Short-Term Effect of Nitrogen Fertilization on Carbon Mineralization during Corn Residue Decomposition in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Studied
2.2. Residues
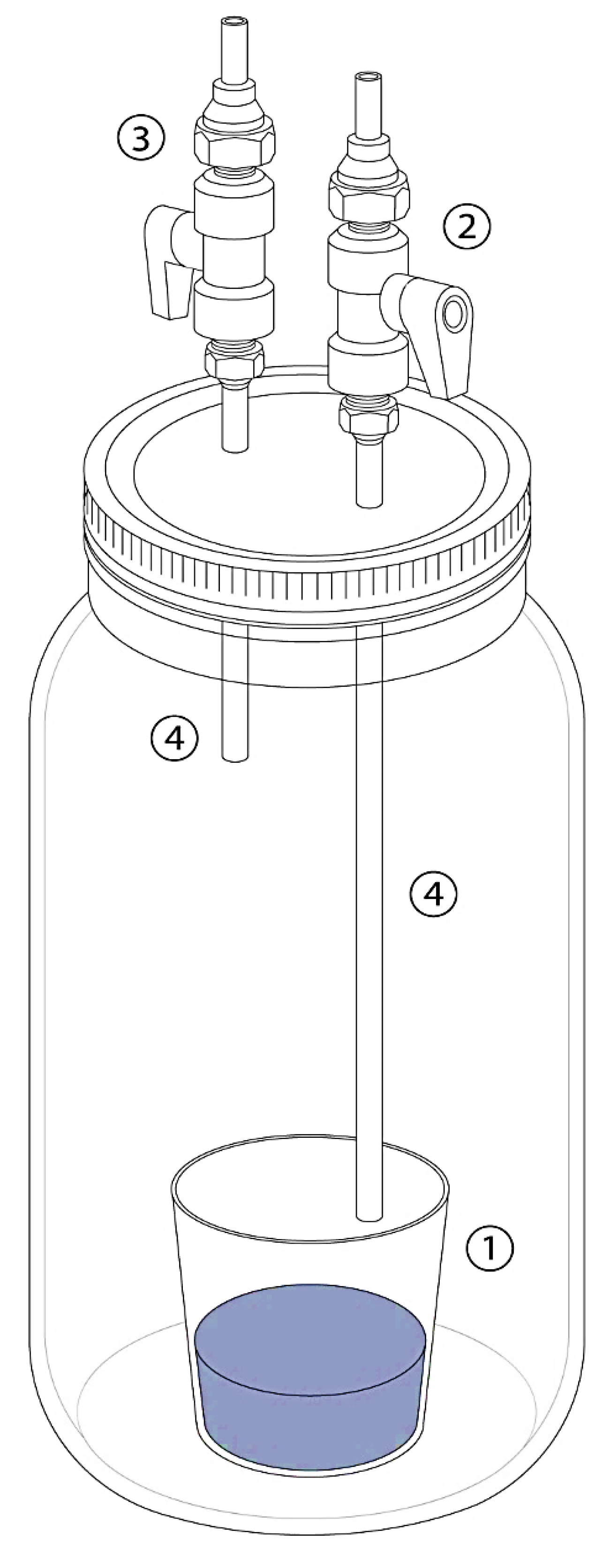
2.3. Incubation Procedure
2.4. Atmospheric Analyses
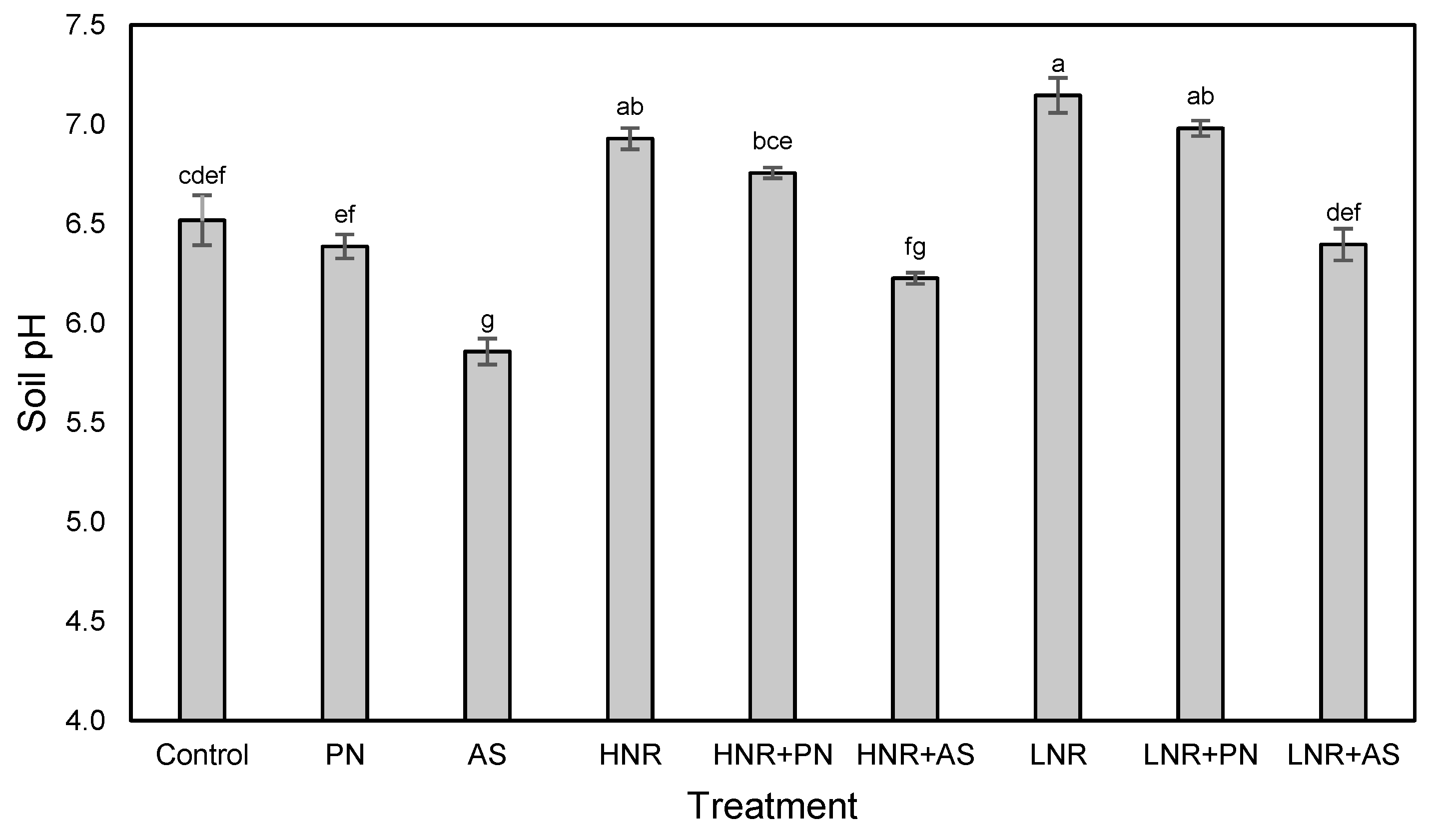
2.5. Soil pH
2.6. Microbial Biomass Parameters
2.7. Enzyme Activities
2.8. Gross N Mineralization and Immobilization
2.9. Statistical Analyses
3. Results and Discussion
3.1. Residue Quality
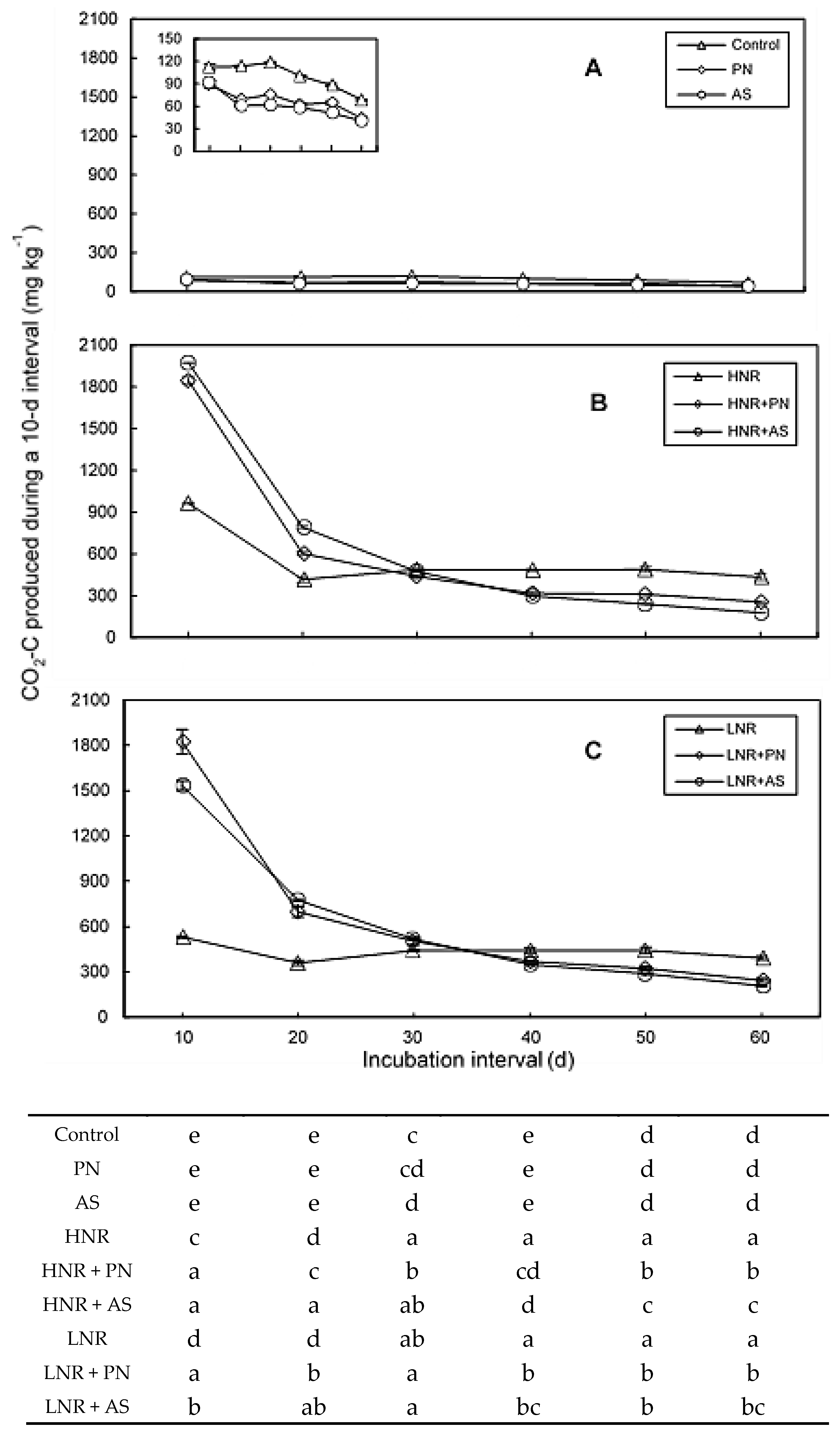
3.2. C Mineralization
3.3. Microbial Parameters
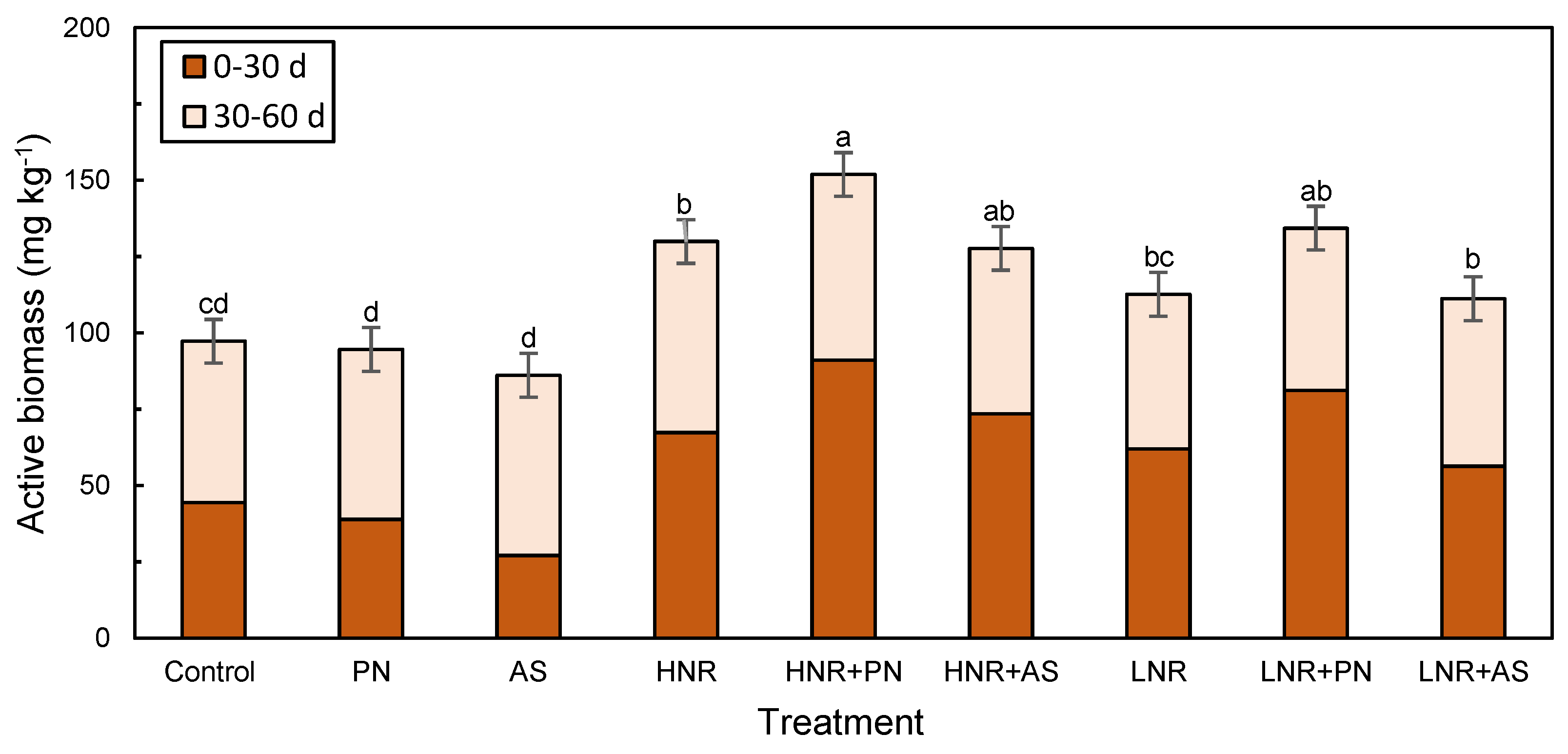
3.3.1. Active Biomass
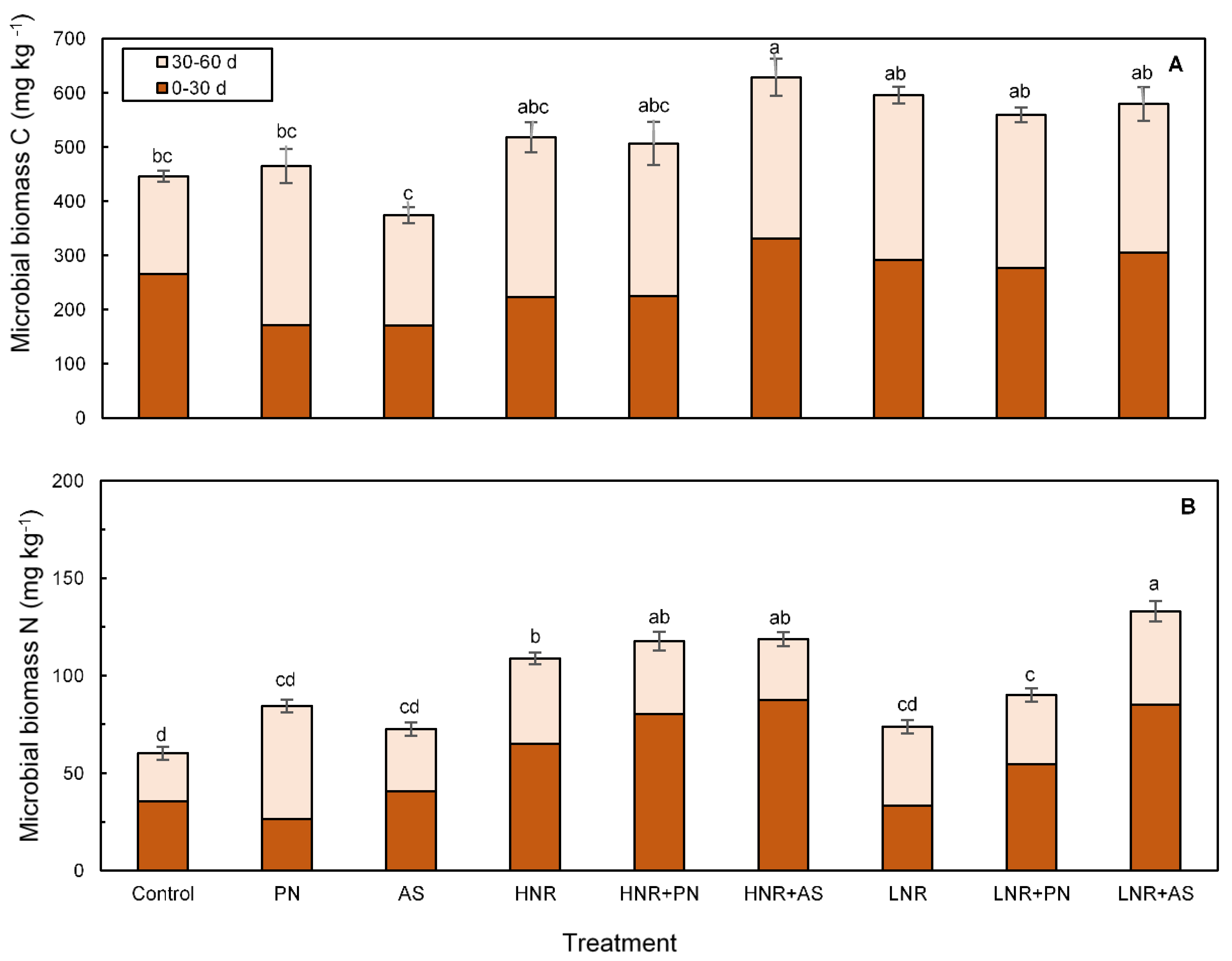
3.3.2. Microbial Biomass C and N
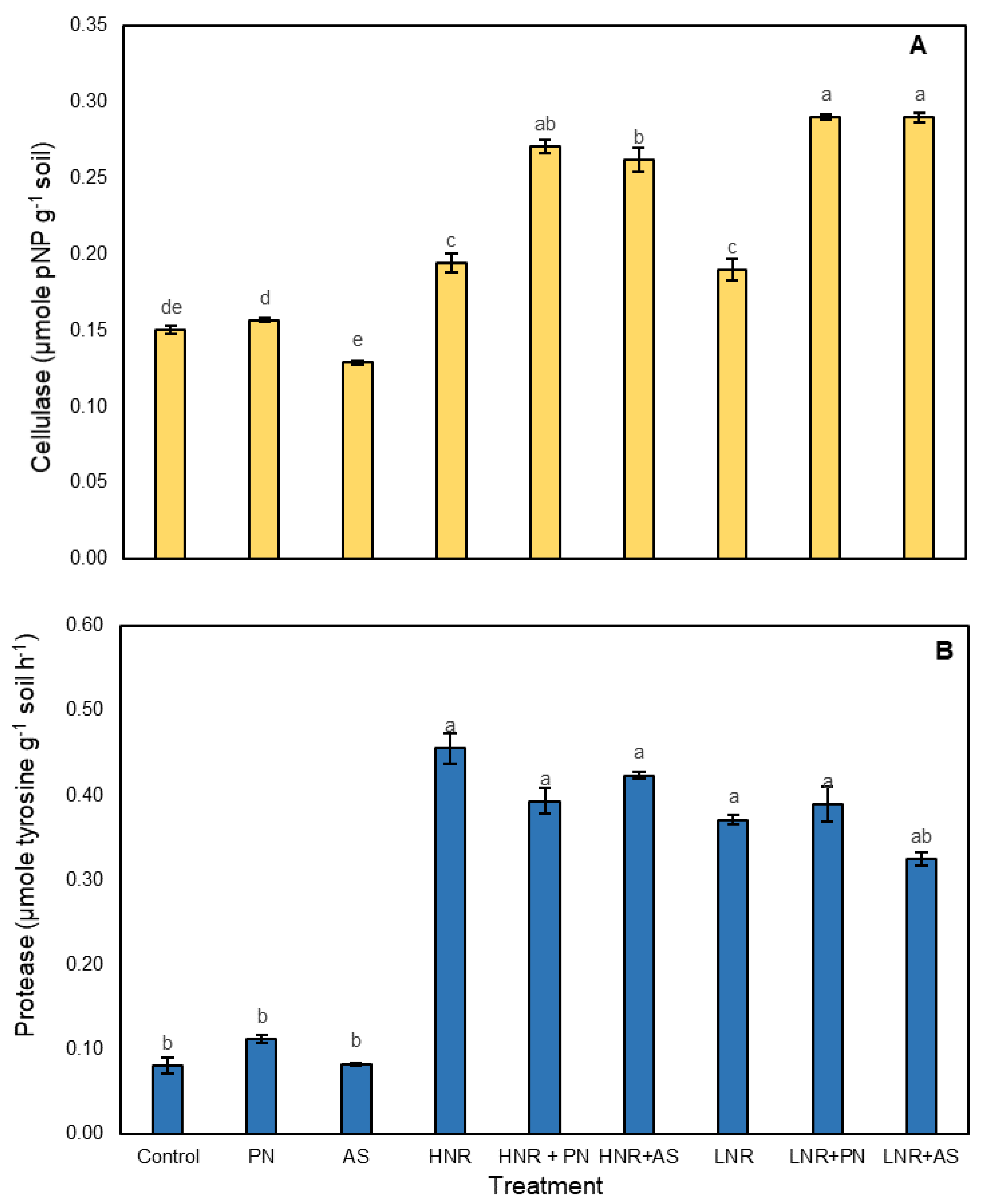
3.3.3. Enzymes Involved in C and N Mineralization
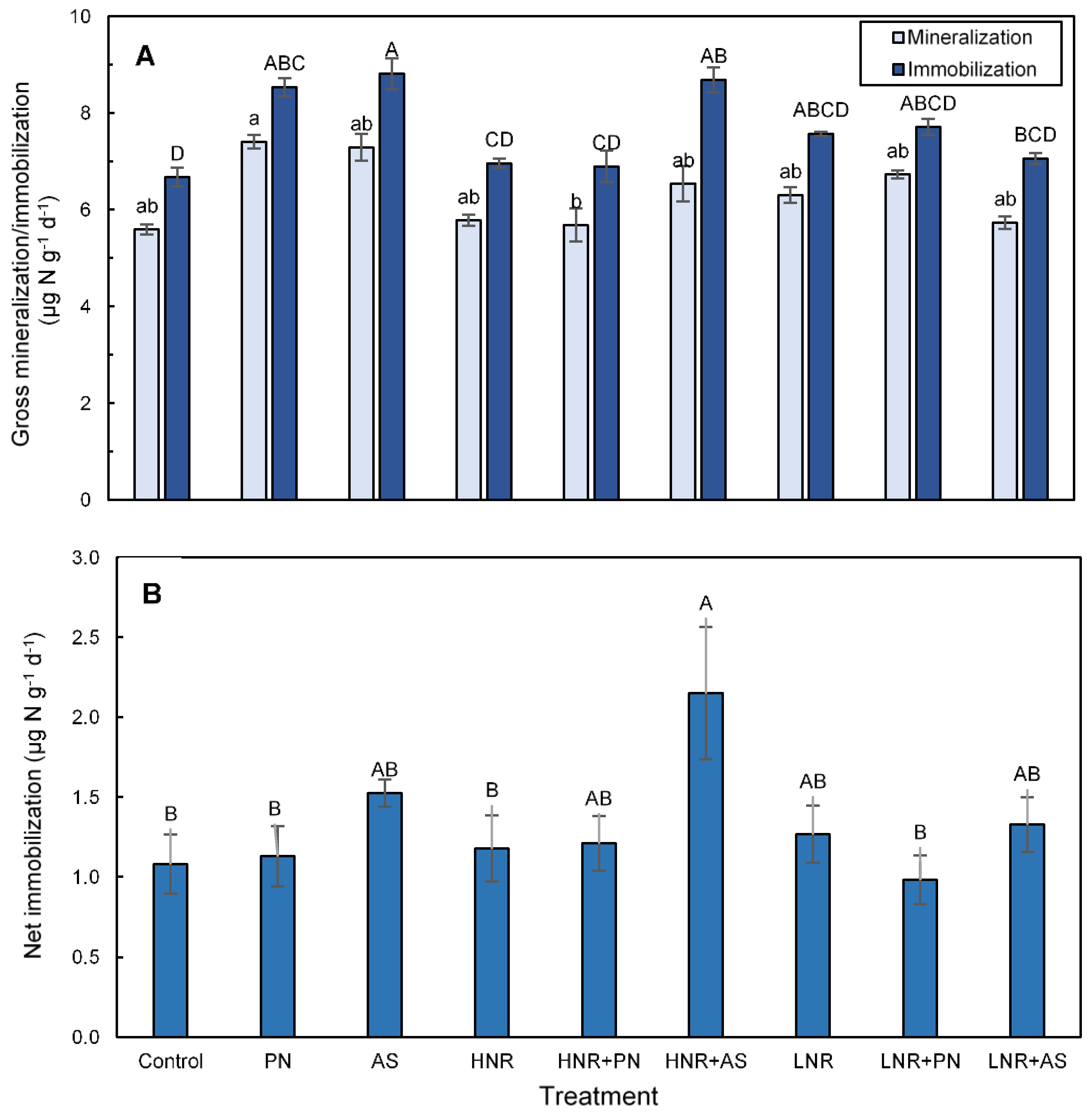
3.4. N Mineralization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental factors affecting the mineralization of crop residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Green, C.J.; Blackmer, A.M.; Horton, R. Nitrogen effects on conservation of carbon during corn residue decomposition in soil. Soil Sci. Soc. Am. J. 1995, 59, 453–459. [Google Scholar] [CrossRef]
