1. Introduction
The classic SIR model was introduced by Kermack and McKendrick in 1927 [
1] as one of the first models in mathematical epidemiology. The model divides a population into three compartments with fractional sizes
S (Susceptibles),
I (Infectious) and
R (Recovered), such that
. The flow diagram between compartments, as given in
Figure 1, leads to the dynamical system
Here,
denotes the recovery rate and
the effective contact rate (i.e., the number of contacts/time leading to infection of a Susceptible, given the contacted was infectious). Members of
R are supposed to be immune forever. Due to (
1),
S decreases monotonically, eventually causing
and
. At the end, the disease dies out,
, and one stays with a nonzero final size
, thus providing a model for
Herd immunity.
To construct models also featuring
endemic scenarios one needs enough supply of susceptibles to keep the incidence
ongoing above a positive threshold. The literature discusses three basic methods to achieve this, see
Figure 2.
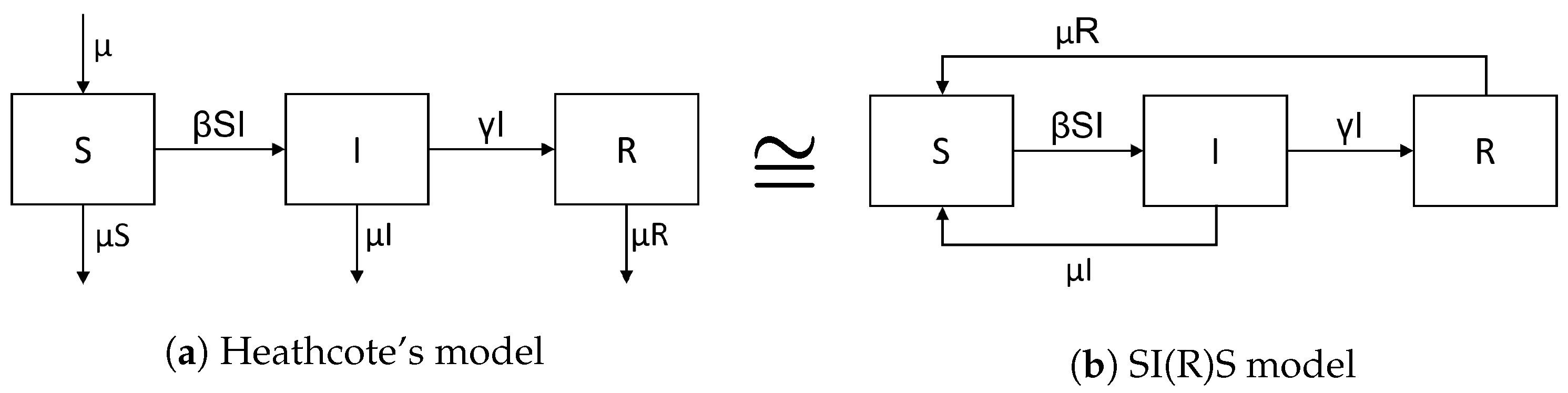
Heathcote’s
classic endemic model adds balanced birth and death rates
to the SIR model and assumes all newborns are susceptible. This leads to a bifurcation from a stable disease-free equilibrium point to a stable endemic scenario when raising the basic reproduction number
above one [
2,
3,
4].
The SIRS model adds an immunity waning flow, from R to S, to the SIR model, leading to the same result.
The SIS model considers recovery without immunity, i.e., a recovery flow from I to S, while putting . Again, this leads to the same result.
In what follows the reader is assumed to be familiar with the basic notions in these models. For a comprehensive and self-contained overview of the history, methods and results in mathematical epidemiology see the textbook by M. Martcheva [
5], wherein an extensive list of references to original papers is also given.
As a starting point for this paper, observe, from
Figure 3, that Heathcote’s model could equivalently be reformulated by disregarding birth and death rates and instead introducing a combined SI(R)S ≡ SIRS/SIS model with flow rates
. More generally, adding Heathcote’s balanced birth and death rates
to a SI(R)S model with independent parameters
apparently becomes equivalent to considering the SI(R)S model without birth and death rates and with shifted parameters
and
[
6].
The aim of this paper is to generalize this observation to homogeneous three-compartment models with the following:
(A) positive susceptibility of the R-compartment describing incomplete immunity (in which case it makes sense to rename and ),
(B) a non-trivial birth matrix and a time varying population size N due to the compartment-dependent constant per capita birth and death rates.
