7 |
| 2018 | 2.1 ± 0.3 | 1.2 ± 0.3 | 2.5 ± 2.1 | 2.0 ± 2.0 | 1.5 ± 0.2 | 1.8 ± 0.2 |
| 2019 | 7.7 ± 1.4 | 7.2 ± 3.5 | 6.3 ± 2.0 | 5.7 ± 2.0 | 5.7 ± 1.3 | 6.4 ± 2.3 |
| CH4-C kg ha−1 yr−1 (Mean ± SE) | ||||
|---|---|---|---|---|
| Mx Effects Model Mean Extrapolated | Seasonally-Based Interpolation | |||
| Year | DWM | Non-DWM | DWM | Non-DWM |
| 2017 | 0.16 ± 0.05 | 0.11 ± 0.05 | 0.18 ± 0.10 | 0.19 ± 0.06 |
| 2018 | 0.16 ± 0.05 | 0.11 ± 0.05 | 0.17 ± 0.15 | 0.15 ± 0.06 |
| 2019 | 0.21 ± 0.05 | 0.16 ± 0.05 | 0.56 ± 0.73 | 0.42 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagedorn, J.G.; Davidson, E.A.; Fisher, T.R.; Fox, R.J.; Zhu, Q.; Gustafson, A.B.; Koontz, E.; Castro, M.S.; Lewis, J. Effects of Drainage Water Management in a Corn–Soy Rotation on Soil N2O and CH4 Fluxes. Nitrogen 2022, 3, 128-148. https://doi.org/10.3390/nitrogen3010010
Hagedorn JG, Davidson EA, Fisher TR, Fox RJ, Zhu Q, Gustafson AB, Koontz E, Castro MS, Lewis J. Effects of Drainage Water Management in a Corn–Soy Rotation on Soil N2O and CH4 Fluxes. Nitrogen. 2022; 3(1):128-148. https://doi.org/10.3390/nitrogen3010010
Chicago/Turabian StyleHagedorn, Jacob G., Eric A. Davidson, Thomas R. Fisher, Rebecca J. Fox, Qiurui Zhu, Anne B. Gustafson, Erika Koontz, Mark S. Castro, and James Lewis. 2022. "Effects of Drainage Water Management in a Corn–Soy Rotation on Soil N2O and CH4 Fluxes" Nitrogen 3, no. 1: 128-148. https://doi.org/10.3390/nitrogen3010010
APA StyleHagedorn, J. G., Davidson, E. A., Fisher, T. R., Fox, R. J., Zhu, Q., Gustafson, A. B., Koontz, E., Castro, M. S., & Lewis, J. (2022). Effects of Drainage Water Management in a Corn–Soy Rotation on Soil N2O and CH4 Fluxes. Nitrogen, 3(1), 128-148. https://doi.org/10.3390/nitrogen3010010







