Efficacy of Peat and Liquid Inoculant Formulations of Bradyrhizobium japonicum Strain WB74 on Growth, Yield and Nitrogen Concentration of Soybean (Glycine max L.)
Abstract
1. Introduction
2. Matrials and Methods
2.1. Liquid and Peat Inoculant Preparation
2.2. Experimental Site
2.3. Soil Analysis and Fertilizer Application
2.4. Experimental Design and Treatment
2.5. Data Collection
2.5.1. Growth and Yield Parameters
2.5.2. Determination of Nitrogen Concentration with Dumas and Fixed Using the 15N Natural Abundance Technique
2.6. Statistical Analysis
3. Results
3.1. Growth and Yield Parameters of Soybean Crop
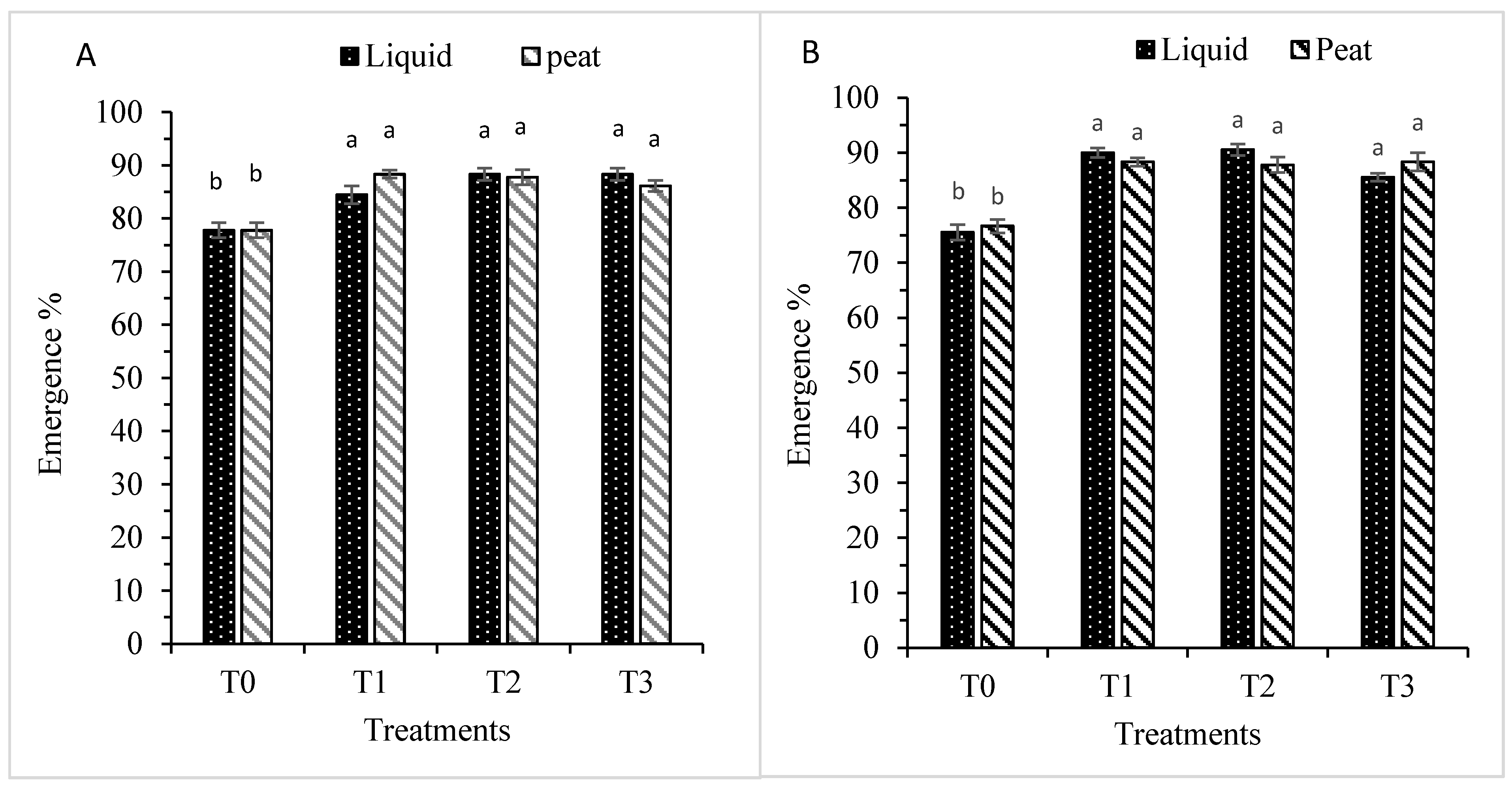
3.1.1. Plant Emergence
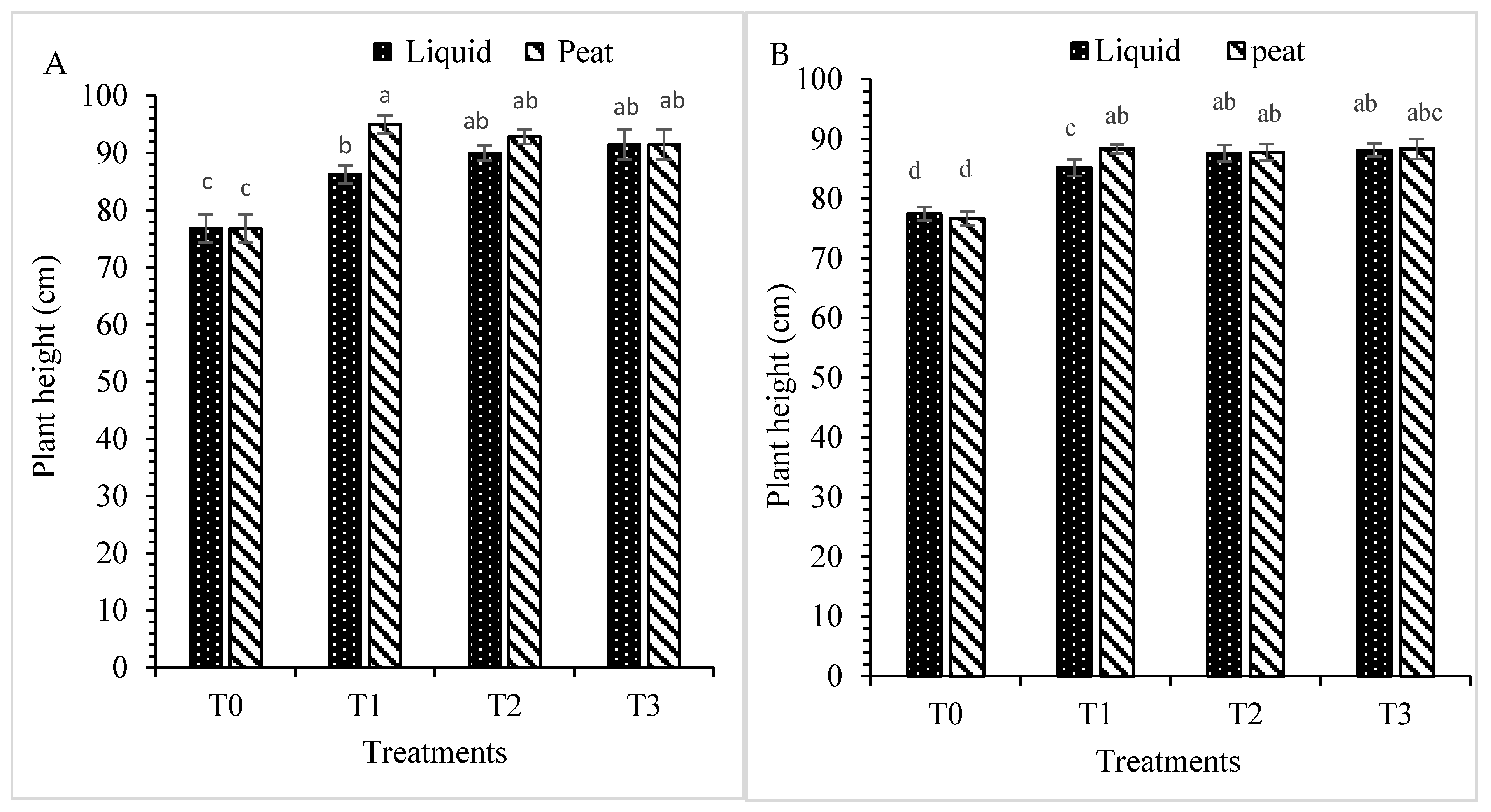
3.1.2. Plant Height