- Moran, K.K.; Six, J.; Horwath, W.R.; van Kessel, C. Role of mineral-nitrogen in residue decomposition and stable soil organic matter formation. Soil Sci. Soc. Am. J. 2005, 69, 1730–1736. [Google Scholar] [CrossRef]
- Lueken, H.; Hutcheon, W.L.; Paul, E.A. The influence of nitrogen on the decomposition of crop residues in the soil. Can. J. Soil Sci. 1962, 42, 276–288. [Google Scholar] [CrossRef][Green Version]
- Henriksen, T.M.; Breland, T.A. Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol. Biochem. 1999, 31, 1121–1134. [Google Scholar] [CrossRef]
- Wang, W.J.; Baldock, J.A.; Dalal, R.C.; Moody, P.W. Decomposition dynamics of plant materials in relation to nitrogen availability and biochemistry determined by NMR and wet-chemical analysis. Soil Biol. Biochem. 2004, 36, 2045–2058. [Google Scholar] [CrossRef]
- Recous, S.; Robin, D.; Darwis, D.; Mary, B. Soil inorganic N availability: Effect on maize residue decomposition. Soil Biol. Biochem. 1995, 27, 1529–1538. [Google Scholar] [CrossRef]
- Foereid, B.; de Neergaard, A.; Høgh-Jensen, H. Turnover of organic matter in a Miscanthus field: Effect of time in Miscanthus cultivation and inorganic nitrogen supply. Soil Biol. Biochem. 2004, 36, 1075–1085. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Kwaw-Mensah, D.; Ci, E. Effect of nitrogen fertilizer application on corn residue decomposition in Iowa. Agron. J. 2017, 109, 2415–2427. [Google Scholar] [CrossRef]
- Alexander, M. Introduction to Soil Microbiology, 2nd ed.; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Rice, C.W.; Tiedje, J.M. Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol. Biochem. 1989, 21, 597–602. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, J.; Watanabe, T.; Funakawa, S. Microbial immobilization of ammonium and nitrate fertilizers induced by starch and cellulose in an agricultural soil. Soil Sci. Plant Nutr. 2021, 67, 89–96. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Wei, J.; Zhang, Y.; Zhang, L.; Chang, J.; Thompson, M.L. Decomposition dynamics and changes in chemical composition of wheat straw residue under anaerobic and aerobic conditions. PLoS ONE 2016, 11, e0158172. [Google Scholar] [CrossRef]
- Asmar, F.; Eiland, F.; Nielsen, N.E. Effect of extracellular-enzyme activities on solubilization rate of soil organic nitrogen. Biol. Fertil. Soils 1994, 17, 32–38. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Sinsabaugh, R.L.; Repert, D.A.; Parkhurst, D.F. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 2009, 53, 87–98. [Google Scholar] [CrossRef]