As a result, we see that, for coinciding birth minus death rates in compartments and , in the dynamics of fractional variables, all demographic parameters become redundant by shifting the remaining parameters appropriately. In particular, transmission coefficients describing -susceptibility are replaced by , where denotes the excess mortality in compartment I. Hence, may possibly become negative.
This result leads to a unifying normalization prescription when always considering these models without vital dynamics and, instead, with two distinguished, and possibly also negative, incidence rates
. When normalized this way, seemingly different models in the literature become isomorphic at coinciding shifted parameters. As an example, the recent results in [
7] follow from earlier results in [
8] (for
) and [
9] (for
).
2. Compartment Models
For simplicity, all maps are supposed to be
. Let
and
be a homogeneous vector field,
for all
and
. Denote
the dual of
and
the dual pairing. Let
be the local flow of
V. For functions
we denote their time derivative along
by
. Let
be a cone and
be a homogeneous function,
, satisfying
on
. In this case, the local flow
naturally projects to a local flow
, leaving the leaves
invariant.
Using
one immediately checks
The vector field
generating
is given by
Clearly,
F is also homogeneous and by putting
we have
.
Now, let us focus specifically on compartment models,= where gives the population in compartment i, the total population and . To guarantee being forward invariant one also needs .
Definition 1. The compartment model is said to have constant per capita demographic rates, if there exists , such that , i.e., . We call the total birth minus death rate in compartment i.
In such models, one usually decouples the time development of
N and analyzes the dynamics of fractional variables
,
. The main observation of this paper states that, in many standard models, the correction term
in Equation (
2) can be absorbed by redefining the parameters determining
V.
Lemma 1. Assume and denote . Putting we have Proof. Use and, therefore, , for all . □
Let us apply this to vector fields
V of the form
where
,
and
. Here,
is the mortality rate in compartment
j,
denotes the number of newborns from compartment
j landing in compartment
i, and the parameters
and
determine the population flow from compartment
j to
i, due to infection transmission, recovery, loss of immunity, vaccination, etc. Thus,
is the total birth rate in compartment
j and
. Forward invariance of the non-negative orthant
for zero birthrates requires (a)
M to be essentially non-negative, i.e.,
for
, whence
, and (b)
for
. Now put
Using
,
,
and
for
, the new parameters
and
have the same properties as
M and
and we obtain
Hence, in the dynamics for fractional variables
, all birth and death rates may be absorbed by redefining
M and
. Note that standard models typically satisfy
, which is consistent with
. On the other hand,
might change sign as compared to epidemiological requirements.
Remark 1. If the vector field V is of the form (4), with replaced by for some function f, and constant excess mortality , then V is no longer homogeneous but still . In this case, Equation (3) still holds with . Hence, the function f does not appear in the definition of and in (5), implying that F in Equation (6) is independent of f and still homogeneous, whence . 3. The Three-Compartment Master Model
As a kind of master example, consider an abstract SI(R)S-type model consisting of three compartments,
,
and
, with total population
. Members of
are infectious, members of
are highly susceptible (not immune) and members of
are less susceptible (partly immune). The flow diagram between compartments is completely symmetric with respect to permuting
, and is depicted in
Figure 4.
The parameters in this model are:
- :
Vaccination rate.
- :
Immunity waning rate.
- :
Number of effective contacts per unit time of a susceptible from .
- :
Recovery rate from .
- :
Mortality rate in .
- :
Mortality rate in .
- :
Probability of a newborn from to be infected.
- :
Rate of newborns from .
- :
Rate of newborns from . These newborns are not supposed to be infected.
- B:
Sum of newborns who are not infected, .
- :
Portion of newborns who are not infected landing in , . So, is the portion of newborns who are not infected and who are vaccinated.
All parameters are assumed to be non-negative. Furthermore,
,
,
and
. Putting
the dynamics is given by
So, in total, this model counts 14 independent parameters. A list of prominent examples is discussed below. Let us now cast this model into the formalism of
Section 2. Putting
and
we have
Here
,
and we get
. So now introduce
to conclude from (
5)
In summary, denoting fractions of the total population by
and
, and assuming the condition
, the dynamics for fractional variables becomes
So, for
, all birth and death rates become redundant and may be absorbed by redefining
,
and
. The price to pay is that
might become negative. Hence, the space of admissible parameters for the system (
14) becomes (Due to the permutation symmetry
, there is no loss, assuming
. The case
is ignored, since, in this case, putting
one can easily check that
obeys the dynamics of a SIS model, which can immediately be solved by separation of variables.):
Concerning the dynamics of fractional variables, any two models mapping to the same set of shifted parameters
become isomorphic. In particular, the case of constant population,
, yields
. In summary, we get
Proposition 1. Referring to the parameter transformation (12) and the normalized dynamics of fractional variables (14), assume , and put . - (i)
If the model with variable population is isomorphic to a model with constant population and transmission coefficients .