3.1.3. Root Length
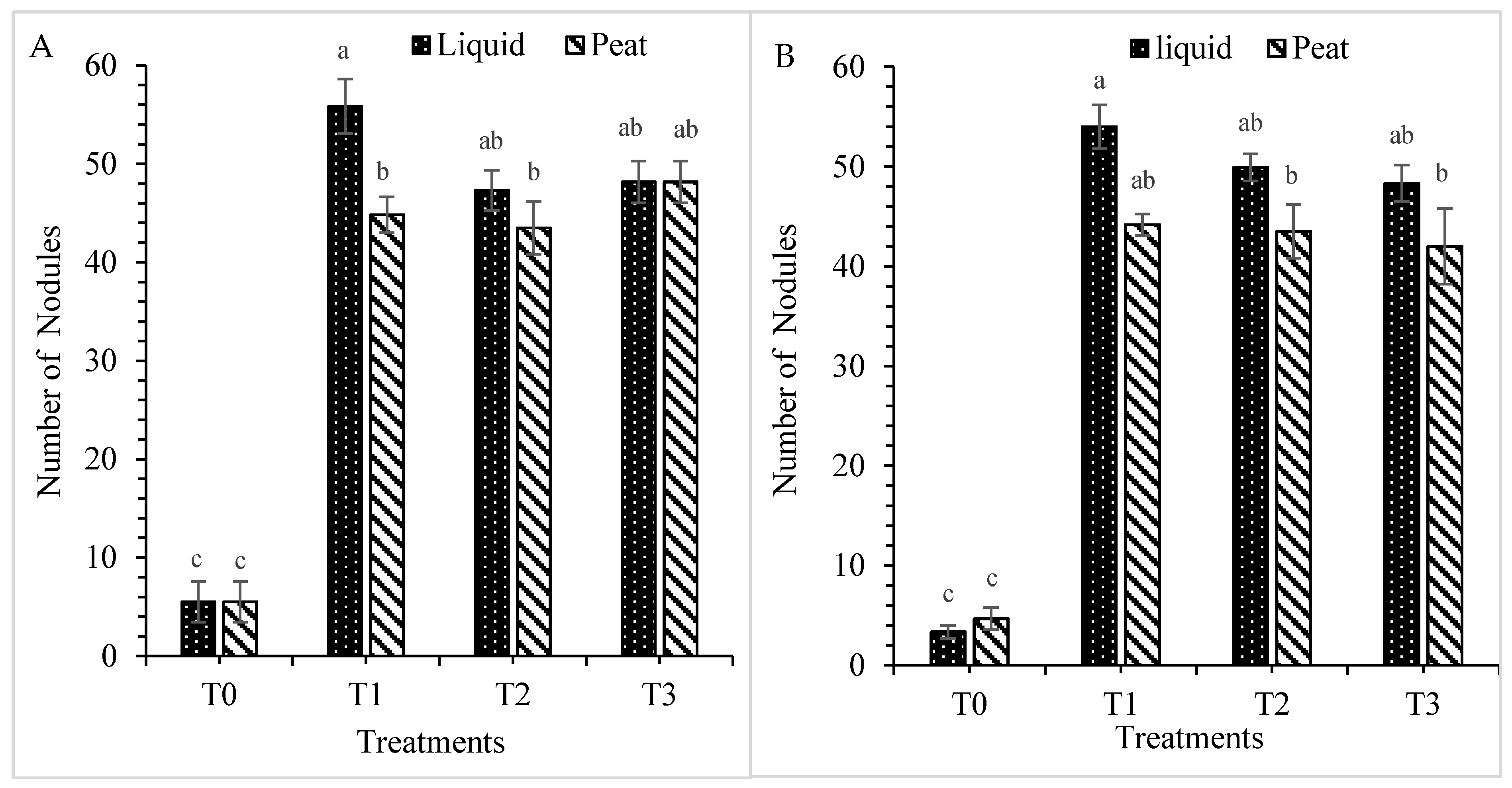
3.1.4. Number of Nodules
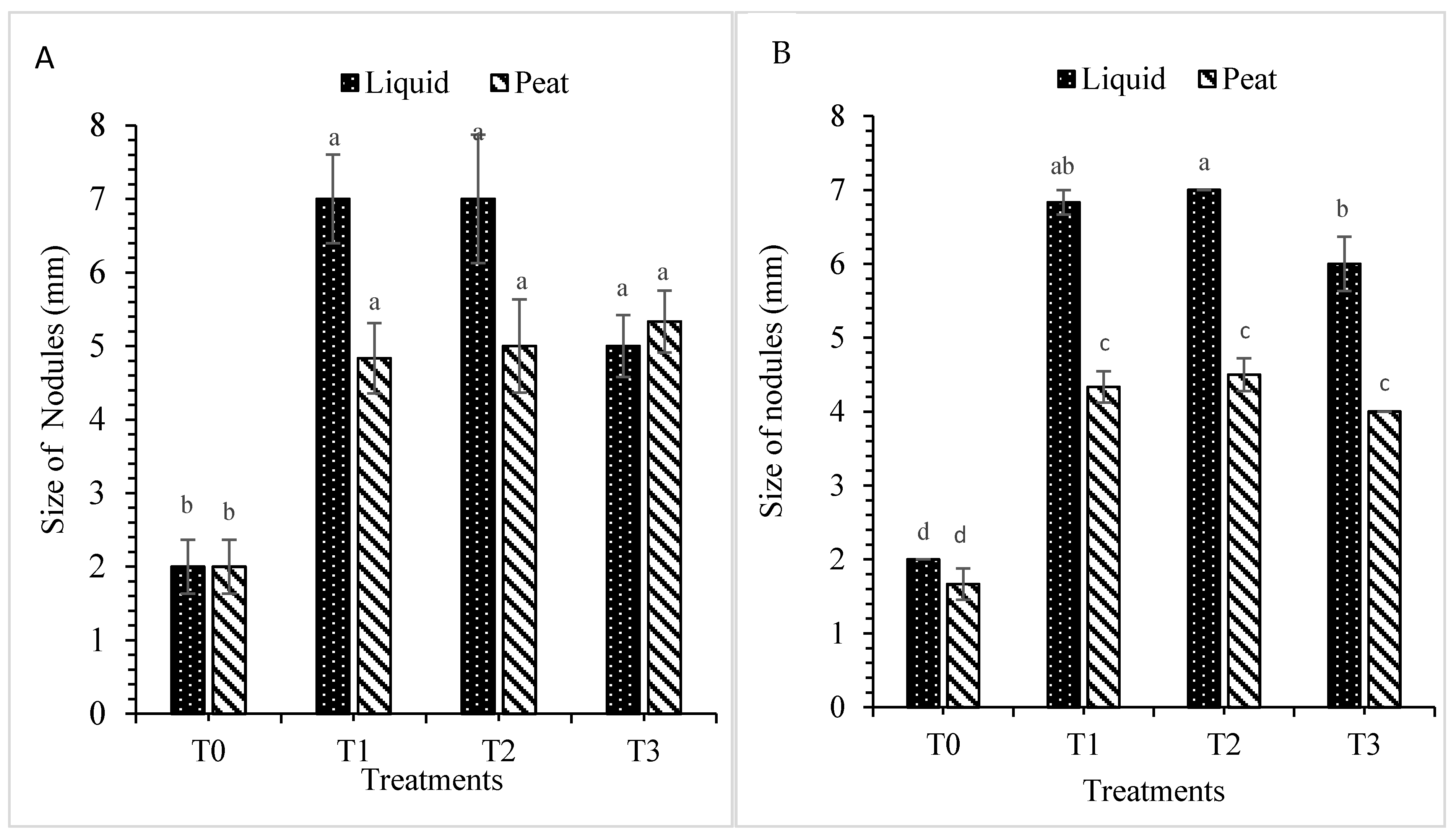
3.1.5. Size of Nodules
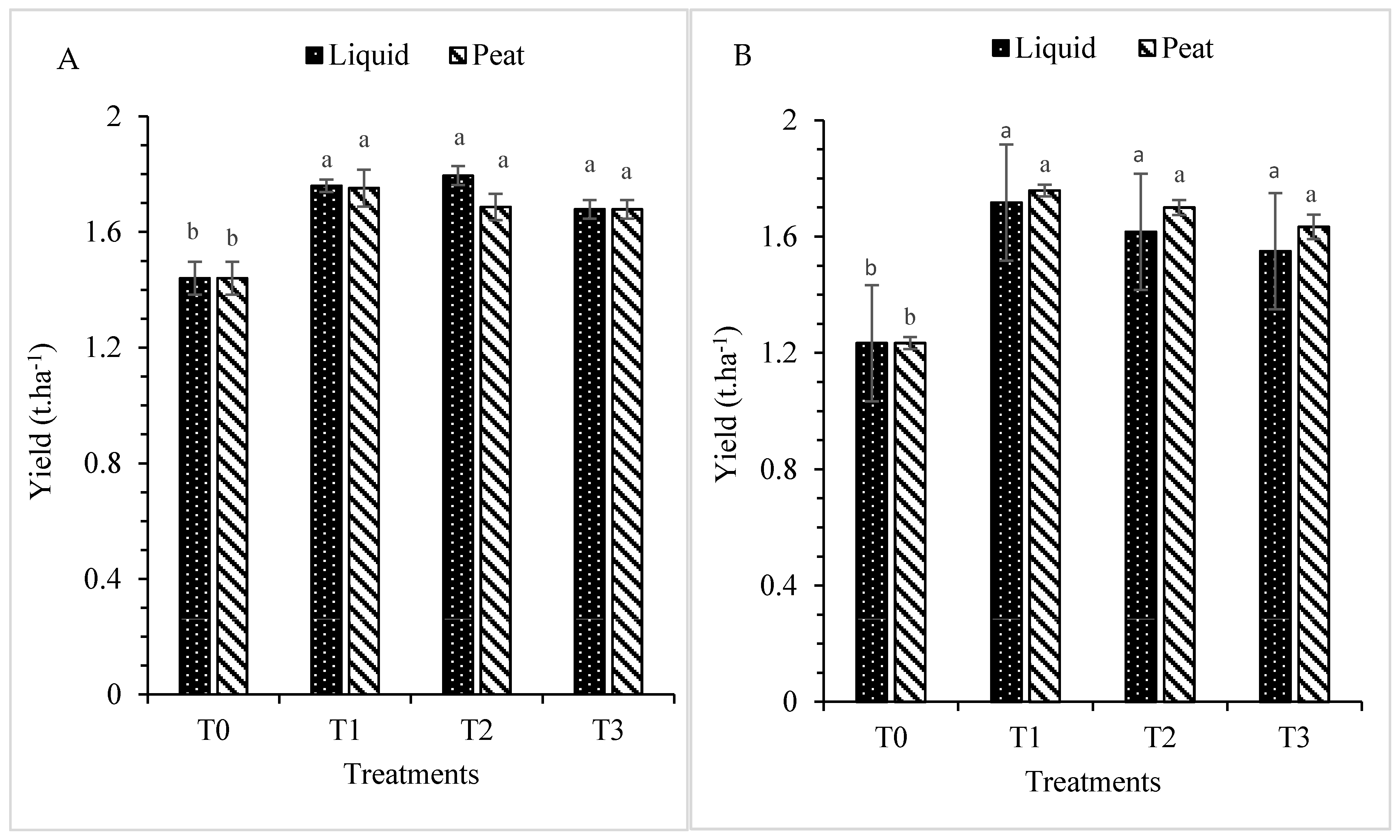
3.1.6. Yield
3.1.7. Nitrogen Concentration with Dumas Analysis
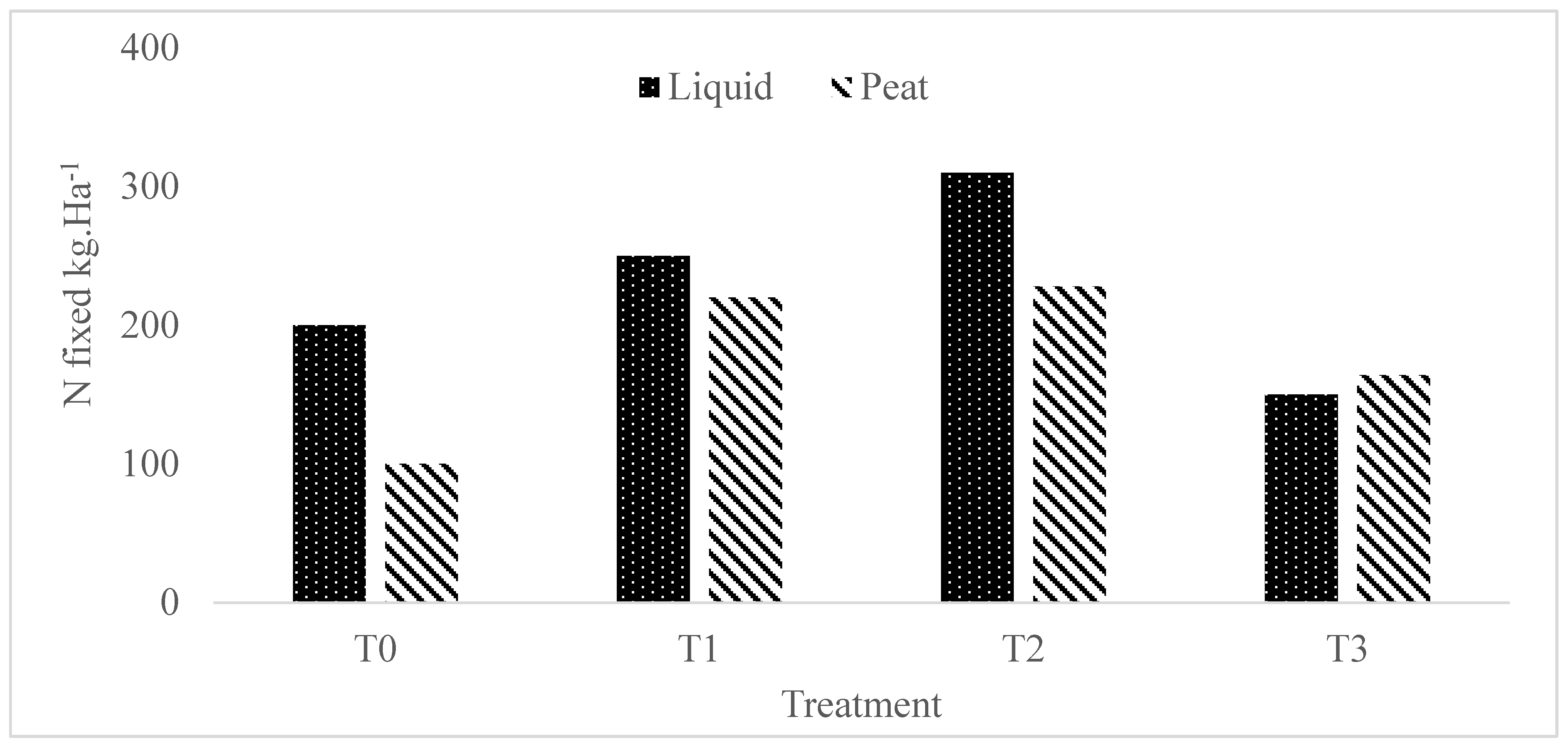
3.1.8. Nitrogen Fixed
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodriĝues-Navarro, D.N.; Oliver, I.M.; Contreras, M.A.; Ruiz-Sainz, J.E. Soybean interactions with soil microbes, agronomical and molecular aspects. Agron. Sustain. Dev. 2011, 31, 173–190. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- United States Department of Agriculture (USDA). World Soybean Production 2020/2021. Available online: World Agricultural.com (accessed on 23 July 2021).
