The Effect of Organic Acid Modification on the Pore Structure and Fractal Features of 1/3 Coking Coal
Abstract
1. Introduction
2. Experimental Methods
2.1. Collection and Preparation of Coal Samples
- (1)
- Collection of Coal Samples
- (2)
- Preparation of Coal Samples
2.2. Acidification and Modification of Coal Samples
- (1)
- Acid Solutions
- (2)
- Acidification Treatment of Coal Samples
- (1)
- Weighing Coal Powder: Using a precision balance, weigh approximately 30 g of coal sample and place it into a sealed container. Label the container for identification.
- (2)
- Preparation of Acid Solutions: Prepare acid solutions according to the concentration requirements listed in Table 1.
- (3)
- Mixing Acid Solution with Coal Powder: Pour 300 mL of the prepared acid solution into the corresponding sealed container. Thoroughly mix the acid solution with the coal sample and allow the mixture to react for 48 h.
- (4)
- Filtration and Drying: Filter the coal sample and rinse it thoroughly with clean water to remove any residual acidic substances. Place the sample in a drying oven set at 35 °C for drying. Once dried, store the sample in a sealed bag, label it, and preserve it for further use.
2.3. Experiment
2.3.1. Low-Temperature N2 Adsorption
2.3.2. FTIR Measurements
2.3.3. SEM Experiment
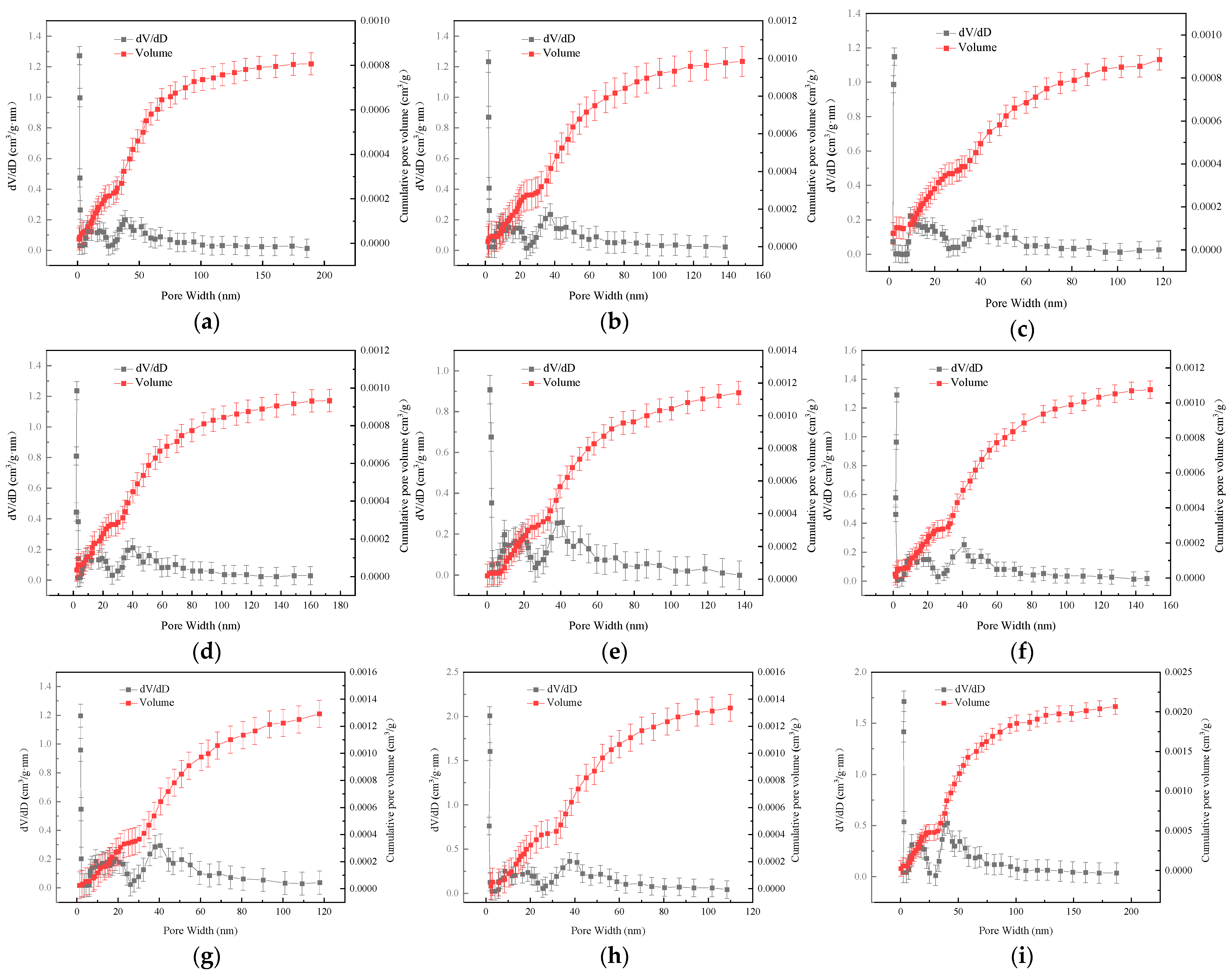
3. Pore Structure
3.1. Characterization Methods for Coal Pores
3.2. Examination of Coal Sample Pore Configuration Characteristics
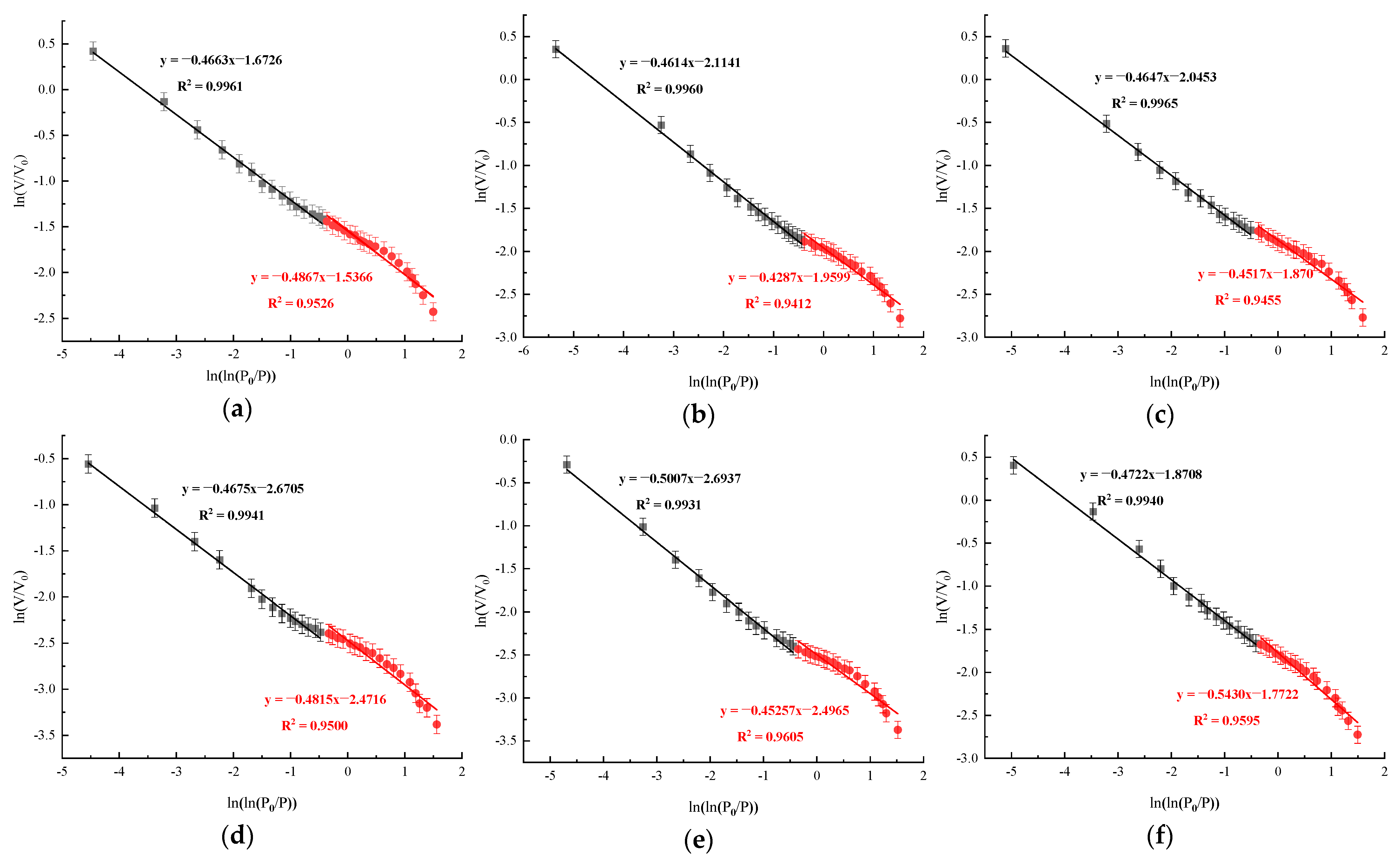
4. Fractal Characteristics of Coal Sample Pores
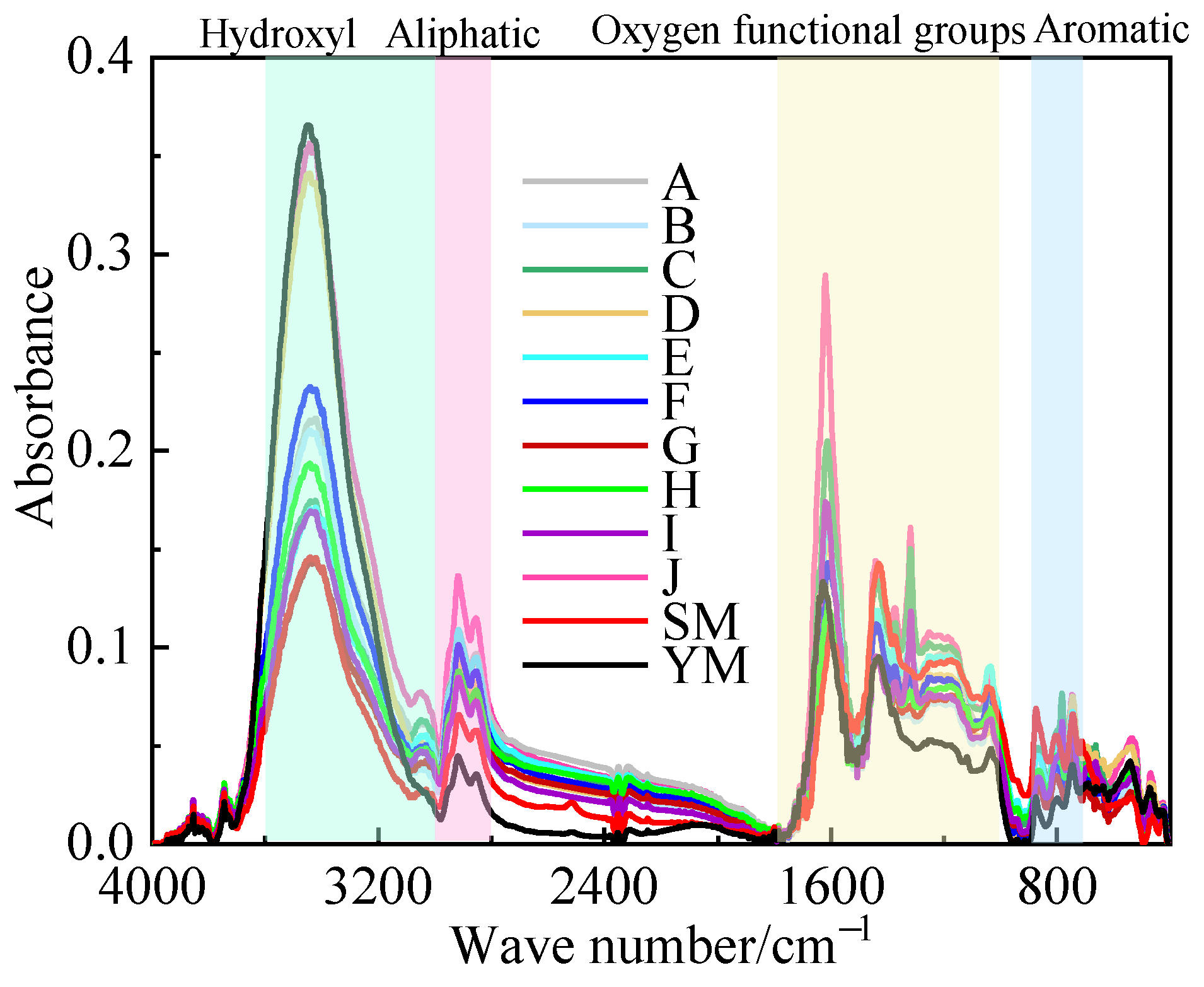
5. FTIR Functional Group Analysis
