Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review
Abstract
1. Introduction
2. Determination of N Rate: Importance of EUF and N Min Methods
3. Nitrogen Fertilization Influence on Vegetative Growth
4. Sugar Beet N Fertilization Management
5. Nitrogen Use Efficiency (NUE) in Sugar Beet Production
6. Influence of Soil Type and N Fertilization on Sugar Beet Yield and Quality
7. Cercospora Leaf Spot and N Fertilizers
8. Leaf N Content
9. Sugar Beet Root Quality
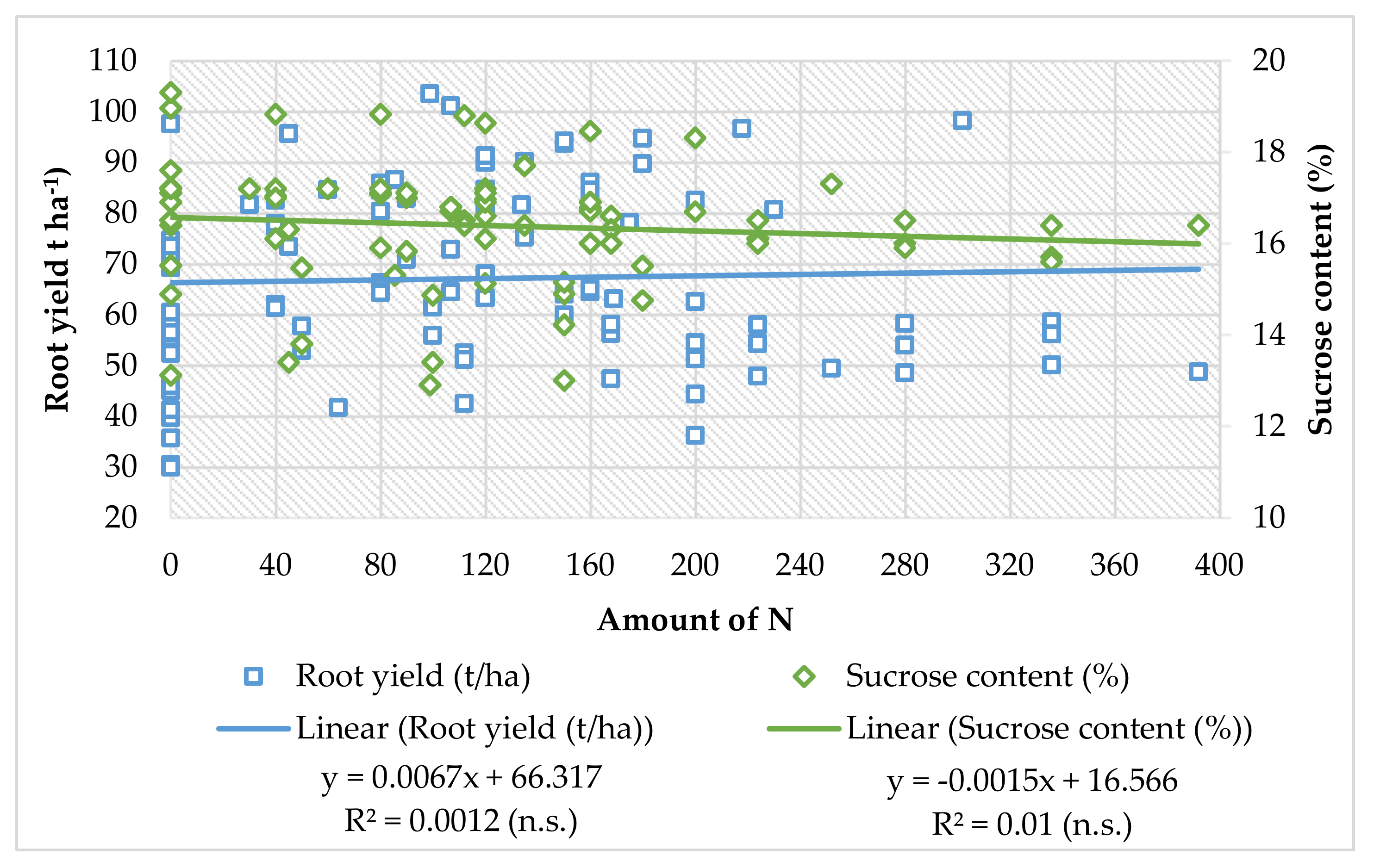
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bruhns, J.; Baron, O.; Maier, K. Sugar Economy Europe 2004; No. Ed. 50; Verlag Dr. Albert Bartens KG: Berlin, Germany, 2003. [Google Scholar]
- Jug, D.; Jug, I.; Brozović, B.; Vukadinović, V.; Stipešević, B.; Đurđević, B. The role of conservation agriculture in mitigation and adaptation to climate change. Poljoprivreda 2018, 24, 35–44. [Google Scholar] [CrossRef]
- Kristek, S.; Brkić, S.; Jović, J.; Stanković, A.; Ćupurdija, B.; Brica, M.; Karalić, K. The application of nitrogen-fixing bacteria in order to reduce the mineral nitrogen fertilizers in sugar beet. Poljoprivreda 2020, 26, 65–71. [Google Scholar] [CrossRef]
- Stošić, M.; Brozović, B.; Vinković, T.; Ravnjak, B.; Kluz, M.; Zebec, V. Soil resistance and bulk density under different tillage system. Poljoprivreda 2020, 26, 17–24. [Google Scholar] [CrossRef]
- Rašovský, M.; Pačuta, V.; Černý, I.; Ernst, D.; Michalska-Klimczak, B.; Wyszyňski, Z. Monitoring of Influence of Biopreparates, Weather Conditions and Variety on Production Parameters of Sugar Beet. Listy Cukrov. A Řepařské 2021, 137, 154. [Google Scholar]
- Varga, I.; Lončarić, Z.; Pospišil, M.; Rastija, M.; Antunović, M. Dynamics of sugar beet root, crown and leaves mass with regard to plant densities and spring nitrogen fertilization. Poljoprivreda 2020, 26, 32–39. [Google Scholar] [CrossRef]
- Jurišić, M.; Radočaj, D.; Plaščak, I.; Rapčan, I. A Comparison of Precise Fertilization Prescription Rates to a Conventional Approach Based on the Open Source GIS Software. Poljoprivreda 2021, 27, 52–59. [Google Scholar] [CrossRef]
- Antunović, M.; Varga, I.; Stipešević, B.; Ranogajec, L. Analýza chorvatského cukrovarnického sektoru a produkce cukrové řepy. Listy Cukrov. A Řepařské 2021, 137, 383–386. [Google Scholar]
- Ernst, D.; Černý, I.; Pačuta, V.; Zapletalová, A.; Rašovský, M.; Skopal, J.; Vician, T.; Šulík, R.; Gažo, J. Yield and Sugar Content of Sugar Beet Depending on Different Soil Tillage Technologies. Listy Cukrov. A Řepařské 2021, 137, 319–324. [Google Scholar]
- Turesson, H.; Andersson, M.; Marttila, S.; Thulin, I.; Hofvander, P. Starch biosynthetic genes and enzymes are expressed and active in the absence of starch accumulation in sugar beet tap-root. BMC Plant Biol. 2014, 14, 104. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Kenter, C. Yield potential of Sugar beet—Have we hit the ceiling? Front. Plant Sci. 2018, 9, 289. [Google Scholar] [CrossRef]
- Franzen, D.W. Delineating nitrogen management zones in a sugarbeet rotation using remote sensing—A review. J. Sugar Beet Res. 2004, 41, 47–60. [Google Scholar] [CrossRef]
- Draycott, A.P.; Christenson, D.R. Nutrients for Sugar Beet Production: Soil-Plant Relationships; CABI: Wallingford, UK, 2003. [Google Scholar]
- Jaćimović, G.; Marinković, B.; Crnobarac, J.; Bogdanović, D.; Kovačev, L.; Danojević, D. Influence of fertilization and nitrate-nitrogen position in soil profile on the sugar beet root yield and quality. J. Agric. Sci. 2008, 53, 83–90. [Google Scholar]