- Luo, R.; Fan, J.; Wang, W.; Luo, J.; Kuzyakov, Y.; He, J.S.; Chu, H.; Ding, W. Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2019, 650, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Dimarogona, M.; Topakas, E.; Christakopoulos, P. Cellulose degradation by oxidative enzymes. Comput. Struct. Biotechnol. J. 2012, 2, e201209015. [Google Scholar] [CrossRef] [PubMed]
- Ajwa, H.A.; Dell, C.J.; Rice, C.W. Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol. Biochem. 1999, 31, 769–777. [Google Scholar] [CrossRef]
- Singh, H.; Singh, K.P. Effect of residue placement and chemical fertilizer on soil microbial biomass under tropical dryland cultivation. Biol. Fertil. Soils 1993, 16, 275–281. [Google Scholar] [CrossRef]
- Muhammad, W.; Vaughan, S.M.; Dalal, R.C.; Menzies, N.W. Crop residues and fertilizer nitrogen influence residue decomposition and nitrous oxide emission from a Vertisol. Biol. Fertil. Soils 2011, 47, 15–23. [Google Scholar] [CrossRef]
- Jansson, S.L. Tracer studies on nitrogen transformations in soil with special attention to mineralization-immobilization relationships. Ann. R. Agric. Coll. Swed. 1958, 24, 101–306. [Google Scholar]
