Abstract
The amino acid glutamine (Gln) is an important assimilatory intermediate between root-derived inorganic nitrogen (N) (i.e., ammonium) and downstream macromolecules, and is a central regulator in plant N physiology. The timing of Gln accumulation after N uptake by roots has been well characterized. However, the duration of availability of accumulated Gln at a sink tissue has not been well defined. Measuring Gln availability would require temporal measurements of both Gln accumulation and its reciprocal depletion. Furthermore, as Gln varies spatially within a tissue, whole-organ in situ visualization would be valuable. Here, the accumulation and subsequent disappearance of Gln in maize seedling leaves (Zea mays L.) was imaged in situ throughout the 48 h after N application to roots of N-deprived plants. Free Gln was imaged by placing leaves onto agar embedded with bacterial biosensor cells (GlnLux) that emit luminescence in the presence of leaf-derived Gln. Seedling leaves 1, 2, and 3 were imaged simultaneously to measure Gln availability across tissues that potentially vary in N sink strength. The results show that following root N fertilization, free Gln accumulates and then disappears with an availability period of up to 24 h following peak accumulation. The availability period of Gln was similar in all seedling leaves, but the amount of accumulation was leaf specific. As Gln is not only a metabolic intermediate, but also a signaling molecule, the potential importance of regulating its temporal availability within plant tissues is discussed.
Keywords:
glutamine; Zea mays; assimilation; nitrogen use efficiency; utilization; biosensor; regulation 1. Introduction
Nitrogen (N) is a limiting plant macronutrient, especially in non-leguminous crops such as maize (Zea mays L.). N is required for the synthesis of macromolecules including nucleic acids, amino acids and chlorophyll [1]. Plant roots uptake soil N, primarily nitrate (NO3−) and ammonium (NH4+), and then assimilate this inorganic N into the amino acid glutamine (Gln) beginning with conversion of nitrate to ammonium. Glutamate (Glu) is then used as a substrate by the glutamine synthetase/glutamine oxoglutarate aminotransferase (GS/GOGAT) cycle to assimilate ammonium, which can become the amide group of Gln [2,3]; this is considered a major route by which inorganic N is incorporated into organic N [4,5,6]. Although the synthesis of other amino acids primarily uses Glu or aspartate (Asp) as the direct substrate for aminotransferases, Gln is also used as a N donor for amino acid synthesis by enzymes such as asparagine synthetase, releasing Glu [2,3,7]. As a primary assimilatory product, Gln displays extremely sensitive and rapid responses to N application in maize tissues; however, because it is a donor of N for downstream metabolism and hence has a transitory role, free Gln forms only a fraction of the steady-state amino acid pool, reported to comprise 4–6% of the total amino acid content in leaves [8,9]. Therefore, the appearance and subsequent disappearance of Gln from plant tissue may serve as a useful biomarker for tracking the progression and rate of N uptake, assimilation and utilization. Although the rate and timing of appearance of assimilatory Gln following N fertilization have been well characterized in many plants grown in laboratory conditions, the rate of consumption and hence availability of free Gln in sink tissues is less defined despite its implications for N metabolism and regulation.
Previously [10], an Escherichia coli biosensor auxotrophic for Gln (named GlnLux) was engineered with a constitutive lux operon to emit luminescence upon exposure to external Gln. Advantages of GlnLux include a low cost and tissue requirement, and simplified tissue preparation steps compared to most biochemical approaches including high-performance liquid chromatography (HPLC). The biosensor cells could be embedded into agar (GlnLux agar), upon which leaves were placed, freeze-thawed to cause Gln leakage, and then incubated at 37 °C to activate the biosensor cells. Following root N fertilization, spatial luminescence-output from maize seedling leaves could then be imaged using a photon-capture camera, corresponding to free Gln tissue-localization [11]. However, in our previous study, there were few timepoints, resulting in low temporal resolution, and furthermore N was applied continuously for 24 h following N deprivation, corresponding to the entire duration of the experiment. As a result, while rapid assimilation to free Gln was observable following N fertilization, the rate of subsequent conversion of free Gln to downstream products could not be discerned.