- FAOSTAT. FAO Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 11 May 2015).
- South African Bureau for Food and Agricultural Policy. 2013.
- Department of Trade and Industry. Industrial Policy Action Plan 2012/13–2014/15; Department of Trade and Industry: Pretoria, South Africa, 2010.
- De Beer, A.; Prinsloo, T. The National Soybean Cultivar Trials in South Africa–34 Years’ Experiences and Progress; Agric Cultural Research Council, Grain Crops Institute: Potchefstroom, South Africa, 2013. [Google Scholar]
- Sahoo, R.K.; Bhardwaj, D.; Tuteja, N. Bio-fertilizers: A sustainable eco-friendly agricultural approach to crop improvement. In Plant Acclimation to Environmental Stress; Springer: New York, NY, USA, 2013; pp. 403–432. [Google Scholar]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as bio-stimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Abaidoo, R.C.; Keyser, H.H.; Singleton, P.W.; Dashiell, K.E.; Sanginga, N. Population size, distribution, and symbiotic characteristics of indigenous Bradyrhizobium spp. that nodulate TGx soybean genotypes in Africa. Appl. Soil Ecol. 2007, 35, 57–67. [Google Scholar] [CrossRef]
- Hussain, K.; Islam, M.; Siddique, M.T.; Hayat, R.; Molisan, S.O. Soybean Growth and Nitrogen Fixation as Affected by Sulfur Fertilization and Inoculation Under rainfed conditions in Pakistan. Int. J. Agric. Biol. 2011, 13, 951–955. [Google Scholar]