- Zaman, M.D.; Di, H.J.; Cameron, K.C.; Frampton, C.M. Gross nitrogen mineralization and nitrification rates and their relationships to enzyme activities and the soil microbial biomass in soils treated with dairy shed effluent and ammonium fertilizer at different water potentials. Biol. Fertil. Soils 1999, 29, 178–186. [Google Scholar] [CrossRef]
- Brye, K.R.; Gower, S.T.; Norman, J.M.; Bundy, L.G. Carbon budgets for a prairie and agroecosystems: Effects of land use and interannual variability. Ecol. Appl. 2002, 12, 962–979. [Google Scholar] [CrossRef]
- Wilts, A.R.; Reicosky, D.C.; Allmaras, R.R.; Clapp, C.E. Long-term corn residue effects: Harvest alternatives, soil carbon turnover, and root-derived carbon. Soil Sci. Soc. Am. J. 2004, 68, 1342–1351. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Peters, J.B.; Nathan, M.V.; Laboski, C.A.M. pH and lime requirement. In Recommended Chemical Soil Test Procedures for the North Central Region; Nathan, M., Gelderman, R., Eds.; University of Missouri: Columbia, MO, USA, 2015; pp. 4.1–4.7. Available online: https://extension.missouri.edu/media/wysiwyg/Extensiondata/Pub/pdf/specialb/sb1001.pdf (accessed on 3 March 2021).