Here, an in situ leaf imaging experiment using GlnLux agar was designed to characterize the timing of Gln availability in maize leaves in the 48 h following a pulse of root N fertilizer. All seedling leaves were imaged simultaneously to measure Gln availability across organs that potentially vary in N sink strength. Nine distinct timepoints were imaged allowing for high temporal resolution.
2. Materials and Methods
2.1. Maize Growth Conditions
Zea mays L. hybrid CG60 X CG102 seed [12] was used. Two independent trials were conducted in a greenhouse set to 28 °C/20 °C day/night (16 h/8 h), with 1000 W high pressure sodium and 1000 W metal halide lamps supplemented with GroLux bulbs (Osram Sylvania Inc., Wilmington, MA, USA). Average light intensity was 768–923 μmol·m−2·s−1 (Trial 1) and 749–896 μmol·m−2·s−1 (Trial 2) (canopy level at noon). Seeds were surface sterilized by soaking for 4 min in 70% ethanol, 2 min in 4% NaClO, and then washed five times in sterile double distilled (dd)H2O. Seeds were transferred to 18-cell (1 seed per cell, 8.5 × 8.5 × 9 cm) growth flats of Turface® (Profile Products, Buffalo Grove, IL, USA), a clay gravel containing only trace plant-available nitrogen (N) [13]. The flats were placed into sub-irrigation trays containing ddH2O with no nutrients. Stiff plastic webbing (L1020 injection molded web tray, Landmark Plastic, Akron, OH, USA) was placed in between the flats and sub-irrigation trays for easy transfer of the flats. Flats were watered as needed, with daily randomization.
Seedlings were grown for seven days, after which the remaining ddH2O was emptied. Flats were moved to different sub-irrigation trays containing modified N-free Hoagland’s nutrient solution (No. 2 Basal Salt Mixture HOP03-1LT, Caisson Labs, Smithfield, UT, USA) in which all other macro and micronutrients were provided as 2.86 mg·L−1 H3BO3, 554.90 mg·L−1 CaCl2, 0.045 mg·L−1 CuCl2, 33.00 mg·L−1 C10H12N2NaFeO8∙3H2O, 240.33 mg·L−1 MgSO4, 1.81 mg·L−1 MnCl2∙4H2O, 0.025 mg·L−1 Na2MoO4∙2H2O, 372.70 mg·L−1 KCl, 136.025 mg·L−1 KH2PO4, and 0.1 mg·L−1 ZnCl2. Seedlings remained in the solution for 24 h before sampling to prevent nutrient imbalance during N application.
2.2. Two Hour Nitrogen Pulse and Leaf Tissue Sampling
Growth flats served as the experimental units for repeated measurements, arranged as a randomized complete block design. The first leaf sampling was conducted just before applying +/−N treatments to the seedlings (time 0; the starting point). Leaves were harvested and placed in liquid N2. Leaf 1 (the first leaf to emerge) was entirely removed at the ligule. Leaves 2 and 3 had not fully developed, and only the completely unfurled segment was harvested. Four replicates of each leaf, from each N treatment, from individual plants grown in separate growth flats were stored at −80 °C until imaging. After the starting timepoint, +/−N treatment was performed by moving all flats to new sub-irrigation trays containing either the same N-free Hoagland’s solution described above (−N) or supplemented with 20 mM N (+N) provided as ammonium nitrate (NH4NO3).
Plant N uptake was permitted for 2 h. Maize leaves were sampled again, as described above, after 1 and 2 h of N uptake. Flats were then removed from the sub-irrigation trays, and a water hose was applied directly over the top of each flat for 5 min, followed by the bottom of each flat for 1 min to flush N present in the growth media. Previous experimental steps were staggered to account for the flushing period. Flats were placed into new sub-irrigation trays of N-free Hoagland’s solution for the remainder of the experiment. Leaf sampling was performed again at 4 h, 6 h, 12 h, 24 h, 36 h and 48 h after the initial transfer to the N-uptake treatments, again by sampling the three different leaves of four replicate plants from each N treatment harvested from separate growth flats. Two additional harvest timepoints were included in Trial 2 at 18 h and 30 h to increase the temporal resolution.