- Kristek, S.; Kristek, A.; Evačić, M. Influence of nitrogen fertilization on sugar beet root yield and quality. Cereal Res. Commun. 2008, 36, 371–374. [Google Scholar]
- Malnou, C.S.; Jaggard, K.W.; Sparkes, D.L. A canopy approach to nitrogen fertilizer recommendations for the sugar beet crop. Eur. J. Agron. 2006, 25, 254–263. [Google Scholar] [CrossRef]
- Pospišil, M. Ratarstvo II. Dio—Industrijsko Bilje; Zrinski: Čakovec, Croatia, 2013. [Google Scholar]
- Lundegårdh, H. Plant Physiology; Oliver and Boyd: Edinburgh, UK; London, UK; T. & A. Constable Ltd.: Edinburgh, UK, 1966. [Google Scholar]
- Hoffmann, C.M.; Kluge-Severin, S. Growth analysis of autumn and spring sown sugar beet. Eur. J. Agron. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Pevalek-Kozlina, B. (Ed.) Fiziologija Bilja; Profil International: Zagreb, Croatia, 2003. [Google Scholar]
- Klotz, K.L.; Finger, F.L. Impact of temperature, length of storage and postharvest disease on sucrose catabolism in sugarbeet. Postharvest Biol. Technol. 2004, 34, 1–9. [Google Scholar] [CrossRef]
- Tarkalson, D.D.; Bjorneberg, D.L.; Camp, S.; Dean, G.; Elison, D.; Foote, P. Improving nitrogen management in Pacific Northwest sugarbeet production. J. Sugar Beet Res. 2016, 53, 14–36. [Google Scholar] [CrossRef]
- Carter, J.N.; Traveller, D.J. Effect of time and amount of nitrogen uptake on sugarbeet growth and yield. Agron. J. 1981, 73, 665–671. [Google Scholar] [CrossRef]
- Mary, B.; Recous, S. Measurement of nitrogen mineralization and immobilization fluxes in soil as a means of predicting net mineralization. Eur. J. Agron. 1994, 3, 291–300. [Google Scholar] [CrossRef]
- Mengel, K. Turnover of organic nitrogen in soils and its availability to crops. Plant Soil 1996, 181, 83–93. [Google Scholar] [CrossRef]
- Nemeth, K. Recent advances in EUF research (1980–1983). Plant Soil 1985, 83, 1–19. [Google Scholar] [CrossRef]
- Nemeth, K.; Bartels, H.; Vogel, M.; Mengel, K. Organic nitrogen compounds extracted from arable and forest soils by electro-ultrafiltration and recovery rates of amino acids. Biol. Fertil. Soils 1988, 5, 271–275. [Google Scholar] [CrossRef]
- Natesan, S.; Ranganathan, V.; Nemeth, K.; Krishnan, V. EUF-analysis of tea soils of Southern India and tea productivity. Plant Soil 1985, 83, 191–198. [Google Scholar] [CrossRef]
- Akinrinde, E.A.; Obigbesan, G.O.; Gaiser, T. Electro-ultrafiltration (EUF) technique in relation to conventional methods of soil testing for the determination of available P, Ca, Mg and NO3-N in some tropical soils. J. Agron. 2006, 5, 375–381. [Google Scholar] [CrossRef][Green Version]
- Wiedeman, H. Soil analysis and N-fertilizer recommendations for growing sugar beet in southern Germany and Austria. In Proceedings of the International Institute for Beet Research 57th Winter Congress, Bruxelles, Belgium, 16–17 February 1994. [Google Scholar]
- Horn, D.; Fürstenfeld, F. Nitrogen fertilizer recommendation for sugar beet according to the EUF soil testing system. In Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Wiklicky, L. Application of the EUF procedure in sugar beet cultivation. Plant Soil 1982, 64, 115–127. [Google Scholar] [CrossRef]
- Scharpf, H.C.; Wehrmann, J. Bedeutung des Mineralstickstoffvorrates des Bodens zu Vegetationsbeginn fur die Bemessung der N Dungung zu Winterweizen. Landwirtschaftliche Forschung. Sonderheft 1976, 52, 109–126. [Google Scholar]