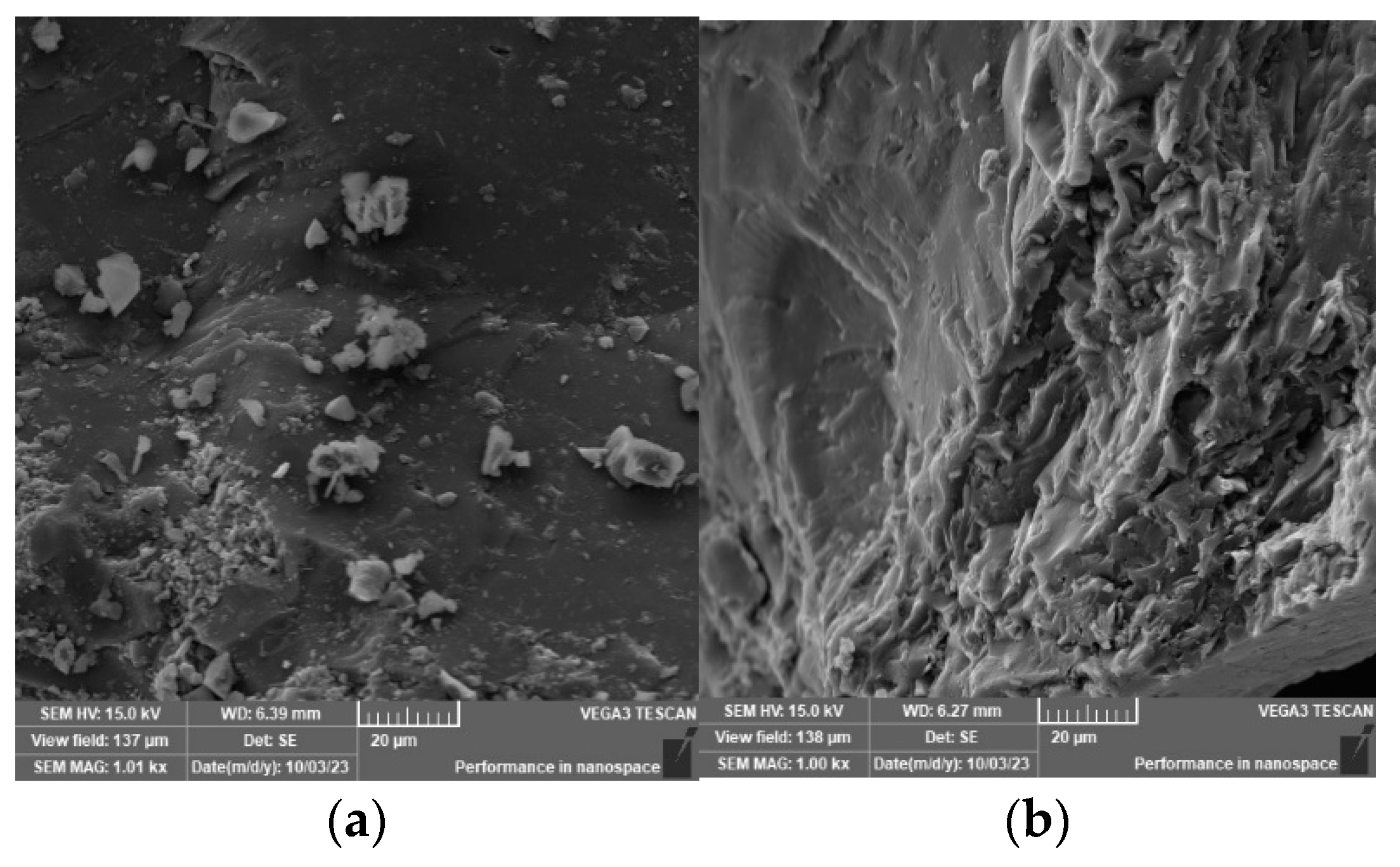
6. SEM Test Results and Analysis
7. Conclusions
- (1)
- The modification treatment with organic acid solution changed the pore structure of the coal samples, resulting in a decrease in the specific surface area, a decrease in the percentage of microporous pore volume and an increase in the percentage of mesoporous pore volume. The pore distribution was optimized and the contribution of transition pores was enhanced, which significantly improved the extraction efficiency of coalbed methane.