2.3. Generating Leaf In Situ Images of Free Glutamine
Direct in situ imaging was performed with GlnLux biosensor cells (Figure 1) as previously described [11]. Briefly, biosensor cells were incorporated into solid GlnLux agar plates (150 × 15 mm Petri dishes). Images of free glutamine (Gln) were generated by placing freeze-thawed leaves on the surface of the GlnLux agar. Plates were incubated at 37 °C for 2.5 h, and imaged with a photon capture camera (7383-0007, Princeton Instruments, Trenton, NJ, USA) pre-cooled to −100 °C with an exposure time of 1000 s. The methodology was validated in a previous publication to ensure that image variability between plates was not significant [11]. Raw image output was converted to false-colour heat maps with WinView software (version 2.5.16.5, Princeton Instruments).
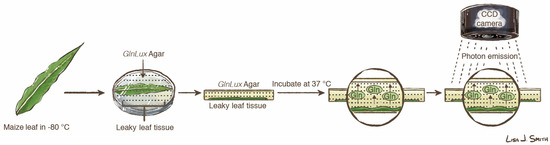
Figure 1.
Schematic image of the GlnLux in situ imaging assay, courtesy of Lisa Smith (University of Guelph). The dots shown in the Petri dish represent GlnLux biosensor cells embedded in the agar. The figure may be re-used under the Creative Commons BY license. CCD, charge-coupled device.
3. Results and Discussion
3.1. GlnLux Imaging Demonstrates the Availability Period of Assimilatory Gln
Roots of seedlings grown without N for 8 days were provided with a transitory 2 h pulse of N fertilizer (Figure 2A and Figure 3A). Immediately before the N fertilizer application, no differences in leaf GlnLux luminescence output were observed between the +/−N treated plants (0 h: Figure 2B and Figure 3B). In Trial 1, GlnLux output from plants treated with the +N solution increased until 12–24 h after the onset of the N pulse (Figure 2B). In Trial 2, additional 18 h and 30 h timepoints were examined (Figure 3B), but peak accumulation occurred in the same 12–24 h period. Pronounced differences between the N treatments persisted until 30 h (Figure 3B). Following accumulation, Gln depletion was of a similar rate, evident by 36 h and complete by 48 h in both Trials 1 and 2 (Figure 2B and Figure 3B). At the latter timepoint, no differences between the +/−N treatments were visible. The results suggest that the availability period of free Gln in maize seedling leaves is 24 h following peak accumulation.
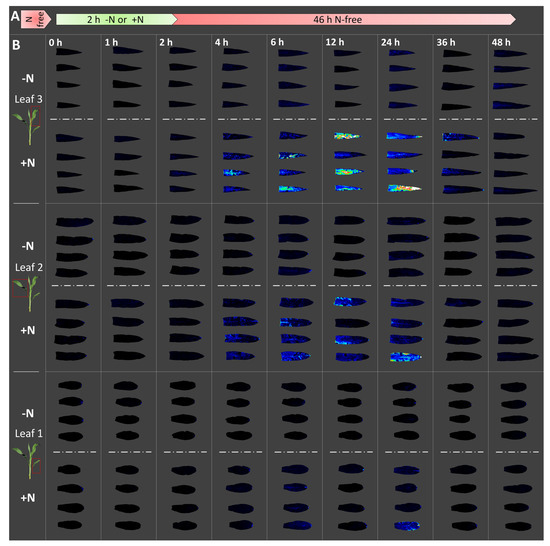
Figure 2.
Luminescence imaging of free Gln from maize seedling leaves: Trial 1 (July 2016). Maize plants were grown in N-free media from germination for 8 d. Plants were then transferred for 2 h to a nutrient solution containing either 0 mM (−N) or 20 mM (+N) total nitrogen provided as ammonium nitrate (A). Three different seedling leaves were harvested and imaged using GlnLux agar (B). Harvesting was performed just prior to application of the −/+N treatments, and twice during the treatments after 1 h and 2 h of N uptake. Following the 2 h N-uptake period, all plants were transferred to N-free nutrient solution. Leaves were again harvested after 4 h, 6 h, 12 h, 24 h, 36 h, and 48 h. Displayed are GlnLux false-colour images of four replicates of each leaf from both the –N (above dashed lines) and +N (below dashed lines) treatments at equivalent harvest timepoints, roughly corresponding to panel A. For display purposes, the three leaves from each plant are arranged along a single column, and the four biological replicates are shown in the same order within each box. The intensity of GlnLux output, from greatest to least, is white, red, yellow, green, and blue, with black indicating absence of GlnLux output. The reader is encouraged to magnify the images.