- Weisany, W.; Raei, Y.; Allahverdipoor, K.H. Role of some of mineral nutrients in biological nitrogen fixation. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 77–84. [Google Scholar]
- Bai, Y.; Zhou, X.; Smith, D.L. Enhanced soybean plant growth resulting from co-inoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci. 2003, 43, 1774–1781. [Google Scholar] [CrossRef]
- Solomon, T.; Pant, L.M.; Angaw, T. Co (Glycine max L. Merill) varieties on nitisols of Bako, Western Ethiopia. ISRN Agron. 2012. [Google Scholar] [CrossRef]
- Bhangoo, M.S.; Albritton, D.J. Nodulating and nonnodulating Soybean isolines response applied nitrogen. Agron. J. 1996, 68, 642–645. [Google Scholar] [CrossRef]
- Mburu, M.W.; Okalebo, J.R.; Lesueur, D.; Pypers, P.; Ng’etich, W.; Mutegi, E.; Nekesa, O.A. Evaluation of biological commercial inoculants on soybean production in Bungoma county, Kenya. In Proceedings of the 10th African Crop Science Conference Proceedings, Maputo, Mozambique, 10–13 October 2011; pp. 605–610. [Google Scholar]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Bio-stimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263. [Google Scholar] [CrossRef] [PubMed]
- Biradar, B.P.; Santhosh, G.P. Role of Polymeric Additives in Formulation, Shelf-life and Bioefficacy of Liquid Inoculant of Pseudomonas fluorescens. Int. J. Pure App. Biosci. 2018, 6, 123–133. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Koppen-Gieger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Soil Classification Working Group. 1991; South Africa.