- (ii)
If it is isomorphic to a variable population SI(R)S model with two recovery flows and and parameters , and .
- (iii)
If it is isomorphic to a SI(R)S model as in (ii) with constant population.
4. Examples from the Literature
For simplicity, from now on let us assume the rate of newborns who are not infected to be compartment-independent, , implying . In this case, one may without loss assume by redefining . Hence, and, in this case, gives the excess mortality in the infectious compartment.
Below there is a list of prominent examples from the literature.
Table 1 maps these examples to the present set of parameters.
- Heth
Heathcote’s classic endemic model [
2,
3,
4] by putting
,
,
,
all other parameters vanish.
- BuDr
The 7-parameter SIRS model with time varying population size in [
10], adds to Heathcote’s model an immunity waning rate
and allows non-balancing mortality and birth rates
.
- SIRI
The 6-parameter SIRI model of [
11], replaces the immunity waning rate
in [
10] with the transmission rate
and also requires
.
- SIRS
The 8-parameter constant population SI(R)S model with vaccination and two recovery flows and . Hence and .
- HaCa
The 6-parameter core system in [
12], with transmission and recovery rates
, a vaccination term
and a constant population with balanced birth and death rates,
and
.
- KZVH
The 7-parameter vaccination model of [
8] adds an immunity waning rate
to the model in [
12].
- LiMa
The 8-parameter SIS-model with vaccination and varying population size of [
9] keeps only
and assumes
. (Actually the authors let
be a function of
N and put
with constant excess mortality
. Still,
disappears when passing to tilde parameters (
12), see also Remark 1.)
- AABH
The 8-parameter SIRS-type model analyzed recently by [
7], keeps only
and all other parameters are positive. The authors allow a varying population size by first discussing the general case of all mortality rates being different and then concentrating on
and
.
Assuming
, and applying the transformations (
12), we acquire a classification in terms of the redundancy-free 6-parameter set
.
†: To be comparable, Equation (19) refers to the sub-case in BuDr, so implies . Also, as in SIRI, but becomes independent.
: For one of the three parameters always is redundant. So .
: For the mapping is bijective.
The dimensions of these parameter spaces are listed in the last column of
Table 1. In summary, we arrive at
Corollary 1. Consider the dynamics of fractional variables in the models of Table 1, for BuDr and AABH under the restriction . Disregarding boundary configurations in parameter space , the following relations hold. - (i)
The model of AABH [7] is isomorphic to the master model (14) and covers all other models. - (ii)
The SIS-type model of LiMa [9], with time-dependent population size, coincides with the subcase of AABH [7]. - (iii)
The constant population model of KZVH [8] coincides with the subcase of AABH [7]. - (iv)
The subcase of AABH [7] reduces to the SI(R)S model (16). - (v)
The models of HaCa [12] and KZVH [8] are isomorphic.
5. Summary
We have seen in Lemma 1 that, in a large class of homogeneous compartment models with constant per capita demographic rates and time-dependent total population
N, the dynamics of fractional variables
can be rewritten such that all demographic parameters become redundant. In this way, various prominent SI(R)S-type models with standard incidence, demographic parameters and possibly susceptible
R-compartments may be normalized, such that the dynamics of fractional variables appear as a sub-case of a master model with zero birth and death rates, see Equations (
16)–(22). Since, apparently, none of the original papers used the identity (
3) of Lemma 1, these relations were not realized before. The price to pay is that, in the normalized master model, infection transmission rates
may also be negative. As a particular example, recent results on backward bifurcation in models with time-varying total population
, coinciding mortality rates
and an excess mortality
by AABH [
7] were already covered by the isomorphic model with constant population of KZVH [
8], published in 2000. The complementary case
turns out to be isomorphic to the variable population SIS model with
, published by LiMa [
9] in 2002.
The normalized master model (
14) is also the starting point of an ongoing analysis of symmetry operations in these kinds of models, giving rise to further parameter reductions, see the work in progress in [
13,
14].