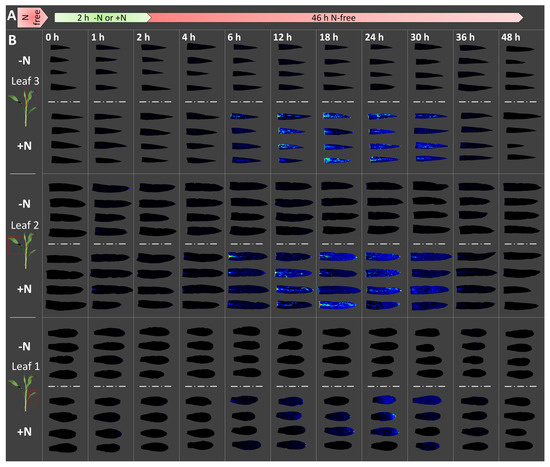
Figure 3.
Luminescence imaging of free Gln from maize seedling leaves: Trial 2 (December 2016). Maize plants were grown in N-free media from germination for 8 d. Plants were then transferred for 2 h to a nutrient solution containing either 0 mM (−N) or 20 mM (+N) total nitrogen provided as ammonium nitrate (A). Three different seedling leaves were harvested and imaged using GlnLux agar (B). Harvesting was performed just prior to application of the −/+N treatments, and twice during the treatments after 1 h and 2 h of N uptake. Following the 2 h N-uptake period, all plants were transferred to N-free nutrient solution. Leaves were again harvested after 4 h, 6 h, 12 h, 18 h, 24 h, 30 h, 36 h, and 48 h. Displayed are GlnLux false-colour images of four replicates of each leaf from both the –N (above dashed lines) and +N (below dashed lines) treatments at equivalent harvest timepoints, roughly corresponding to panel A. For display purposes, the three leaves from each plant are arranged along a single column, and the four biological replicates are shown in the same order within each box. The intensity of GlnLux output, from greatest to least, is white, red, yellow, green, and blue, with black indicating absence of GlnLux output. The reader is encouraged to magnify the images.
The results infer the timing by which free Gln is consumed by downstream reactions (e.g., conversion to other amino acids). Future experiments might be conducted in which the molecular regulation of enzymes such as asparagine synthetase and their products are described in a similar timecourse. For example, glutamate, asparagine, and aspartate are important downstream products of assimilatory Gln [7], and their quantification may provide a layer of data with which to annotate GlnLux images. Additionally, genetic mutants or chemical inhibitors [14,15] that block Gln conversion, or N15 labeling experiments [9] would provide confirmation that reduction of GlnLux output over time is the result of Gln conversion to downstream molecules. The exact timing of Gln disappearance may also be characterized with analytical chemistry methods [9], or an alternative GlnLux methodology which analyzes plant extracts for more quantitative results [11].
3.2. The Timing of Gln Accumulation and Depletion Occurs Symmetrically along the Leaf-Age Sink Gradient
GlnLux imaging was performed simultaneously in all leaves to detect differences in Gln accumulation and depletion among leaves with potential high N sink strength (youngest growing leaf 3) and low sink strength (leaf 1) [16,17]. There was not a leaf-specific effect in terms of the timing of GlnLux accumulation (all peaked at 12–24 h) and depletion (common disappearance by 48 h) but intensity differences were observed (Figure 2B and Figure 3B). Specifically, Gln accumulation was greatest in leaf 3 (youngest leaf), followed by leaf 2, then leaf 1 (oldest leaf) (Figure 2B and Figure 3B), likely to fulfill the extra nutrient demand required to support growth of the younger leaves [16,17,18,19]. GlnLux output persisted slightly longer in leaf 3 in Trial 1 than Trial 2 (Figure 2B) for an unknown reason, either biological or technical.
One interpretation of these results is that Gln shuttling to N sinks is regulated to maintain a metabolic balance between supporting the N requirement for growth in young tissue, with maintaining the ongoing metabolic needs of older leaves (e.g., photosynthesis) which were non-senescent at the assayed stage. These results are consistent with those of a previous publication in terms of the onset of timing of peak Gln accumulation across maize seedling leaves [11], but also demonstrate that the timing of Gln depletion and hence Gln availability is similar across the leaf-age sink gradient.
