1. Introduction
Compartmental models for infectious diseases separate a population into various classes according to the stages of infection. The reflected rates of transition between compartments are stated by time derivatives of the population sizes in each compartment, so such models are formulated by differential equations. Mathematical models in which the rates of transfer between compartments depend on the sizes of compartments in the past or at the moment of transfer require working with differential, integral, or integro-differential equations.
The early and foundational work on compartmental models in mathematical epidemiology is based on Kermack and McKendrick [
1]. In 1927, they introduced a compartmental epidemic model based on basic transfers between divided groups in a population. To explain the compartments, they divided the population into three groups, designated
S,
I, and
R, to describe the compartments.
denotes the number of individuals who are susceptible to the disease at time
t, in other words, those who are not yet infected and do not have any immunity.
represents the number of infectious individuals, that is, members of
can spread the disease to susceptible individuals with effective contact.
shows the number of recovered individuals, who have immunity against the pathogen and there thus is no probability of spread of infection via these individuals.
Based on the model given by Kermack and McKendrick, mathematical epidemiology has been developed with numerous studies providing various contributions to the literature in this field (e.g., [
2,
3,
4]).
Vaccination of susceptible individuals against infection is one of the effective control measures in the process related to the struggle with diseases. So far, numerous studies have been introduced explaining the effects of vaccination on the spread of diseases (see [
5,
6,
7,
8] and references therein).
Fractional order systems are modeled with fractional order differential equations containing derivatives of non-integer order. These systems make significant contributions to the study of the behavior of dynamic systems in many fields such as physics, electrochemistry, biology, engineering, mechanics, economics, mathematical epidemiology, and ecology.
The reason for choosing to work with these systems is that fractional order derivatives provide a better fit to real data in these application areas and overcome the limitations of classical integer order derivatives.
In this context, in recent times, studies on the necessity of using fractional order equations, including fractional order derivatives or integrals in order for model problems to better reflect the events in our daily lives and to establish more realistic models, have increased considerably [
9,
10,
11,
12].
For dynamical systems that reflect situations where memory effects are important, fractional order equations are more suitable than integer order equations. Due to the memory effect, the non-integer models integrate all previous information from the past that makes them able to predict and translate the epidemic models more accurately. For this reason, fractional order models are more reliable and helpful in real phenomena than the classical models [
10]. To summarize, the fractional epidemic models are the generalizations of the integer-order models and provide useful information any time desired. Additionally, in the real-world explanation, the integer order derivative does not explore the dynamics between two different points. For example, there are many TB models in the literature that are based on ordinary (or delay) integer-order derivative. However, such models have some limitations, as they do not provide any information about the memory and learning mechanism. To deal with such failures of classical local differentiation, different concepts on differentiation with non-local or fractional orders have been developed in the existing literature. Thus, working with fractional equations offers a more realistic pathway to the epidemic models. Some authors have extended classical epidemic models to fractional-order epidemic systems and discussed the stability of equilibrium [
9,
13,
14].
In recent years, some fractional-order differential equation models of infectious disease dynamics have been introduced with the Caputo derivation. In 2018, Saif Ullah et al. [
10] proposed a fractional Caputo model of TB infection and validated it with the real data of TB incidence cases from 2002 to 2017 in the Khyber Pakhtunkhwa province of Pakistan. The authors then showed through numerical simulations that the proposed fractional model provides a better fit to real data than classical models.
Alqahtani et al. [
15] introduced a Caputo Fractional Chlamydia pandemic model and investigated the stability analysis of the
-fractional order model along with the sensitivity analysis. Their simulations showed that memory effects, represented by the fractional order (
), significantly impact disease spread, with memory-driven acceleration increasing transmission potential.
El Hajji and Sayari [
16] have investigated a
model of infectious disease transmission in a chemostat and study on the model’s local and global stabilities with a profound analysis.
Özdemir and colleagues [
11] studied a
model under the influence of vaccination for an infectious disease by generalizing it with the Caputo fractional derivative. They focused on the effect of fractional parameter and noted that the increasing effect of vaccination was observed at decreasing values of the fractional order.
Gökbulut et al. [
12] proposed a fractional order
model for studying the COVID-19 pandemic and used Caputo fractional derivatives with the purpose of observing a memory effect.