- (2)
- The surface fractal dimension of the coal samples increased and the spatial fractal dimension decreased after the organic acidification modification treatment, indicating that the organic acidic liquid can simplify the pore structure of coal and improve pore connectivity, thus enhancing the transport capacity of coalbed methane.
- (3)
- The chemical action of the organic acid solution of the coal sample results in a reduction in hydroxyl and oxygen-containing functional groups, which reduces the number of adsorption sites on the surface of the coal, thus weakening the adsorption capacity of methane and making it more susceptible to desorption.
- (4)
- Through comparative analysis, the improvement effects of different organic acids on the pores of coal samples were significantly different. Among them, oxalic acid and citric acid have better modification effects than hydrochloric acid, while citric acid has the best performance, which can optimize the pore structure and enhance the efficiency of coalbed methane extraction more effectively. Therefore, it is recommended to give priority to citric acid, followed by oxalic acid, in the acidification modification of coal reservoirs in order to obtain a more significant effect of production increase.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, L.; Jiang, Y.; He, X.; Dou, L.; Zhao, K.; Zhao, X.; Wang, K.; Yu, Q.; Lu, X.; Li, H. Research progress in key technologies for accurate identification, monitoring and early warning of typical dynamic disaster risk in coal mines. J. China Coal Soc. 2018, 43, 306–318. [Google Scholar] [CrossRef]
- Meng, Z.; Lu, Y. Experimental study on stress-permeability of high rank coal samples before and after hydraulic fracturing. Coal Sci. Technol. 2023, 51, 353–360. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Zhao, Y.; Liao, X.; Li, J.; Zhao, Z.; Liu, Q. Multi-scale pore structure transformation of shale under mixed acid acidification method. Arab. J. Chem. 2023, 16, 104937. [Google Scholar] [CrossRef]
- Zheng, C.; Liao, F.; Xue, S.; Jiang, B.; Gong, X.; Han, B.; Chen, Z. Experimental investigation on pores and permeability variation characteristics of coal treated by chemical solvents. Geoenergy Sci. Eng. 2023, 223, 211514. [Google Scholar] [CrossRef]
- Pi, Z.; Dong, Z.; Li, R.; Wang, Y.; Li, G.; Zhang, Y.; Peng, B.; Meng, L.; Fu, S.; Yin, G. Low-Field NMR Experimental Study on the Effect of Confining Pressure on the Porous Structure and Connectivity of High-Rank Coal. ACS Omega 2022, 7, 14283–14290. [Google Scholar] [CrossRef]
- Wang, N.; Li, S. Study on coal mechanical damage law and anti-reflection effects under fracturing fluid corrosion affecting. Coal Sci. Technol. 2022, 50, 150–156. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, B.; Yang, W.; Li, H.; Lin, M. Fracture and pore development law of coal under organic solvent erosion. Fuel 2022, 307, 121815. [Google Scholar] [CrossRef]
- Wang, P.; Liu, R.; Tang, M.; Zhang, W.; Gui, Z. Experimental study on atomization characteristics and dust suppression efficiency of high-pressure spray in underground coal mine. J. China Coal Soc. 2015, 40, 24–30. [Google Scholar] [CrossRef]
- An, W.; Wang, L. Mechanical properties and modification of coal under the action of surfactant. J. China Coal Soc. 2020, 45, 74–86. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, P.; Liu, R.; Pei, Y.; Wu, G. An experimental study on wetting of coal dust by surfactant solution. Adv. Mater. Sci. Eng. 2020, 2020, 1567461. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Yue, J. Study on wettability of coal by different surfactants. Saf. Coal Mines 2019, 50, 22–25. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Z.; Li, R.; Liu, X.; Wang, H.; Gong, S. Effects of acid-based fracturing fluids with variable hydrochloric acid contents on the microstructure of bituminous coal: An experimental study. Energy 2022, 244, 122621. [Google Scholar] [CrossRef]