3.3. The Timing of Gln Availability May Be Specific to the Conditions Used
In general, these timing results should be interpreted cautiously. The exact timing of Gln accumulation/disappearance and hence availability may be specific to the conditions of this study. The results might be different with other developmental stages, genotypes, or environmental conditions. Previous experiments have also demonstrated that the tissue location of N assimilation (i.e., root vs. shoot) is specific to the inorganic soil N concentration [20,21,22] and source (i.e., ammonium vs. nitrate) [8,23,24]. In this study, GlnLux output across N treatments and timepoints was generally greater in Trial 1 (Figure 2B) compared to Trial 2 (Figure 3B), which were conducted in July and December 2016, respectively. Despite the controlled greenhouse environment, plants in July (summer) received higher light intensities, day lengths, and temperatures, compared to December (winter). N uptake is in part regulated by the rate of carbon fixation and photosynthesis, which ensures the adequate supply of carbon skeletons for assimilatory products [25,26]. These physiological responses to environmental variation may have contributed to observed differences between the trials.
Despite the variable nature of N metabolism, various studies have consistently shown that GS and/or Gln rapidly accumulate within minutes to hours after application of N following a period of N starvation [21,22,23,27,28], consistent with the in situ visualization presented here. It is worth noting that free Gln in maize leaves does not represent all of N absorbed by roots—for example, nitrate can also be transported to foliage for storage [29,30,31,32].
3.4. Implications of a Limited Gln-Availability Period in Maize Leaf Tissue
GlnLux imaging suggests that after inorganic N from soil is assimilated and transported in the shoot, a portion accumulates as free Gln in leaves. Following peak accumulation, free Gln remains up to 24 h before downstream utilization. Assimilatory processes can be influenced by different growing conditions, tissue ages, and genetic characteristics, as recently demonstrated and reviewed [11]. However, the literature has indicated 24 h as an important timepoint for a variety of plant species grown under different conditions. In an older study [8], leaf levels of nitrate and especially ammonia similarly began to plateau after 24 h following N fertilization in maize grown from seed for two weeks under N deprivation. The same study also showed that nitrate reductase activity peaked at the 24 h timepoint and then decreased [8]. The 24 h time-frame of Gln (and nitrate, ammonia) availability at a N sink may have implications for our understanding of N metabolism. It is well known that N uptake and assimilation genes, enzymes and metabolites such as Gln are under circadian control [25,26,33]. However, it may be that plants regulate the availability period of free Gln not because it is an intermediate to build other N-containing molecules—in this role, it may be advantageous for free Gln to persist locally. Rather, plants may regulate the persistence of Gln because it acts as a critical signaling molecule of the internal N status which can, for example, negatively regulate root N transporters [34,35]. An inverse correlation has been observed between the Gln concentration and nitrate transporter proteins in the roots of multiple species [28,36,37,38]. When Gln was administered to maize seedlings via tip-cut leaves, root N uptake was shown to decrease [37]. In addition to transporter regulation, increased Gln was shown to control at least 35 general stress response genes in rice [39,40]. Increased Gln has also been implicated in coordinating sulphate and N nutrition in barley [41], and to exert control over the C4 photosynthetic pathway in maize [42]. Therefore, the ~24 h period of Gln availability may be an important interval during which free Gln (or lack thereof) can affect a range of plant metabolic processes. In the future, GlnLux may be used to help probe the relationship between free Gln and these other metabolic processes, allowing metabolic/transcriptomic heatmaps to be overlaid with both temporal and spatial resolution.
4. Conclusions
Following root nitrogen fertilization, based on in situ imaging, the availability period of assimilatory Gln in maize seedling leaves was shown to be 24 h after peak accumulation. The temporal availability period of glutamine was similar in all seedling leaves, however the amount of Gln accumulation was leaf specific. As Gln is not only a metabolic intermediate, but also a signaling molecule, this temporal availability period may also have importance in the regulation of other plant processes.