- Wehrmann, J.V.; Scharpf, H.C. Der Mineralstickstoffgehalt des Bodens als Maßstab für den Stickstoffdüngerbedarf (N min-Methode). Plant Soil 1979, 52, 109–126. [Google Scholar] [CrossRef]
- Vukadinović, V.; Lončarić, Z. Ishrana Bilja. Poljoprivredni Fakultet u Osijeku; Josip Juraj Strossmayer University of Osijek: Osijek, Croatia, 1997. [Google Scholar]
- Bertić, B.; Vukadinović, V. Filozofija Gnojidbe Sve Što Treba Znati o Gnojidbi; Studio HS Internet d.o.o. Osijek: Osijek, Croatia, 2013. [Google Scholar]
- Jaradat, A.A.; Rinke, J. Modeling sugar content of farmer-managed sugar beets (Beta vulgaris L.). Commun. Biometry Crop Sci. 2012, 7, 23–34. [Google Scholar]
- Hoffmann, C.M.; Kluge-Severin, S. Light absorption and radiation use efficiency of autumn and spring sown sugar beets. Field Crops Res. 2010, 119, 238–244. [Google Scholar] [CrossRef]
- Kristek, A.; Halter, J. Djelovanje vegetacijskog prostora na porast lišća šećerne repe i prinos korijena. Agron. Glas. 1988, 2–3, 79–94. [Google Scholar]
- Stanaćev, S. Šećerna Repa; Nolit: Beograd, Serbia, 1979. [Google Scholar]
- Kristek, A.; Liović, I. Ritam rasta šećerne repe u uvjetima 1987. godine. Poljopr. Aktualnosti 1988, 30, 173–185. [Google Scholar]
- Jelić, S.; Antunović, M.; Bukvić, G.; Varga, I.; Iljkić, D. Impact of plant density on growth, yield and quality of sugar beet. Listy Cukrov. A Řepařské 2019, 135, 107. [Google Scholar]
- Manderscheid, R.; Pacholski, A.; Weigel, H.J. Effect of free air carbon dioxide enrichment combined with two nitrogen levels on growth, yield and yield quality of sugar beet: Evidence for a sink limitation of beet growth under elevated CO2. Eur. J. Agron. 2010, 32, 228–239. [Google Scholar] [CrossRef]
- Lüdecke, H. Šećerna Repa; Poljoprivredni Nakladni Zavod Zagreb: Zagreb, Croatia, 1956. [Google Scholar]
- Kristek, A.; Kristek, S.; Varga, I.; Drmić, Z. Rezultati u proizvodnji šećerne repe u zavisnosti od izbora hibrida i broja tretiranja fungicida. Poljoprivreda 2015, 21, 15–22. [Google Scholar] [CrossRef]
- Vukadinović, V.; Jug, I.; Đurđević, B. Ekofiziologija Bilja. In Poljoprivredni Fakultet u Osijeku; Sveučilište Josipa Jurja Strossmayera u Osijeku Zebra: Vinkovci, Croatia, 2014. [Google Scholar]
- Müller-Linow, M.; Pinto-Espinosa, F.; Scharr, H.; Rascher, U. The leaf angle distribution of natural plant populations: Assessing the canopy with a novel software tool. Plant Methods 2015, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Kenter, C.; Hoffman, C.M. Seasonal patterns of sucrose concentration in relation to other quality parameters of sugar beet (Beta vulgaris L.). J. Sci. Food Agric. 2006, 86, 62–70. [Google Scholar] [CrossRef]
- Drachovská, M.; Sandera, K. Fysiologie Cukrovky; Nakladatelství Československé Akademie věd: Prague, Czech Republic, 1959. [Google Scholar]
- Märländer, B.; Hoffmann, C.M.; Koch, H.J.; Ladewig, E.; Merkes, R.; Petersen, J.; Stockfisch, N. Environmental Situation and Yield Performance of the Sugar Beet Crop in Germany: Heading for Sustainable Development. J. Agron. Crop Sci. 2003, 189, 201–226. [Google Scholar] [CrossRef]
- Tóth, M.; Pokrivčák, J.; Smutka, L.; Dvořák, M.; Pulkrábek, J. Economic aspects of sugar beet production and biodiversity: Effects of ban on neonicotinoids use. Listy Cukrov. Řepařské 2022, 138, 116–120. [Google Scholar]
- Pospišil, A.; Pospišil, M. The Effect of Organic Fertilizers on the Spelt Yield and the Yield of its Components. Poljoprivreda 2021, 27, 37–43. [Google Scholar] [CrossRef]