This paper reveals and analyzes a novel epidemic model, considering the vaccination effect on the spread of any disease. As is known, the immunity obtained through vaccination may not be permanent, and the immunization periods of vaccinated individuals may not be the same. Thus, even if they were vaccinated at the same time, while the immunity of some of the individuals may continue, the others may lose their immunity and become susceptible to the epidemic. This situation is particularly relevant for diseases such as pertussis or measles, where vaccine effectiveness may decrease over time. In this study, prepared by taking all these facts into consideration, a mathematical epidemic model reflecting that the protection period provided by the vaccine effect may vary from person to person, is presented. This novel fractional epidemic model is formed by aid of a system of distributed delay nonlinear fractional integro-differential equations. Unlike conventional models, which assume a uniform rate of immunity loss, this view taken into consideration in the proposed model accounts for individual heterogeneity in immune response, thereby offering a more detailed and realistic portrayal of population dynamics.
This study introduces significant innovation by integrating fractional calculus to enhance the explanatory capacity of the model. This approach allows for modeling non-local and memory effects often observed in immunological responses. Additionally, the study employs a distributed delay approach, which allows for a more realistic representation of immunity loss by considering the variability in how long individuals retain immunity post-vaccination, increasing the applicability of the models to real-world vaccination strategies. Furthermore, the expansion to include fractional-order derivatives of the classical and models provides greater flexibility in defining immunity loss, allowing for a finer-grained control over the rate of immunity loss and capturing subtle and non-linear dynamics often missed by classical models.
For model analysis, first, disease-free and endemic equilibrium points were determined. Then the basic reproduction number related to the model was obtained by using the next generation matrix method. It is also shown that the disease-free and the endemic equilibrium are locally and globally asymptotically stable for and , respectively.
Local stabilities of equilibrium points have been researched by analyzing the corresponding characteristic equation. To prove global stabilities of equilibrium, the LaSalle Invariance Principle, associated with the Lyapunov function and Dulac Criteria, respectively, has been used.
Lastly, an assessment regarding the minimum vaccination ratio of new members required for the elimination of the disease in the population has been carried out. After providing examples for the selection of the distribution function, reflecting that the protection period provided by the vaccine effect, which may vary from person to person, the variation of has been simulated for a specific selection of parameters in the model. Finally, the sensitivity indices of the parameters affecting have been calculated, and this situation has also been visually supported.
2. Some Basic Mathematical Properties and Model Structure
The definitions of the fractional integral and Caputo fractional derivative are defined as follows [
17].
Definition 1. Suppose that and x is an integrable function. Then the fractional integral of x of order α is defined as The Caputo fractional derivative of
x of order
is defined as
where
. When
is an integer number,
is a usual derivative of order
of
If
x is a function of class
its fractional derivative is represented by
Specifically, we write
instead of
It is obvious that the Caputo fractional derivative of order
of
x is
for
On the other hand, let us recall some properties of Laplace transforms. The Laplace transform of the Caputo fractional derivative [
9] is given by
Additionally, the Laplace transform of the Mittag–Leffler function defined by the power series
holds the following equality [
9]:
Theorem 1. The equilibrium solutions of the Caputo fractional differential equations system areThe Jacobian matrix evaluated at equilibrium points ensures local asymptotic stability if its eigenvalues satisfy [15,18] Theorem 2. Let be an equilibrium point for , and let Ω be a domain containing Let be a continuously differentiable function such thathold for all , and where functions are continuous positive definite functions on Then the equilibrium point of system is uniformly asymptotically stable [13,14]. Model Structure
We introduce a new fractional
mathematical compartmental model expressed by the system of the following nonlinear fractional integro-differential equations, with all parameters being positive:
where
and functions
,
,
, and
represent the numbers of the susceptible, vaccinated, infectious, and recovered individuals at time
t, respectively. The total population size is
and
for all
. Additionally, all these functions are non-negative.
In the model, all newborn individuals become involved in the population by entering to S at a constant rate . denotes the effective contact rate between infectious and susceptible or vaccinated individuals. is the natural death rate in each compartment, and is the death rate welded from the outbreak. The rate of vaccinated individuals within susceptible individuals is represented by q. Additionally, denotes the transition rate from the infectious compartment to compartment R.
Vaccination may not always be completely effective for individuals. Therefore, in this model, we suppose that the vaccine provides a temporary immunity effect or a permanent effect for some individuals. In this context, we use the distribution parameter to mean the protection period caused by vaccination. Essentially, the term designates the protection efficacy of the vaccine. That is, means that the vaccine is completely ineffective. Additionally, means that the vaccinated individuals have only partial protection. Otherwise, it means that the vaccine is wholly effective against the pathogen.