- Wang, K.; Ding, C.; Jiang, S.; Zhengyan, W.; Shao, H.; Zhang, W. Application of the addition of ionic liquids using a complex wetting agent to enhance dust control efficiency during coal mining. Process. Saf. Environ. Prot. 2019, 122, 13–22. [Google Scholar] [CrossRef]
- Ni, G.; Li, S.; Rahman, S.; Xun, M.; Wang, H.; Xu, Y.; Xie, H. Effect of nitric acid on the pore structure and fractal characteristics of coal based on the low-temperature nitrogen adsorption method. Powder Technol. 2020, 367, 506–516. [Google Scholar] [CrossRef]
- Balucan, R.D.; Turner, L.G.; Steel, K.M. Acid-induced mineral alteration and its influence on the permeability and compressibility of coal. J. Nat. Gas Sci. Eng. 2016, 33, 973–987. [Google Scholar] [CrossRef]
- Liu, P.; Li, Z.; Zhang, X.; Li, J.; Miao, G.; Cao, S.; Li, S. Study on the inhibition effect of citric acid on coal spontaneous combustion. Fuel 2022, 310, 122268. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Zheng, Q. Highly selective separation of vanadium over iron from stone coal by oxalic acid leaching. J. Ind. Eng. Chem. 2017, 45, 241–247. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, H.; Cheng, W.; Cui, W.; Chen, Y. Porosity and fracture changes of bituminous coal under action of dry–wet cycles in acid environment. Energy Fuels 2024, 38, 3792–3803. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Y.; Li, S.; Zhang, Y.; Zhang, Q.; Xu, C.; Sun, B.; Liu, R. Effects of SDBS and BS-12 on functional groups/wettability of multicomponent acidified lignite. J. Mol. Liq. 2023, 390, 122908. [Google Scholar] [CrossRef]
- Banerjee, R.; Mohanty, A.; Chakravarty, S.; Chakladar, S.; Biswas, P. A single-step process to leach out rare earth elements from coal ash using organic carboxylic acids. Hydrometallurgy 2021, 201, 105575. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Shao, Y. Effect of demineralization on Yimin lignite by experiments and molecular simulation techniques. J. Mol. Struct. 2022, 1269, 133837. [Google Scholar] [CrossRef]
- Xue, S.; Huang, Q.; Wang, G.; Bing, W.; Li, J. Experimental study of the influence of water-based fracturing fluids on the pore structure of coal. J. Nat. Gas Sci. Eng. 2021, 88, 103863. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Zhang, D.; Zou, D.; Du, J.; Wang, Z.; Li, C. Study on pore and fissure structure characteristics of deep soft coal rock. Geofluids 2021, 2021, 1475926. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, B. Main occurrence form of methane in coal: Micropore filling. J. China Coal Soc. 2021, 46, 2933–2948. [Google Scholar] [CrossRef]
- Jin, K.; Cheng, Y.; Liu, Q.; Zhao, W.; Wang, L.; Wang, F.; Wu, D. Experimental Investigation of Pore Structure Damage in Pulverized Coal: Implications for Methane Adsorption and Diffusion Characteristics. Energy Fuels 2016, 30, 10383–10395. [Google Scholar] [CrossRef]
- Liu, K.Q.; Ostadhassan, M.; Kong, L.Y. Multifractal characteristics of Longmaxi Shale pore structures by N2 adsorption: A model comparison. J. Pet. Sci. Eng. 2018, 168, 330–341. [Google Scholar] [CrossRef]
- Ji, B.; Tao, X.; Ji, Y. Barrier Option Pricing in the Sub-Mixed Fractional Brownian Motion with Jump Environment. Fractal Fract. 2022, 6, 244. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Wang, G.; Wang, E.; Ju, S.; Qin, C. Experimental study of effect of slickwater fracturing on coal pore structure and methane adsorption. Energy 2022, 239, 122421. [Google Scholar] [CrossRef]