- Tan, Z.X.; Lal, R.; Wiebe, K.D. Global soil nutrient depletion and yield reduction. J. Sustain. Agric. 2005, 26, 123–146. [Google Scholar] [CrossRef]
- Pan, B.; Lam, S.K.; Mosier, A.; Luo, Y.; Chen, D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agriculture. Ecosyst. Environ. 2016, 232, 283–289. [Google Scholar] [CrossRef]
- Cucina, M.; De Nisi, P.; Sordi, S.; Adani, F. Sewage Sludge as N-Fertilizers for Crop Production Enabling the Circular Bioeconomy in Agriculture: A Challenge for the New EU Regulation 1009/2019. Sustainability 2021, 13, 13165. [Google Scholar] [CrossRef]
- Lukas, V.; Neudert, L.; Širůček, P.; Novák, J.; Elbl, J. Effect of variable rate application of phosphorus and potassium fertilizers in sugar beet. Listy Cukrov. A Řepařské 2021, 137, 417–422. [Google Scholar]
- de Souza Braz, A.M.; da Costa, M.L.; Ramos, S.J.; Dall’Agnol, R.; Fernandes, A.R. Long Term Application of Fertilizers in Eastern Amazon and Effect on Uranium and Thorium Levels in Soils. Minerals 2021, 11, 994. [Google Scholar] [CrossRef]
- Issukindarsyah, I.; Sulistyaningsih, E.; Indradewa, D.; Putra, E.T.S. The Effect of Ammonium Nitrate Ratio and Support Types on the NPK Uptake and Growth of Black Pepper (Piper nigrum L.) in Field Conditions. Poljoprivreda 2021, 27, 25–33. [Google Scholar] [CrossRef]
- Varga, I.; Kerovec, D.; Engler, M.; Popović, B.; Lončarić, Z.; Iljkić, D.; Antunović, M. Determination N-NO3− in sugar beet leaves). Listy Cukrov. A Řepařské 2022, 138, 69–72. [Google Scholar]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Harrison, R.B.; Footen, P.W.; Strahm, B.D. Deep soil horizons: Contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. For. Sci. 2011, 57, 67–76. [Google Scholar]
- Hoffmann, C.M.; Blomberg, M. Estimation of leaf area index of Beta vulgaris L. based on optical remote sensing data. J. Agron. Crop Sci. 2004, 190, 197–204. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Maslaris, N. Leaf allometry and prediction of specific leaf area (SLA) in sugar beet (Beta vulgaris L.) cultivar. Photosynthetica 2008, 46, 351–355. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Saulioti, E.; Maslaris, N.; Papakosta, D. Genotypic response to regrowth of defoliated sugar beets after re-watering in a water-limited environment: Effects on yield and quality. Int. J. Plant Prod. 2009, 3, 1–18. [Google Scholar]
- Putnik-Delić, M. Fiziološki i Molekularni Aspekti Tolerantnosti Šećerne Repe Prema Suši. Doctoral Dissertation, University of Novi Sad, Novi Sad, Serbia, 2013. [Google Scholar]
- Kosterj, A.; Repka, J. Quantitative indicators of growth, production process and yield-formation of sugar-beet. Rostl. Vyrob. 1993, 39, 1077–1086. [Google Scholar]
- Tsialtas, J.T.; Maslaris, N. Leaf physiological traits and its relation with sugar beet cultivar success in two contrasting environments. Int. J. Plant Prod. 2012, 6, 15–36. [Google Scholar]
- Kristek, A.; Kristek, S.; Antunović, M.; Varga, I.; Katušić, J.; Besek, Z. Utjecaj tipa tla i gnojidbe dušikom na prinos i kvalitetu korijena šećerne repe. Poljoprivreda 2011, 17, 16–22. [Google Scholar]
- Brentrup, F.; Küsters, J.; Kuhlmann, H.; Lammel, J. Application of the Life Cycle Assessment methodology to agricultural production: An example of sugar beet production with different forms of nitrogen fertilizers. Eur. J. Agron. 2001, 14, 221–233. [Google Scholar] [CrossRef]
- Draycott, A.P. Sugar Beet; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Last, P.J.; Draycott, A.P.; Messem, A.B.; Webb, D.J. Effects of nitrogen fertilizer and irrigation on sugar beet at Broom’s Barn 1973–8. J. Agric. Sci. 1983, 101, 185–205. [Google Scholar] [CrossRef]