On the other hand, we assume that the protection provided by the vaccination may vary according to the individual. Therefore, we use a distribution function f to reflect this fact into the model. f is a distribution function, and is the ratio of the individuals whose protection period provided by the vaccine is Classically, it is supposed that is non-negative for each and f is continuous on , and f satisfies . The term represents the number of surviving individuals at time t who have been vaccinated at time and have protection period .
Of course, since it is thought that the vaccine may not provide full protection, some vaccinated individuals may still be susceptible to infection. Therefore, as a result of a sufficiently effective contact with infectious individuals, a vaccinated individual who no longer has any protection enters the infectious compartment. To reflect this transition to the compartment I, the expression has been used in the model.
4. Sensitivity Analysis
It is difficult to completely eradicate an epidemic in a population in a short period of time. Considering that many negative situations are brought about by the disease, attempts to reduce the spread of the disease have great importance. In this context, with various control measures to be implemented, lowering the value is an important goals. Therefore, it is of great importance to examine the effect of parameters on the change of and to apply control measures in this direction.
Now we will focus on the sensitivity analysis of
. Sensitivity analysis clarifies how effective each parameter is to disease transmission. To observe whether the parameters that affect the basic reproduction number have a positive or negative effect, we will explore the normalized forward sensitivity index of
The normalized forward sensitivity index of the variable
with respect to the parameter
is defined as
by using partial derivatives, where
represents the basic parameters constituting
.
Then
and
Additionally, we have
and