- Yi, M.; Cheng, Y.; Wang, C.; Wang, Z.; Hu, B.; He, X. Effects of composition changes of coal treated with hydrochloric acid on pore structure and fractal characteristics. Fuel 2021, 294, 120506. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Y.; Zhang, D.; Liu, Z.; Wang, Z.; Jiang, Z. Influence of tectonic evolution on pore structure and fractal characteristics of coal by low pressure gas adsorption. J. Nat. Gas. Sci. Eng. 2021, 87, 103788. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, F.; Ma, L.; Jiang, P.; Li, B.; Ren, J.; Lv, R.; Liu, G.; Song, Z.; Chang, P.; et al. Fractal Characterization of a Multi-Scale Pore Structure in Ultra-Deep Coal Seams. Fractal Fract. 2025, 9, 250. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Liu, J.; Wang, Y.; Guo, L.; Wu, Z.; Tian, Y. A Fractal Characteristics Analysis of the Pore Throat Structure in Low-Permeability Sandstone Reservoirs: A Case Study of the Yanchang Formation, Southeast Ordos Basin. Fractal Fract. 2025, 9, 224. [Google Scholar] [CrossRef]
- Zheng, C.; Li, J.; Xue, S.; Jiang, B.; Liu, B. Experimental study on changes in components and pore characteristics of acidified coal treated by organic solvents. Fuel 2023, 353, 129215. [Google Scholar] [CrossRef]




| Acid Solution | Solution Concentration (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YM | SM | A | B | C | D | E | F | G | H | I | J | |
| HA | / | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AA | / | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| GA | / | 0 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 |
| OA | / | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 |
| CA | / | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 5 |
| Samples | Average Pore Size/nm | BET Specific Surface Area/(m2/g) | BJH Pore Volume /(104 cm3/g) | Proportion of Pore Volume in Different Pore Size Ranges/% | ||
|---|---|---|---|---|---|---|
| Micropores | Transitional Pores | Mesopores | ||||
| YM | 14.3509 | 0.4415 | 16.5024 | 14.62 | 79.87 | 5.51 |
| SM | 15.0254 | 0.4045 | 20.8896 | 12.77 | 79.54 | 7.69 |
| A | 18.6597 | 0.3913 | 20.0790 | 10.67 | 81.10 | 8.23 |
| B | 19.6766 | 0.3858 | 21.9271 | 10.45 | 80.98 | 8.57 |
| C | 17.9554 | 0.4086 | 21.5613 | 12.02 | 80.13 | 7.86 |
| D | 17.0052 | 0.3991 | 23.8201 | 11.70 | 80.21 | 8.09 |
| E | 18.4808 | 0.3902 | 20.3019 | 10.71 | 80.76 | 8.53 |
| F | 19.7517 | 0.3854 | 19.4417 | 10.36 | 79.74 | 9.90 |
| G | 21.3328 | 0.2939 | 15.6756 | 8.83 | 80.21 | 10.96 |
| H | 24.2460 | 0.2327 | 15.9081 | 7.06 | 79.49 | 13.45 |
| I | 22.4552 | 0.2492 | 10.7666 | 6.89 | 81.99 | 11.12 |
| J | 23.7204 | 0.2654 | 13.6148 | 6.53 | 79.56 | 13.91 |
| Samples | = 0–0.5 | = 0.5–1 | ||
|---|---|---|---|---|
| 1 | R2 | 2 | R2 | |
| YM | 2.4473 | 0.9961 | 2.6335 | 0.9526 |
| SM | 2.4466 | 0.9960 | 2.6296 | 0.9412 |
| A | 2.6697 | 0.9965 | 2.4460 | 0.9455 |
| B | 2.6593 | 0.9941 | 2.4426 | 0.9500 |
| C | 2.6277 | 0.9931 | 2.4381 | 0.9605 |
| D | 2.6698 | 0.9940 | 2.4096 | 0.9595 |
| E | 2.6546 | 0.9944 | 2.4415 | 0.9587 |
| F | 2.6688 | 0.9973 | 2.4177 | 0.9592 |
| G | 2.6885 | 0.9950 | 2.4410 | 0.9763 |
| H | 2.7915 | 0.9961 | 2.3459 | 0.9912 |
| I | 2.7656 | 0.9949 | 2.4271 | 0.9960 |
| J | 2.8031 | 0.9963 | 2.3712 | 0.9675 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Cai, F. The Effect of Organic Acid Modification on the Pore Structure and Fractal Features of 1/3 Coking Coal. Fractal Fract. 2025, 9, 283. https://doi.org/10.3390/fractalfract9050283
Fan J, Cai F. The Effect of Organic Acid Modification on the Pore Structure and Fractal Features of 1/3 Coking Coal. Fractal and Fractional. 2025; 9(5):283. https://doi.org/10.3390/fractalfract9050283
Chicago/Turabian StyleFan, Jiafeng, and Feng Cai. 2025. "The Effect of Organic Acid Modification on the Pore Structure and Fractal Features of 1/3 Coking Coal" Fractal and Fractional 9, no. 5: 283. https://doi.org/10.3390/fractalfract9050283
APA StyleFan, J., & Cai, F. (2025). The Effect of Organic Acid Modification on the Pore Structure and Fractal Features of 1/3 Coking Coal. Fractal and Fractional, 9(5), 283. https://doi.org/10.3390/fractalfract9050283