- Starke, P.; Hoffmann, C.M. Yield Parameters of Beta Beets as a Basis to Estimate the Biogas Yield; Nutzung von Zuckerrüben für die Biogaserzeugung–Definition der Qualität sowie ertragsrelevante Parameter von Rübe, Blatt und Schossern; Institut für Zuckerrübenforschung: Gottingen, Germany, 2014; Volume 42. [Google Scholar]
- Starke, P.; Hoffmann, C.M. Yield parameters of Beta beets as a basis to estimate the biogas yield. Sugar Ind. 2014, 139, 169–176. [Google Scholar] [CrossRef]
- Monreal, J.A.; Jiménez, E.T.; Remesal, E.; Morillo-Velarde, R.; García-Mauriñoa, S.; Echevarría, C. Proline content of sugar beet storage roots: Response to water deficit and nitrogen fertilization at field conditions. Environ. Exp. Bot. 2007, 60, 257–267. [Google Scholar] [CrossRef]
- Vielemeyer, H.-P.; Lux, H.; Weege, K.-H. Einfluß des zeitlichen N-Angebots auf den Ertragsbildungsprozeß der Zuckerrübe. Arch. Acker-Pflanzenbau Bodenkd. Berl. 1986, 30, 131–137. [Google Scholar]
- Yadav, M.R.; Kumar, R.; Parihar, C.M.; Yadav, R.K.; Jat, S.L.; Ram, H.; Meena, R.K.; Singh, M.; Birbal; Verma, A.P.; et al. Strategies for improving nitrogen use efficiency: A review. Agric. Rev. 2017, 38, 29–40. [Google Scholar] [CrossRef]
- Melino, V.J.; Tester, M.A.; Okamoto, M. Strategies for engineering improved nitrogen use efficiency in crop plants via redistribution and recycling of organic nitrogen. Curr. Opin. Biotechnol. 2022, 73, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, P.; Chiodi, C.; Broccanello, C.; Concheri, G.; Biancardi, E.; Pavli, O.; Skaracis, G. Sustainability of the sugar beet crop. Sugar Tech 2019, 21, 703–716. [Google Scholar] [CrossRef]
- Atlason, R.S.; Lehtinen, T.; Davíðsdóttir, B.; Gísladóttir, G.; Brocza, F.; Unnþórsson, R.; Ragnarsdóttir, K.V. Energy return on investment of Austrian sugar beet: A small-scale comparison between organic and conventional production. Biomass Bioenergy 2015, 75, 267–271. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Changes in N composition of sugar beet varieties in response to increasing N supply. J. Agron. Crop Sci. 2005, 191, 138–145. [Google Scholar] [CrossRef]
- Laufer, D.; Nielsen, O.; Wilting, P.; Koch, H.J.; Märländer, B. Yield and nitrogen use efficiency of fodder and sugar beet (Beta vulgaris L.) in contrasting environments of northwestern Europe. Eur. J. Agron. 2016, 73, 124–132. [Google Scholar] [CrossRef]
- Monteiro, F.; Frese, L.; Castro, S.; Duarte, M.C.; Paulo, O.S.; Loureiro, J.; Romeiras, M.M. Genetic and genomic tools to asssist sugar beet improvement: The value of the crop wild relatives. Front. Plant Sci. 2018, 9, 74. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Feć, M.; Łukowiak, R.; Szczepaniak, W. Row method of sugar beet (Beta vulgaris L.) fertilization with multicomponent fertilizer based on urea-ammonium nitrate solution as a way to increase nitrogen efficiency. J. Cent. Eur. Agric. 2010, 11, 225–234. [Google Scholar]
- Hergert, G.W. Sugar beet fertilization. Sugar Tech 2010, 12, 256–266. [Google Scholar] [CrossRef]
- Barłóg, P.; Nowacka, A.; Błaszyk, R. Effect of zinc band application on sugar beet yield, quality and nutrient uptake. Plant Soil Environ. 2016, 62, 30–35. [Google Scholar] [CrossRef]
- Bronson, K.F.; Scharf, P.C.; Kitchen, N.R. Use of GIS-Based Site-Specific Nitrogen Management for Improving Energy Efficiency; USDA-ARS; UNL Faculty: Lincoln, NE, USA, 2011; Volume 677, pp. 359–384.