With increasing values of the parameters that have positive indices , increases, so the spread of the disease progresses in the population. On the other hand, the parameters in which its sensitivity indices are negative cause a decrease in . That is, the average number of secondary infection cases decreases, while these parameters increase, so the spread of the disease starts to decrease. In the next section, we will present an example for sensitivity analysis after choosing the distribution function.
5. Some Examples for Distribution Function and
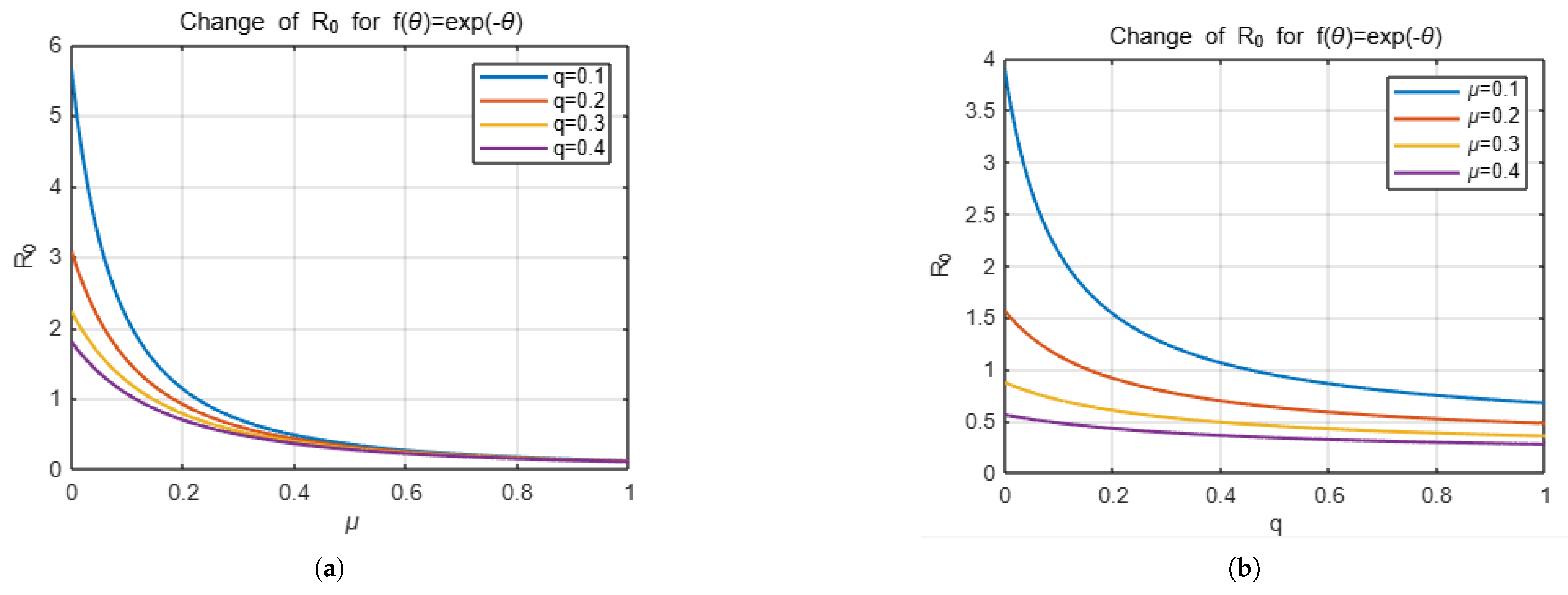
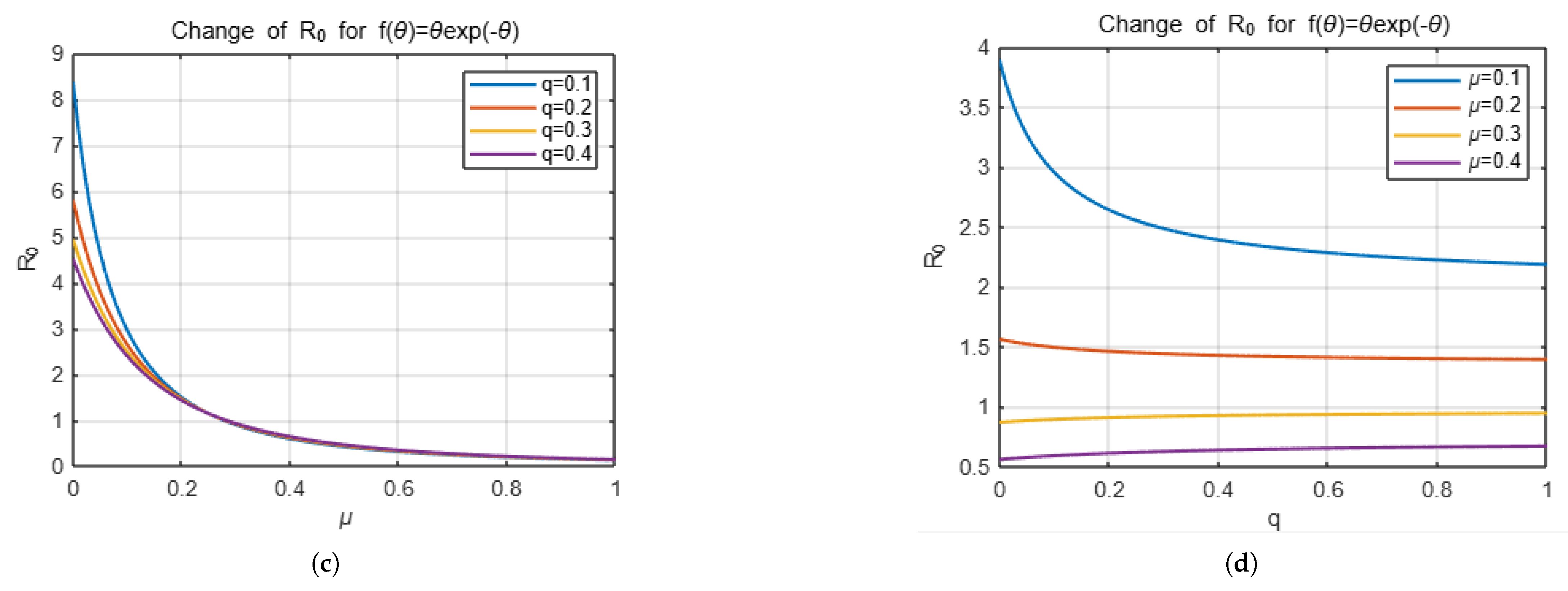
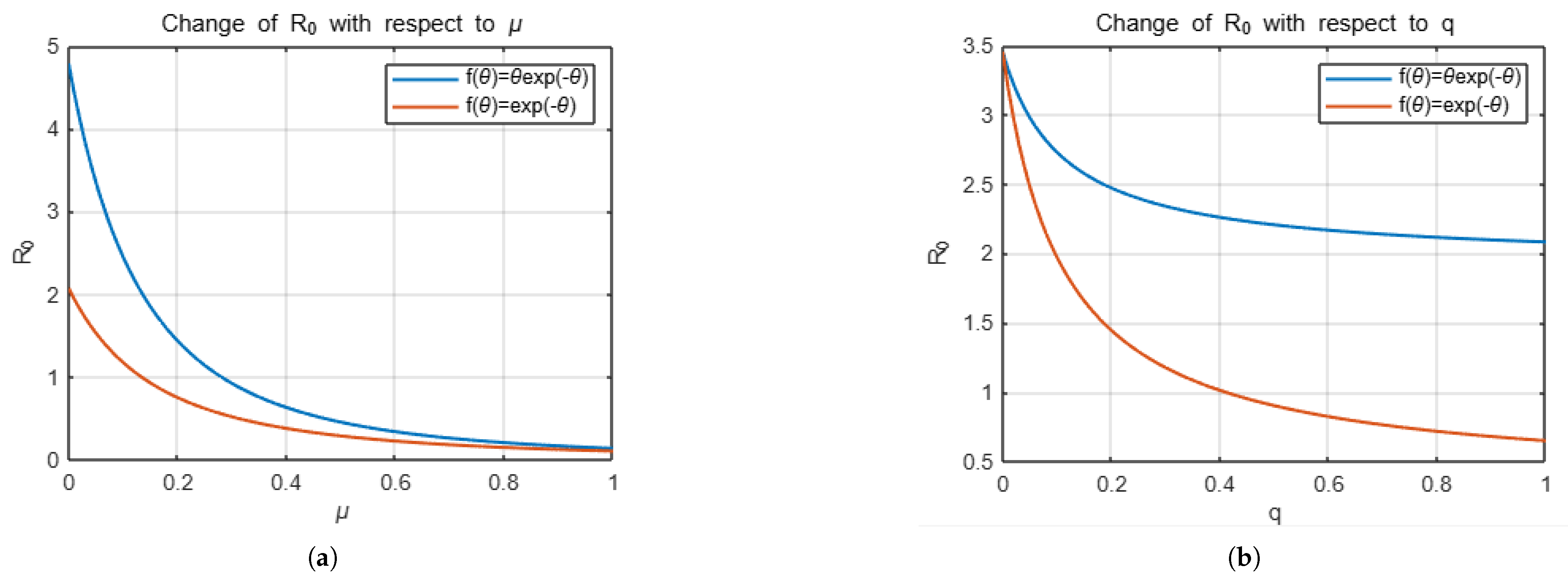
Example 1. If we take the distribution function f as , thenandTherefore, we obtain as Example 2. If we take f as , thenand we obtainTherefore, we can write as Now we will focus on presenting the effect of the choice of distribution function on the number of secondary infections with some simulations. For this, we will present the effect of the variables q, which is related to vaccination, and , which is decisive in the distribution function, which determine . The simulation below shows how the change in the parameters q and , respectively, affects for two different distribution functions, keeping the other parameters that determine constant except for these two parameters.
In the rest of the article, we will use the following parameters given in [
25] (
Table 1).
The
Figure 1 and
Figure 2 represent the changes in
with respect to the
q and
variables depending on the choices of
f.
As mentioned in the previous section, parameters
and
exhibit negative indices, while
display positive indices.
Table 2 and
Figure 3 reflect this case.
A small example can be given to explain the meaning of these values. As can be seen in
Table 2, sensitivity indices of
and
F are −0.4523 and 0.2291, respectively. This means that, if
is increased by 10%, then
is decreased by 4.523%. Similary, a 10% increase in
F results in a 2.291% increase in
.
6. Conclusions