- Mebius, L.J. A rapid method for the determination of organic carbon in soil. Anal. Chim. Acta 1960, 22, 120–124. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen‒total. In Methods of Soil Analysis Part 3-Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar] [CrossRef]
- Stevens, W.B.; Mulvaney, R.L.; Khan, S.A.; Hoeft, R.G. Improved diffusion methods for nitrogen and 15nitrogen analysis of Kjeldahl digests. J. AOAC Int. 2000, 83, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Mulvaney, R.L.; Hoeft, R.G. A simple soil test for detecting sites that are nonresponsive to nitrogen fertilization. Soil Sci. Soc. Am. J. 2001, 65, 1751–1760. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464. [Google Scholar] [CrossRef]
- Bremner, J.M.; Shaw, K. Denitrification in soil. I. Methods of investigation. J. Agric. Sci. 1958, 51, 40–52. [Google Scholar] [CrossRef]
- Pordesimo, L.O.; Edens, W.C.; Sokhansanj, S. Distribution of aboveground biomass in corn stover. Biomass Bioenerg. 2004, 26, 337–343. [Google Scholar] [CrossRef]
- Harper, S.H.T.; Lynch, J.M. The chemical components and decomposition of wheat straw leaves, internodes and nodes. J. Sci. Food Agric. 1981, 32, 1057–1062. [Google Scholar] [CrossRef]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Protein; Circular No. 183; USDA: Washington, DC, USA, 1931. [Google Scholar]
- Horgan, B.P.; Mulvaney, R.L.; Branham, B.E. Determination of atmospheric volume for direct field measurement of denitrification in soil cores. Soil Sci. Soc. Am. J. 2001, 65, 511–516. [Google Scholar] [CrossRef][Green Version]
- Van de Werf, H.; Verstraete, W. Estimation of active soil microbial biomass by mathematical analysis of respiration curves: Calibration of the test procedure. Soil Biol. Biochem. 1987, 19, 261–265. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Margenot, A.J.; Nakayama, Y.; Parikh, S.J. Methodological recommendations for optimizing assays of enzyme activities in soil samples. Soil Biol. Biochem. 2018, 125, 350–360. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Jesmin, T.; Margenot, A.J.; Mulvaney, R.L. A comprehensive method for casein-based assay of soil protease activity. Commun. Soil Sci. Plant Anal. 2021, in press. [Google Scholar]
- Khan, S.A.; Mulvaney, R.L.; Mulvaney, C.S. Accelerated diffusion methods for inorganic-nitrogen analysis of soil extracts and water. Soil Sci. Soc. Am. J. 1997, 61, 936–942. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Fohringer, C.L.; Bojan, V.J.; Michlik, M.M.; Herzog, L.F. A commercial system for automated nitrogen isotope-ratio analysis by the Rittenberg technique. Rev. Sci. Instrum. 1990, 61, 897–903. [Google Scholar] [CrossRef]
- Kirkham, D.; Bartholomew, W.V. Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci. Soc. Am. Proc. 1954, 18, 33–34. [Google Scholar] [CrossRef]
- Paleontological Statistics Software Package for Education and Data Analysis (PAST). Available online: https://past.en.lo4d.com/windows (accessed on 27 July 2021).