Acknowledgments
We thank Dietmar Scholz and Sue Couling (University of Guelph) for assistance in maintaining the greenhouse experiments. We thank Elizabeth A. Lee (University of Guelph) for the gift of maize seed. T.L.G. was supported in part by scholarships from the University of Guelph, and an Alexander Graham Bell Canada Graduate Scholarship award from the Natural Sciences and Engineering Research Council of Canada (NSERC). Grants were awarded to M.N.R. from the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA), Grain Farmers of Ontario and the NSERC CRD Program.
Author Contributions
T.L.G. and M.N.R. conceived and designed the experiments; T.L.G. performed the experiments, analyzed the data, and wrote the paper. M.N.R. edited the paper.
Conflicts of Interest
A US patent has been issued on the GlnLux biosensor technology (US 61/499286) and a conflict of interest may be present, however the technology is not currently commercialized.
References
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.M.; Habash, D. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Lea, P.J. The pathway of nitrogen assimilation in plants. Phytochemistry 1976, 15, 873–885. [Google Scholar] [CrossRef]
- Joy, K.W. Ammonia, glutamine, and asparagine: A carbon-nitrogen interface. Can. J. Bot. 1988, 66, 2103–2109. [Google Scholar] [CrossRef]
- Cren, M.; Hirel, B. Glutamine synthetase in higher plants: Regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol. 1999, 40, 1187–1193. [Google Scholar] [CrossRef]
- Lam, H.M.; Coschigano, K.T.; Oliveira, I.C.; Melo-Oliveira, R.; Coruzzi, G.M. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 569–593. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, B.; Sugiyama, T. Effects of nitrate and ammonium on gene expression of phosphoenolpyruvate carboxylase and nitrogen metabolism in maize leaf tissue during recovery from nitrogen stress. Plant Physiol. 1992, 98, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Cañas, R.A.; Yesbergenova-Cuny, Z.; Simons, M.; Chardon, F.; Armengaud, P.; Quilleré, I.; Cukier, C.; Gibon, Y.; Limami, A.M.; Nicolas, S.; et al. Exploiting the genetic diversity of maize using a combined metabolomic, enzyme activity profiling, and metabolic modeling approach to link leaf physiology to kernel yield. Plant Cell 2017, 29, 919–943. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, M.J.; Soliman, S.S.M.; Raizada, M.N. Bacterial whole-cell biosensor for glutamine with applications for quantifying and visualizing glutamine in plants. Appl. Environ. Microbiol. 2012, 78, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Goron, T.L.; Raizada, M.N. Biosensor-based spatial and developmental mapping of maize leaf glutamine at vein-level resolution in response to different nitrogen rates and uptake/assimilation durations. BMC Plant Biol. 2016, 16, 230. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.; Earl, H.; Lee, E.A.; Lukens, L. The genetic architecture of flowering time and related traits in two early flowering maize lines. Crop Sci. 2011, 51, 146–156. [Google Scholar] [CrossRef]
- Goron, T.L.; Bhosekar, V.K.; Shearer, C.R.; Watts, S.; Raizada, M.N. Whole plant acclimation responses by finger millet to low nitrogen stress. Front. Plant Sci. 2015, 6, 652. [Google Scholar] [CrossRef] [PubMed]
- Monselise, E.B.I.; Kost, D. 15N NMR spectroscopic study of ammonium ion assimilation by Spirodela oligorrhiza—Lemnaceae—As affected by light and carbon supply in green and extoliated plants. Israel J. Plant Sci. 1998, 46, 255–264. [Google Scholar] [CrossRef]
- Oaks, A.; Sivasankar, S.; Goodfellow, V.J. The specificity of methionine sulfoximine and azaserine inhibition in plant tissues. Phytochemistry 1998, 49, 355–357. [Google Scholar] [CrossRef]
- Meiri, A.; Silk, W.K.; Lauchli, A. Growth and deposition of inorganic nutrient elements in developing leaves of Zea mays L. Plant Physiol. 1992, 99, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Poethig, R.S. Phase change and the regulation of developmental timing in plants. Science 2003, 301, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Neves-Piestun, B.G.; Bernstein, N. Salinity-induced changes in the nutritional status of expanding cells may impact leaf growth inhibition in maize. Funct. Plant Biol. 2005, 32, 141–152. [Google Scholar] [CrossRef]