- Clay, D.E.; Shanahan, J.F. GIS Applications in Agriculture, Volume Two: Nutrient Management for Energy Efficiency; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Last, P.J.; Draycott, A.P. Growth and yield of sugar beet on contrasting soils in relation to nitrogen supply: II. Growth, uptake and leaching of nitrogen. J. Agric. Sci. 1975, 85, 27–37. [Google Scholar] [CrossRef]
- Pospišil, M.; Pospišil, A.; Rastija, M. Effect of plant density and nitrogen rates upon the leaf area of seed sugar beet on seed yield and quality. Eur. J. Agron. 2000, 12, 69–78. [Google Scholar] [CrossRef]
- Marinković, B.; Crnobarac, J.; Jaćimović, G.; Rajić, M.; Latković, D.; Aćin, V. Sugar yield and technological quality of sugar beet at different levels of nitrogen fertilization. Res. J. Agric. Sci. 2010, 42, 162–167. [Google Scholar]
- Hoffmann, C.M.; Märländer, B. Composition of harmful nitrogen in sugar beet (Beta vulgaris L.)—Amino acids, betaine, nitrate—As affected by genotype and environment. Eur. J. Agron. 2005, 22, 255–265. [Google Scholar] [CrossRef]
- Malnou, C.S.; Jaggard, K.W.; Sparkes, D.L. Nitrogen fertilizer and the efficiency of the sugar beet crop in late summer. Eur. J. Agron. 2008, 28, 47–56. [Google Scholar] [CrossRef]
- Marinković, B.J.; Crnobarac, J.Ž. Dependence of sugarbeet quality and yield on the application of NPK nutrients. Acta Period. Technol. 2000, 31, 345–350. [Google Scholar]
- Bolton, M.D.; Birla, K.; Rivera-Varas, V.; Rudolph, K.D.; Secor, G.A. Characterization of CbCyp51 from field isolates of Cercospora beticola. Phytopathology 2012, 102, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kristek, S.; Jović, J.; Zmaić, K.; Kraljičak, Ž.; Kišpal, H.; Bešlo, D.; Horvat, D.; Stjepanović, B.; Rašić, B. Problem of Development of Resistance to Some Fungicide Active Sub stances Intended for Suppressing of Cercospora beticola Sacc. Listy Cukrov. A Řepařské 2017, 133, 222–226. [Google Scholar]
- Vogel, J.; Kenter, C.; Holst, C.; Märländer, B. New generation of resistant sugar beet varieties for advanced integrated management of Cercospora leaf spot in central Europe. Front. Plant Sci. 2018, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Ereš, H.; Dujković, A.; Vrandečić, K. Cercospora leaf spot. Glas. Zaštite Bilja 2021, 44, 52–54. [Google Scholar] [CrossRef]
- Byford, W.J. Ramularia beticola in sugar-beet seed crops in England. J. Agric. Sci. 1975, 85, 369–375. [Google Scholar] [CrossRef]
- Wieczorek, T.M.; Jørgensen, L.N.; Hansen, A.L.; Munk, L.; Justesen, A.F. Early detection of sugar beet pathogen Ramularia beticola in leaf and air samples using qPCR. Eur. J. Plant Pathol. 2014, 138, 775–785. [Google Scholar] [CrossRef][Green Version]
- Mcfarlane, J.S.; Bardin, R.; Snyder, W.C. An Alternaria leaf spot of the sugar beet. Proc. Am. Soc. Sugarbeet Technol. 1954, 8, 241–245. [Google Scholar]
- Rosenzweig, N.; Hanson, L.E.; Mambetova, S.; Jiang, Q.W.; Guza, C.; Stewart, J.; Somohano, P. Fungicide sensitivity monitoring of Alternaria spp. causing leaf spot of sugarbeet (Beta vulgaris) in the Upper Great Lakes. Plant Dis. 2019, 103, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.R.; Haque, M.E.; Bloomquist, M.; Bhuiyan, M.Z.R.; Brueggeman, R.; Zhong, S.; Sharma Poudel, R.; Gross, T.; Hakk, P.; Leng, Y.; et al. First Report of Alternaria Leaf Spot Caused by Alternaria tenuissima on Sugar Beet (Beta vulgaris) in Minnesota, USA. Plant Dis. 2020, 104, 580. [Google Scholar] [CrossRef]
- Koenick, L.B.; Vaghefi, N.; Knight, N.L.; du Toit, L.J.; Pethybridge, S.J. Genetic diversity and differentiation in Phoma betae populations on table beet in New York and Washington States. Plant Dis. 2019, 103, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Chand, N.; Jones, E.E.; Casonato, S. Pathogenicity of Phoma betae isolates from red beet (Beta vulgaris) at seed farms in Canterbury, New Zealand. Plant Prot. 2019, 72, 21–26. [Google Scholar] [CrossRef][Green Version]
- Agarwal, P.C.; Dev, U.; Rani, I.; Khetarpal, R.K. Seed-borne fungi detected in sugar beet seeds imported into India during last three decades. Plant Health Prog. 2006, 7, 2. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Barto, E.K.; Menexes, G.; Rillig, M.C. Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathol. 2013, 62, 961–969. [Google Scholar] [CrossRef]
- Huber, D.M.; Haneklaus, S. Managing nutrition to control plant disease. Landbauforsch. Volkenrode 2007, 57, 313. [Google Scholar]