- Jackson, M.C. Soil Chemical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1975. [Google Scholar]
- Argaw, A. Evaluation of co-inoculation of Bradyrhizobium japonicum and Phosphate solubilizing Pseudomonas spp. effect on soybean (Glycine max L. Merr.) in Assossa Area. J. Agric. Sci. Technol. 2012, 14, 213–224. [Google Scholar]
- Thao, T.Y. Need for and benefits of soybean inoculation of the south Vietnam. In Proceedings of the National Conference of Soybean, VASI-CSIRO (Vietnam-Australia), Hanoi, Vietnam, 22–23 March 2001. [Google Scholar]
- Souleimanov, A.; Prithiviraj, B.; Smith, D.L. The major Nod factor of Bradyrhizobium japonicum promotes early growth of soybean and corn. J. Exp. Bot. 2002, 53, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Tairo, E.V.; Ndakidemi, P.A. Yields and economic benefits of soybean (Glycine max L.) as affected by Bradyrhizobium japonicum inoculation and phosphorus supplementation. Am. J. Res. Commun. 2013, 1, 159–172. [Google Scholar]
- Zerpa, M.; Mayz, J.; Méndez, J. Effects of Bradyrhizobium japonicum inoculants on soybean (Glycine max (L.) Merr.) growth and nodulation. Ann. Biol. Res. 2013, 4, 193–199. [Google Scholar]
- Dwivedi, S.L.; Sahrawat, K.L.; Upadhyaya, H.D.; Mengoni, A.; Galardini, M.; Bazzicalupo, M.; Biondi, E.G.; Hungria, M.; Kaschuk, G.; Blair, M.W.; et al. Advances in host plant and rhizobium genomics to enhance symbiotic nitrogen fixation in grain legumes. Adv. Agron. 2015, 129, 1–116. [Google Scholar]
- Lamptey, S.; Ahiabor BD, K.; Yeboah, S.; Osei, D. Effect of rhizobium inoculants and reproductive growth stages on shoot biomass and yield of soybean (Glycine max (L.) merril). J. Agric. Sci. 2014, 6, 44. [Google Scholar] [CrossRef]
- Kumaga, F.K.; Ofori, K. Response of soybean (Glycine max (L.) Merrill) to Bradyrhizobia inoculation and phosphorus application. Int. J. Agric. Biol. 2004, 6, 324–327. [Google Scholar]
- De Bruin, J.L.; Pedersen, P.; Conley, S.P.; Gaska, J.M.; Naeve, S.L.; Kurle, J.E.; Elmore, R.W.; Giesler, L.J.; Abendroth, L.J. Probability of yield response to inoculants in fields with a history of soybean. Crop Sci. 2010, 50, 265–272. [Google Scholar] [CrossRef]
- Vitosh, M.L. Soybean Inoculation in Michigan; Department of Crop and Soil Sciences, Michigan State University: Ann Arbor, MI, USA, 1997. [Google Scholar]
- Egamberdiyeva, D.; Qarshieva, D.; Davranov, K. Growth and yield of soybean varieties inoculated with Bradyrhizobium spp in N-deficient calcareous soils. Biol. Fertil. Soils 2004, 40, 144–146. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Soe, K.M.; Moe, K.; Yamakawa, T. Effects of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of mung bean, cowpea, and soybean. Agronomy 2019, 9, 77. [Google Scholar] [CrossRef]
- Javaid, A.; Mahmood, N. Growth, nodulation and yield response of soybean to biofertilizers and organic manures. Pak. J. Bot. 2010, 42, 863–871. [Google Scholar]
- Atieno, M.; Herrmann, L.; Okalebo, R.; Lesueur, D. Efficiency of different formulations of Bradyrhizobium japonicum and effect of co-inoculation of Bacillus subtilis with two different strains of Bradyrhizobium japonicum. World J. Microbiol. Biotechnol. 2012, 28, 2541–2550. [Google Scholar] [CrossRef] [PubMed]