- Terman, G.L.; Noggle, J.C. Nutrient concentration changes in corn as affected by dry matter accumulation with age and response to applied nutrients. Agron. J. 1973, 65, 941–945. [Google Scholar] [CrossRef]
- Perry, L.J., Jr.; Olson, R.A. Yield and quality of corn and grain sorghum grain and residues as influenced by N fertilization. Agron. J. 1975, 67, 816–818. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Wegenast, T.; Longin, C.F.; Xu, X.; Melchinger, A.E.; Chen, S. Effect of N supply on stalk quality in maize hybrids. Field Crops Res. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Russell, A.E.; Cambardella, C.A.; Laird, D.A.; Jaynes, D.B.; Meek, D.W. Nitrogen fertilizer effects on soil carbon balances in Midwestern U.S. agricultural systems. Ecol. Appl. 2009, 19, 1102–1113. [Google Scholar] [CrossRef]
- Jantalia, C.P.; Halvorson, A.D. Nitrogen fertilizer effects on irrigated conventional tillage corn yields and soil carbon and nitrogen pools. Agron. J. 2011, 103, 871–878. [Google Scholar] [CrossRef]
- Chen, S.F.; Mowery, R.A.; Scarlata, C.J.; Chambliss, C.K. Compositional analysis of water-soluble materials in corn stover. J. Agric. Food Chem. 2007, 55, 5912–5918. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Shi, A.D.; Marschner, P. Soil respiration and microbial biomass after residue addition are influenced by the extent by which water-extractable organic C was removed from the residues. Eur. J. Soil Biol. 2014, 63, 28–32. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhao, B.Z.; Zhang, J.B. Effects of maize residue quality and soil water content on soil labile organic carbon fractions and microbial properties. Pedosphere 2016, 26, 829–838. [Google Scholar] [CrossRef]
- Schulze, W. Stickstoffdüngung und Zuckerbildung bei Mais. Albrecht-Thaer-Arch 1964, 8, 711–723. [Google Scholar] [CrossRef]
- Baghdadi, A.; Balazadeh, M.; Kashani, A.; Golzardi, F.; Gholamhoseini, M.; Mehrnia, M. Effect of pre-sowing and nitrogen application on forage quality of silage corn. Agron. Res. 2017, 15, 11–23. [Google Scholar]
- Gallagher, M.E.; Hockaday, W.C.; Masiello, C.A.; Snapp, S.; McSwiney, C.P.; Baldock, J.A. Biochemical suitability of crop residues for cellulosic ethanol: Disincentives to nitrogen fertilization in corn agriculture. Environ. Sci. Technol. 2011, 45, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, D.L.; Sinsabaugh, R.L. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 2006, 76, 151–174. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Bornemann, L.; Amelung, W. Microbial nitrogen mining affects spatio-temporal patterns of substrate-induced respiration during seven years of bare fallow. Soil Biol. Biochem. 2017, 104, 175–184. [Google Scholar] [CrossRef]
- Rader, L.F.; White, L.M.; Whittaker, C.W. The salt index‒A measure of the effect of fertilizers on the concentration of the soil solution. Soil Sci. 1943, 55, 201–218. [Google Scholar] [CrossRef]
- Pathak, H.; Rao, D.L.N. Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biol. Biochem. 1998, 30, 695–702. [Google Scholar] [CrossRef]
- Adviento-Borbe, M.A.A.; Doran, J.W.; Drijber, R.A.; Dobermann, A. Soil electrical conductivity and water content affect nitrous oxide and carbon dioxide emissions in intensively managed soils. J. Environ. Qual. 2006, 35, 1999–2010. [Google Scholar] [CrossRef]
- Rousk, J.; Elyaagubi, F.K.; Jones, D.L.; Godbold, D.L. Bacterial salt tolerance is unrelated to soil salinity across an arid agroecosystem salinity gradient. Soil Biol. Biochem. 2011, 43, 1881–1887. [Google Scholar] [CrossRef]
- Pierre, W.H. Nitrogenous fertilizers and soil acidity: I. Effect of various nitrogenous fertilizers on soil reaction. J. Am. Soc. Agron. 1928, 20, 254–269. [Google Scholar] [CrossRef]
- McAndrew, D.W.; Malhi, S.S. Long-term N fertilization of a solonetzic soil: Effects on chemical and biological properties. Soil Biol. Biochem. 1992, 24, 619–623. [Google Scholar] [CrossRef]
- Malhi, S.S.; Nyborg, M.; Harapiak, J.T.; Heier, K.; Flore, N.A. Increasing organic C and N in soil under bromegrass with long-term N fertilization. Nutr. Cycl. Agroecosyst. 1997, 49, 255–260. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Anderson, T.H. Adaptive responses of soil microbial communities under experimental acid stress in controlled laboratory studies. Appl. Soil Ecol. 1999, 11, 207–216. [Google Scholar] [CrossRef]
- Chandra, P.; Bollen, W.B. Effect of wheat straw, nitrogenous fertilizers, and carbon-to-nitrogen ratio on organic decomposition in a sub-humid soil. J. Agric. Food Chem. 1960, 8, 19–24. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol. Biochem. 2010, 42, 2336–2338. [Google Scholar] [CrossRef]
- Schmidt, U.; Schmidt, G. Zur Frage der Mineralisation von Ernterückständen in Abhängigkeit vom Stickstoffgehalt. Z. Pflernähr. Düng. Bodenk. 1963, 101, 99–109. [Google Scholar] [CrossRef]
- Reinertsen, S.A.; Elliott, L.F.; Cochran, V.L.; Campbell, G.S. Role of available carbon and nitrogen in determining the rate of wheat straw decomposition. Soil Biol. Biochem. 1984, 16, 459–464. [Google Scholar] [CrossRef]
- Janzen, H.H.; Kucey, R.M.N. C, N, and S mineralization of crop residues as influenced by crop species and nutrient regime. Plant Soil 1988, 106, 35–41. [Google Scholar] [CrossRef]
- Sharma, M.P.; Habib, A. Decomposition rate of crop residue and their nutrients release pattern under different nitrogen levels in Inceptisol. Ann. Agric. Res. New Ser. 2005, 26, 441–446. [Google Scholar]
- Sakala, W.D.; Cadisch, G.; Giller, K.E. Interactions between residues of maize and pigeonpea and mineral N fertilizers during decomposition and N mineralization. Soil Biol. Biochem. 2000, 32, 679–688. [Google Scholar] [CrossRef]
- Conde, E.; Cardenas, M.; Ponce-Mendoza, A.; Luna-Guido, M.L.; Cruz-Mondragón, C.; Dendooven, L. The impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol. Biochem. 2005, 37, 681–691. [Google Scholar] [CrossRef]