In this study, a mathematical epidemic model reflecting that the protection period provided by the vaccine effect may vary from person to person is presented with its analysis. This novel fractional epidemic model is formed by the aid of a system of distributed delay nonlinear Caputo fractional integro-differential equations.
Firstly, equilibrium points of formed system are found and the basic reproduction number of the model are determined as
Thus, it is proved that System (
10) has a unique disease-free equilibrium point
when
and there is a unique endemic equilibrium
when
. Moreover, it has been shown that, in a population with infectious individuals, the disease will never disappear when
. Thus, it has been reinforced that the introduced model is consistent and that
is meaningful.
Additionally, the global behavior of System (
10) was examined. Firstly, it is proved that all solutions of the system tend toward
by constituting the appropriate Lyapunov function for
Then, when
and
, the proof of global stability of
has been presented by the Dulac criterion and the Poincaré–Bendixson theorem, which are commonly used in two-dimensional population models.
When the effect of the parameters in the model on , it can be seen that the parameters that can reduce and can be controlled partially or completely are q and
is the recovery (or treatment) rate, and q is the vaccination rate. Controlling the vaccination rate (q) is both easier and less costly than the recovery rate (). In addition, considering that vaccinating the entire population may not be possible in real terms and is costly, knowing the minimum vaccination level that must be achieved to eliminate the disease is extremely important. In this study, a result is also given regarding the minimum vaccination rate that must be achieved to eliminate the disease.
Possible future work could consist of adding the optimal control problem to the model or using other derivative definitions. Additionally, authors working in the field of applied mathematics can conduct numerical simulations for the model and their comparative analysis.