| Chemical and Physical Properties | Bioclimatic Zone 1 | Bioclimatic Zone 2 |
|---|---|---|
| pH (H2O) | 4.6 | 5.19 |
| CEC (cmol (+) kg−1) | 3.2 | 2.3 |
| Organic matter content (%) | 1.3 | 0.8 |
| P Bray1 (mg kg−1) | 6 | 10 |
| Ca (mg kg−1) | 410 | 278 |
| Na (mg kg−1) | 18 | 19 |
| K (mg kg−1) | 153 | 66 |
| Mg (mg kg−1) | 83 | 86 |
| S (mg kg−1) | 5 | 6.39 |
| N (mg kg−1) | 700 | 800 |
| Density (g mL−1) | 1.1 | 1.2 |
| Clay (%) | 22 | 18 |
| Sand (%) | 68 | 73 |
| Silt (%) | 10 | 9 |
| Month | Avg. T (°C) | Minimum Temp (°C) | Maximum Temp (°C) | Rainfall (mm) |
|---|---|---|---|---|
| December | 21.9 | 15.9 | 28 | 98 |
| January | 21.3 | 14.9 | 27.8 | 129 |
| February | 21 | 14.6 | 27.5 | 88 |
| March | 19.7 | 13 | 26.5 | 76 |
| April | 16.8 | 9.3 | 24.3 | 44 |
| May | 13.3 | 4.8 | 21.8 | 15 |
| June | 10 | 1 | 19.1 | 7 |
| July | 10.1 | 1.1 | 19.2 | 7 |
| August | 12.9 | 3.7 | 22.1 | 6 |
| September | 16.5 | 7.9 | 25.2 | 20 |
| October | 19 | 11.4 | 26.7 | 68 |
| November | 20.1 | 13.3 | 26.9 | 112 |
| Month | Avg. T (°C) | Minimum Temp (°C) | Maximum Temp (°C) | Rainfall\(mm) |
|---|---|---|---|---|
| December | 23.9 | 18.3 | 29.5 | 97 |
| January | 23.4 | 16.9 | 29.9 | 110 |
| February | 22.9 | 16.5 | 29.4 | 88 |
| March | 21.9 | 14.8 | 28.4 | 68 |
| April | 18.9 | 11.2 | 26.1 | 47 |
| May | 14.7 | 6.1 | 23.4 | 19 |
| June | 11.7 | 2.3 | 21.1 | 7 |
| July | 11.7 | 2.1 | 21.4 | 5 |
| August | 14.7 | 4.8 | 24.3 | 5 |
| September | 18.5 | 9.5 | 27.5 | 14 |
| October | 21.3 | 13.4 | 29.2 | 55 |
| November | 21.9 | 15 | 28.9 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatabazi, A.; Vorster, B.J.; Mvondo-She, M.A.; Mangwende, E.; Mangani, R.; Hassen, A.I. Efficacy of Peat and Liquid Inoculant Formulations of Bradyrhizobium japonicum Strain WB74 on Growth, Yield and Nitrogen Concentration of Soybean (Glycine max L.). Nitrogen 2021, 2, 332-346. https://doi.org/10.3390/nitrogen2030023
Gatabazi A, Vorster BJ, Mvondo-She MA, Mangwende E, Mangani R, Hassen AI. Efficacy of Peat and Liquid Inoculant Formulations of Bradyrhizobium japonicum Strain WB74 on Growth, Yield and Nitrogen Concentration of Soybean (Glycine max L.). Nitrogen. 2021; 2(3):332-346. https://doi.org/10.3390/nitrogen2030023
Chicago/Turabian StyleGatabazi, Auges, Barend Juan Vorster, Mireille Asanzi Mvondo-She, Edgar Mangwende, Robert Mangani, and Ahmed Idris Hassen. 2021. "Efficacy of Peat and Liquid Inoculant Formulations of Bradyrhizobium japonicum Strain WB74 on Growth, Yield and Nitrogen Concentration of Soybean (Glycine max L.)" Nitrogen 2, no. 3: 332-346. https://doi.org/10.3390/nitrogen2030023
APA StyleGatabazi, A., Vorster, B. J., Mvondo-She, M. A., Mangwende, E., Mangani, R., & Hassen, A. I. (2021). Efficacy of Peat and Liquid Inoculant Formulations of Bradyrhizobium japonicum Strain WB74 on Growth, Yield and Nitrogen Concentration of Soybean (Glycine max L.). Nitrogen, 2(3), 332-346. https://doi.org/10.3390/nitrogen2030023