- Chen, H.; Billen, N.; Stahr, K.; Kuzyakov, Y. Effects of nitrogen and intensive mixing on decomposition of 14C-labelled maize (Zea mays L.) residue in soils of different land use types. Soil Till. Res. 2007, 96, 114–123. [Google Scholar] [CrossRef]
- Potthoff, M.; Dyckmans, J.; Flessa, H.; Muhs, A.; Beese, F.; Joergensen, R.G. Dynamics of maize (Zea mays L.) leaf straw mineralization as affected by the presence of soil and the availability of nitrogen. Soil Biol. Biochem. 2005, 37, 1259–1266. [Google Scholar] [CrossRef]
- Allison, F.E.; Murphy, R.M. Comparative rates of decomposition in soil of wood and bark particles of several hardwood species. Soil Sci. Soc. Am. Proc. 1962, 26, 463–466. [Google Scholar] [CrossRef]
- Sain, P.; Broadbent, F.E. Decomposition of rice straw in soils as affected by some management factors. J. Environ. Qual. 1977, 6, 96–100. [Google Scholar] [CrossRef]
- Fog, K. The effect of added nitrogen on the rate of decomposition of organic matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Alvarez, C.R.; Alvarez, R. Short-term effects of tillage systems on active soil microbial biomass. Biol. Fertil. Soils 2000, 31, 157–161. [Google Scholar] [CrossRef]
- De Nobili, M.; Contin, M.; Mondini, C.; Brookes, P.C. Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol. Biochem. 2001, 33, 1163–1170. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Tao, B.; Wang, J.; Zhu, X.; Wang, X. Short-term responses of soil enzyme activities and carbon mineralization to added nitrogen and litter in a freshwater marsh of Northeast China. Eur. J. Soil Biol. 2014, 61, 72–79. [Google Scholar] [CrossRef]
- Li, L.J.; Zhu-Barker, X.; Ye, R.; Doane, T.A.; Horwath, W.R. Soil microbial biomass size and soil carbon influence the priming effect from carbon inputs depending on nitrogen availability. Soil Biol. Biochem. 2018, 119, 41–49. [Google Scholar] [CrossRef]
- Bach, E.M.; Hofmockel, K.S. Coupled carbon and nitrogen inputs increase microbial biomass and activity in prairie bioenergy systems. Ecosystems 2015, 18, 417–427. [Google Scholar] [CrossRef]
- Paul, G.C.; Solaiman, A.R. Changes of microbial biomass carbon and nitrogen in upland sugarcane soil amended with different organic materials. Commun. Soil Sci. Plant Anal. 2005, 35, 2433–2447. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.K.; Khizar, A. Microbial biomass carbon and nitrogen transformations in a loam soil amended with organic–inorganic N sources and their effect on growth and N-uptake in maize. Ecol. Eng. 2012, 39, 123–132. [Google Scholar] [CrossRef]
- Mgelwa, A.S.; Hu, Y.L.; Xu, W.B.; Ge, Z.Q.; Yu, T.W. Soil carbon and nitrogen availability are key determinants of soil microbial biomass and respiration in forests along urbanized rivers of southern China. Urban For. Urban Green. 2019, 43, 126351. [Google Scholar] [CrossRef]
- Suto, M.; Tomita, F. Induction and catabolite repression mechanisms of cellulase in fungi. J. Biosci. Bioeng. 2001, 92, 305–311. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 32, 1309–1315. [Google Scholar] [CrossRef]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Landi, L.; Renella, G.; Giagnoni, L.; Nannipieri, P. Activities of proteolytic enzymes. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 247–260. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Regulation of extracellular protease activity in soil in response to different sources and concentrations of nitrogen and carbon. Soil Biol. Biochem. 2008, 40, 3040–3048. [Google Scholar] [CrossRef]
- Geisseler, D.; Joergensen, R.G.; Ludwig, B. Potential soil enzyme activities are decoupled from microbial activity in dry residue-amended soil. Pedobiologia 2012, 55, 253–261. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Mishra, S.; Di, H.J.; Cameron, K.C.; Monaghan, R.; Carran, A. Gross nitrogen mineralisation rates in pastural soils and their relationships with organic nitrogen fractions, microbial biomass and protease activity under glasshouse conditions. Biol. Fertil. Soils 2005, 42, 45–53. [Google Scholar] [CrossRef]
- Green, C.J.; Blackmer, A.M. Residue decomposition effects on nitrogen availability to corn following corn or soybean. Soil Sci. Soc. Am. J. 1995, 59, 1065–1070. [Google Scholar] [CrossRef]

| Property | Value | Reference |
|---|---|---|
| pH (soil:water ratio, 1:1) | 6.6 | [27] |
| Organic C (g kg−1) | 21.5 | [28] |
| Total N (g kg−1) | 1.8 | [29,30] |
| C:N ratio | 12.2 | |
| Potentially mineralizable N (mg kg−1) | 258 | [31] |
| Bioavailable P (mg kg−1) | 22 | [32] |
| Sand (g kg−1) | 158 | [33] |
| Silt (g kg−1) | 595 | [33] |
| Clay (g kg−1) | 247 | [33] |
| Water-holding capacity (WHC, mL kg−1) | 635 | [34] |
| Proximate Analysis (g kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Residue Mixture | Cropping System | Organic C (g kg−1) | Total N (g kg−1) | Crude Protein (g kg−1) | C:N | Water- Soluble Fraction | Lignin | Cellulose + Hemicellulose |
| High N | Corn−corn (fertilized) | 407 | 6.7 | 42 | 61 | 28.0 | 14.5 | 53.7 |
| Low N | Corn−soybean (unfertilized) | 404 | 4.3 | 27 | 94 | 18.0 | 13.8 | 52.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesmin, T.; Mitchell, D.T.; Mulvaney, R.L. Short-Term Effect of Nitrogen Fertilization on Carbon Mineralization during Corn Residue Decomposition in Soil. Nitrogen 2021, 2, 444-460. https://doi.org/10.3390/nitrogen2040030
Jesmin T, Mitchell DT, Mulvaney RL. Short-Term Effect of Nitrogen Fertilization on Carbon Mineralization during Corn Residue Decomposition in Soil. Nitrogen. 2021; 2(4):444-460. https://doi.org/10.3390/nitrogen2040030
Chicago/Turabian StyleJesmin, Tanjila, Dakota T. Mitchell, and Richard L. Mulvaney. 2021. "Short-Term Effect of Nitrogen Fertilization on Carbon Mineralization during Corn Residue Decomposition in Soil" Nitrogen 2, no. 4: 444-460. https://doi.org/10.3390/nitrogen2040030
APA StyleJesmin, T., Mitchell, D. T., & Mulvaney, R. L. (2021). Short-Term Effect of Nitrogen Fertilization on Carbon Mineralization during Corn Residue Decomposition in Soil. Nitrogen, 2(4), 444-460. https://doi.org/10.3390/nitrogen2040030