- Hu, Y.; Burucs, Z.; von Tucher, S.; Schmidhalter, U. Short-term effects of drought and salinity on mineral nutrient distribution along growing leaves of maize seedlings. Environ. Exp. Bot. 2007, 60, 268–275. [Google Scholar] [CrossRef]
- Andrews, M. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ. 1986, 9, 511–519. [Google Scholar]
- Sakakibara, H.; Kawabata, S.; Takahashi, H.; Hase, T.; Sugiyama, T. Molecular cloning of the family of glutamine synthetase genes from maize: Expression of genes for glutamine synthetase and ferredoxin-dependent glutamate synthase in photosynthetic and non-photosynthetic tissues. Plant Cell Physiol. 1992, 33, 49–58. [Google Scholar]
- Redinbaugh, M.G.; Campbell, W.H. Glutamine synthetase and ferredoxin-dependant glutamate synthase expression in the maize (Zea mays) root primary response to nitrate. Plant Physiol. 1993, 101, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, R.; Li, M.; Snustad, D.P. Root- and shoot-specific responses of individual glutamine synthetase genes of maize to nitrate and ammonium. Plant Mol. Biol. 1994, 26, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Okumoto, S.; Zhang, X.; Ervin, E. Circadian patterns of the major nitrogen metabolism-related enzymes and metabolites in creeping bentgrass and the influence of cytokinin and nitrate. Crop Sci. 2011, 51, 2145–2154. [Google Scholar] [CrossRef]
- Magalhães, J.R.; Ju, G.C.; Rich, P.J.; Rhodes, D. Kinetics of 15NH4+ Assimilation in Zea mays. Plant Physiol. 1990, 94, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, J.J.; Zhuo, D.; Siddiqi, M.Y.; Schjoerring, J.K.; Touraine, B.; Glass, A.D.M. Regulation of high-affinity nitrate transporter genes and high-affinity nitrate influx by nitrogen poolis in roots of barley. Plant Physiol. 2000, 123, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ivanko, S.; Ingversen, J. Investigation on the assimilation of nitrogen by maize roots and the transport of some major nitrogen compounds by xylem sap. III. Transport of nitrogen compounds by xylem sap. Physiol. Plant 1971, 24, 355–363. [Google Scholar] [CrossRef]
- Millard, P. The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ. 1988, 11, 1–8. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Brien, A.O.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutie, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. A natural light/dark cycle regulation of carbon-nitrogen metabolism and gene expression in rice shoots. Front. Plant Sci. 2016, 7, 1318. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.M.; Zhou, L. Carbon and nitrogen sensing and signaling in plants: Emerging “matrix effects”. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef]
- Touraine, B.; Daniel-Vedele, F.; Forde, B.G. Nitrate uptake and its regulation. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–36. [Google Scholar]
- Zhuo, D.; Okamoto, M.; Vidmar, J.J.; Glass, A.D.M. Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J. 1999, 17, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Pal’ove-Balang, P.; Mistrik, I. Control of nitrate uptake by phloem-translocated glutamine in Zea mays L. seedlings. Plant Biol. 2002, 4, 440–445. [Google Scholar] [CrossRef]
- Nazoa, P.; Vidmar, J.J.; Tranbarger, T.J.; Mouline, K.; Damiani, I.; Tillard, P.; Zhuo, D.; Glass, A.D.M.; Touraine, B. Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: Responses to nitrate, amino acids and developmental stage. Plant Mol. Biol. 2003, 52, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Chung, T.; Juo, Y.; Hsieh, M. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Chung, T.; Hsieh, M. Gene expression profiling of rice seedlings in response to glutamine treatment. Genom. Data 2015, 6, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Karmoker, J.L.; Clarkson, D.T.; Saker, L.R.; Rooney, J.M.; Purves, J.V. Sulphate deprivation depresses the transport of nitrogen to the xylem and the hydraulic conductivity of barley (Hordeum vulgare L.) roots. Planta 1991, 185, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, B.; Suzuki, I.; Burnell, J.N.; Sugiyama, T. Glutamine induces the N-dependent accumulation of mRNAs encoding phosphoenolpyruvate carboxylase and carbonic anhydrase in detached maize leaf tissue. Plant Physiol. 1992, 100, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).