- Long, D.H.; Lee, F.N.; TeBeest, D.O. Effect of nitrogen fertilization on disease progress of rice blast on susceptible and resistant cultivars. Plant Dis. 2000, 84, 403–409. [Google Scholar] [CrossRef]
- Liu, X.; Lyu, S.; Sun, D.; Bradshaw, C.J.; Zhou, S. Species decline under nitrogen fertilization increases community-level competence of fungal diseases. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162621. [Google Scholar] [CrossRef] [PubMed]
- Skaracis, G.N.; Pavli, O.I.; Biancardi, E. Cercospora leaf spot disease of sugar beet. Sugar Tech 2010, 12, 220–228. [Google Scholar] [CrossRef]
- Schmittgen, S. Effects of Cercospora Leaf Spot Disease on Sugar Beet Geno-Types with Contrasting Disease Susceptibility. Ph.D. Thesis, Universitäts-und Landesbibliothek der Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany, 2015. [Google Scholar]
- Vereijssen, J. Cercospora Leaf Spot in Sugar Beet. Epidemiology, Life Cycle Components and Disease Management. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2004. [Google Scholar]
- Vereijssen, J.; Schneider, J.H.; Termorshuizen, A.J. Root infection of sugar beet by Cercospora beticola in a climate chamber and in the field. Eur. J. Plant Pathol. 2005, 112, 201–210. [Google Scholar] [CrossRef]
- Vukčević, R. Nova primena aviometode na zaštiti šećerne repe od cerkospore. Agron. Glas. 1964, 14, 245–256. [Google Scholar]
- Westerveld, S.M.; McKeown, A.W.; McDonald, M.R. Relationship between nitrogen fertilization and Cercospora leaf spot and Alternaria leaf blight of carrot. HortScience 2008, 43, 1522–1527. [Google Scholar] [CrossRef]
- Makheti Mutebi, C.; Atieno Ondede, D. Effect of nitrogen nutrition on the intensity of Cercospora leaf spot of Mulberry. Int. J. Hortic. Sci. Technol. 2021, 8, 335–342. [Google Scholar]
- Bergmann, W. Nutritional Disorders of Plants: Visual and Analytical Diagnosis; Jena Gustav Fischer Verlag: New York, NY, USA, 1992. [Google Scholar]
- Mäck, G.; Hoffmann, C.M.; Märländer, B. Nitrogen compounds in organs of two sugar beet genotypes (Beta vulgaris L.) during the season. Field Crops Res. 2007, 102, 210–218. [Google Scholar] [CrossRef]
- Bilir, B.; Saltalı, K. The Effect of Nitrogen-Boron Application and Time on the Nitrate Content of Sugar Beet Leaves Used as Animal Feed. Turk. J. Agric. Food Sci. Technol. 2021, 9, 395–400. [Google Scholar]
- Grzebisz, W.; Przygocka-Cyna, K.; Łukowiak, R.; Biber, M. An evaluation of macronutrient nutritional status of sugar beets in critical stages of growth in response to foliar application of multi-micronutrient fertilizers. J. Elem. 2010, 15, 493–507. [Google Scholar] [CrossRef][Green Version]
- Pi, Z.; Stevanato, P.; Sun, F.; Yang, Y.; Sun, X.; Zhao, H.; Geng, G.; Yu, L. Proteomic changes induced by potassium deficiency and potassium substitution by sodium in sugar beet. J. Plant Res. 2016, 129, 527–538. [Google Scholar] [CrossRef]
- Hampe, T.; Marschner, H. Effect of sodium on morphology, water relations and net photosynthesis of sugar beet leaves. Z. Pflanzenphysiol. 1982, 108, 151–162. [Google Scholar] [CrossRef]
- Marinković, B.; Crnobarac, J. Zavisnost kvaliteta i prinosa šećerne repe od primene NPK hraniva. Acta Period. Technol. 2000, 31, 345–350. [Google Scholar]
- Milić, S.; Pejić, B.; Maksimović, L. Uticaj navodnjavanja i đubrenja na sadržaj šećera i prinos korena šećerne repe. J. Sci. Agric. Res. 2006, 67, 5–12. [Google Scholar]
- Hergert, G.W.; Nielsen, R.A. Comparison of strip tillage versus broadcast N application for sugar beets. In Proceedings of the 2009 Biennial Meeting, Denver, CO, USA, 2 April 2009. [Google Scholar]
- Barłóg, P.; Grzebisz, W.; Peplinski, K.; Szczepaniak, W. Sugar beet response to balanced nitrogen fertilization with phosphorus and potassium. Part II. Dynamics of beet quality. Bulg. J. Agric. Sci 2013, 19, 1311–1318. [Google Scholar]
- Abdelaal, K.A.; Sahar, F.T. Response of sugar beet plant (Beta vulgaris L.) to mineral nitrogen fertilization and bio-fertilizers. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 677–688. [Google Scholar]
- Pulkrábek, J.; Brinar, J.; Javor, T.; Dvořák, P.; Bečková, L.; Kuchtová, P.; Hubáčková, J. Experience with variable fertilization of sugar beet. Listy Cukrov. A Reparske 2021, 137, 184–193. [Google Scholar]
- Leilah, A.A.; Khan, N. Interactive Effects of Gibberellic Acid and Nitrogen Fertilization on the Growth, Yield, and Quality of Sugar Beet. Agronomy 2021, 11, 137. [Google Scholar] [CrossRef]
- Pogłodziński, R.; Barłóg, P.; Grzbisz, W. Effect of nitrogen and magnesium sulfate application on sugar beet yield and quality. Plant Soil Environ. 2021, 67, 507–513. [Google Scholar] [CrossRef]
- Varga, I.; Lončarić, Z.; Pospišil, M.; Rastija, M.; Antunović, M. Changes of Nitrate Nitrogen in Sugar Beet Petioles Fresh Tissue during Season with Regard to Nitrogen Fertilization and Plant Population. Listy Cukrov. A Řepařské 2020, 136, 198–204. [Google Scholar]
- Last, P.J.; Tinker, P.B.H. Nitrate nitrogen in leaves and petioles of sugar beet in relation to yield of sugar and juice purity. J. Agric. Sci. 1968, 71, 383–392. [Google Scholar] [CrossRef]
- Steinke, K.; Bauer, C. Enhanced Efficiency Fertilizer Effects in Michigan Sugarbeet Production. J. Sugar Beet Res. 2017, 54, 2–19. [Google Scholar] [CrossRef]
- Varga, I.; Lončarić, Z.; Kristek, S.; Kulundžić, A.M.; Rebekić, A.; Antunović, M. Sugar Beet Root Yield and Quality with Leaf Seasonal Dynamics in Relation to Planting Densities and Nitrogen Fertilization. Agriculture 2021, 11, 407. [Google Scholar] [CrossRef]
- Idris, M.; Baha, E.; Wael, A.M.; Abubaker Haroun, M.A. Effect of Nitrogen Fertilizer and Plant Spacing on Vegetative Growth of Sugar Beet (Beta vulgaris). J. Agron. Res. 2021, 4, 6–13. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Szczepaniak, W.; Peplinski, K. Sugar beet response to balanced nitrogen fertilization with phosphorus and potassium. Part II. Dynamics of beet quality. Bulg. J. Agric. Sci. 2014, 20, 1326–1333. [Google Scholar]
- Lentz, R.D.; Lehrsch, G.A. Nitrogen availability and uptake by sugarbeet in years following a manure application. Int. J. Agron. 2012, 2012, 120429. [Google Scholar] [CrossRef]
- Lauer, J.G. Plant density and nitrogen rate effects on sugar beet yield and quality early in harvest. Agron. J. 1995, 87, 586–591. [Google Scholar] [CrossRef]
| Country | Nitrogen Amount | Root Yield (t ha−1) | Sucrose Content (%) | Reference |
|---|---|---|---|---|
| Serbia | 50 | 57.7 | 13.8 | Marinković and Crnobarac [123] 1 |
| 100 | 55.9 | 13.4 | ||
| 150 | 59.9 | 13.0 | ||
| Serbia | 90 | 70.8 | 17.0 | Milić et al. [124] 2 |
| 120 | 82.9 | 16.6 | ||
| 150 | 93.7 | 14.9 | ||
| 180 | 94.7 | 15.5 | ||
| United Kingdom | 0 | 67.7 | 16.8 | Malnou et al. [16] 3 |
| 40 | 72.2 | 16.8 | ||
| 80 | 76.8 | 16.7 | ||
| 120 | 78.2 | 16.7 | ||
| 160 | 78.6 | 16.5 | ||
| Nebraska (SAD) | 0 | 56.2 | 17.2 | Hergert and Nielsen [125] 4 |
| 39 | 61.3 | 16.8 | ||
| 79 | 64.4 | 16.6 | ||
| 118 | 64.8 | 16.1 | ||
| 157 | 63.3 | 16.2 | ||
| 196 | 64.3 | 16.1 | ||
| 235 | 61.8 | 14.5 | ||
| Poland | 75 | 57.4 | 16.6 | Barłóg et al. [126] 5 |
| 125 | 54.7 | 16.8 | ||
| Republic of Croatia | 46 | 61.4 | 15.8 | Kristek et al. [68] 6 |
| 60 | 62.8 | 15.8 | ||
| 73 | 61.2 | 15.6 | ||
| 87 | 65.2 | 15.6 | ||
| 100 | 63.3 | 15.4 | ||
| Egypt | 0 | 29.5 | 16.3 | Abdelaal and Sahar [127] 7 |
| 35 | 40.8 | 16.6 | ||
| 70 | 61.4 | 16.9 | ||
| 105 | 63.4 | 17.1 | ||
| Czech Republic | 0 | 45.3 | 14.3 | Pulkrábek et al. [128] 8 |
| 70 | 50.7 | 14.6 | ||
| 130 | 53.3 | 14.2 | ||
| Egypt | 165 | 59.9 | 20.4 | Leilah and Khan [129] 9 |
| 220 | 67.2 | 18.9 | ||
| 275 | 74.3 | 18.0 | ||
| Poland | 0 | 65.2 | 18.1 | Pogłodziński et al. [130] 10 |
| 40 | 72.3 | 17.9 | ||
| 80 | 75.4 | 18.0 | ||
| 120 | 76.4 | 17.8 | ||
| 160 | 73.3 | 17.6 | ||
| 200 | 72.5 | 17.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, I.; Jović, J.; Rastija, M.; Markulj Kulundžić, A.; Zebec, V.; Lončarić, Z.; Iljkić, D.; Antunović, M. Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review. Nitrogen 2022, 3, 170-185. https://doi.org/10.3390/nitrogen3020013
Varga I, Jović J, Rastija M, Markulj Kulundžić A, Zebec V, Lončarić Z, Iljkić D, Antunović M. Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review. Nitrogen. 2022; 3(2):170-185. https://doi.org/10.3390/nitrogen3020013
Chicago/Turabian StyleVarga, Ivana, Jurica Jović, Mirta Rastija, Antonela Markulj Kulundžić, Vladimir Zebec, Zdenko Lončarić, Dario Iljkić, and Manda Antunović. 2022. "Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review" Nitrogen 3, no. 2: 170-185. https://doi.org/10.3390/nitrogen3020013
APA StyleVarga, I., Jović, J., Rastija, M., Markulj Kulundžić, A., Zebec, V., Lončarić, Z., Iljkić, D., & Antunović, M. (2022). Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review. Nitrogen, 3(2), 170-185. https://doi.org/10.3390/nitrogen3020013








