Abstract
In this study, we explore a recent biological model created to analyze the behavior of cancer cells by administering a dose of a drug containing anti-PD-L1 and IL-10 with the Caputo and Atangana–Baleanu derivative in the Caputo sense (ABC). Using the Caputo derivative in order to examine the stability of the non-linear system, we are able to demonstrate that it is existent and unique, and to introduce several numeric data obtained for the fractional values in MATLAB by using the Adams–Bashforth–Moulton (ABM) method. Additionally, by using the predictor–corrector approach, the numerical results from the system with ABC derivative will be produced. As a result, it has been observed that immune system cells that are exposed to single-dose drug with fractional order effectively combat cancer cells. The tumor cells decrease by and for the system generalized by the Caputo and ABC derivative, respectively, for the order .
1. Introduction
There is no doubt that cancer is becoming a more popular topic for recent research, as the numbers of people contracting this disease are rising and the deaths that are caused by it are increasing as well. The function of the immune system, the body’s defense system, is to recognize and kill cancer cells by stopping their progress. However, cancer cells may escape the notice of the immune system because the interaction between the PD-L1 proteins located on the surface of cancer cells and the PD-1 molecules located on the surface of T lymphocytes is impacted by a debilitation in the activation of lymphocytes; this leads to a halt in the immune response. Lately, sights have been set on enabling the immune system to recognize and eliminate cancer cells by use of anti-PD-L1 inhibitors in order to prevent cancer cells from escaping the immune system. Specifically, immune checkpoint inhibitors, anti-PD-L1, are designed to enhance the effectiveness of T cell activation by blocking negative pathways that are associated with T cell activation [1,2,3,4,5]. As for the IL-10 cytokine, it provides the immune system with the opportunity to fight the cancer cells more effectively by enhancing the number and efficiency of CD8+T cells [6,7]. Aside from this, the use of IL-10 is also beneficial for controlling the growth of tumors [8].
Mathematical modeling, also referred to as the reinterpretation of real-world problems with equations, is currently one of the tools used by scientists to predict the onset of diseases that cause problems.
There are many mathematical models that are very close to the reality, but they still cannot precisely describe reality. Thus, in order to provide better fit to real-world data, there is a need to build more accurate models that are able to account for this. Numerous studies have shown that fractional-order derivative modeling of real-life processes is more accurate and reliable when compared to the integer or classical order circumstances [9,10,11,12,13,14,15,16,17,18]. Caputo fractional derivatives are also useful when dealing with real-life issues since they allow the application of boundary conditions and beginning conditions to be incorporated into the analysis. There are many important features of fractional analysis, including the ability to describe and analyze problems that arise in the fields of physics [19], biology [20], engineering [21,22,23,24], as well as other science disciplines through mathematical modeling [25,26,27,28,29,30,31,32,33,34,35]. In recent times, we have encountered models that involve the use of fractional derivatives to mathematically analyze complex biological processes involving hereditary properties and memory effects, such as diseases [36,37,38,39,40,41,42,43,44,45] that are being produced by fractional derivative.
There are many academics who are currently working on developing the theory of fractional calculus by creating various types of fractional derivative operators with the non-singular kernels in order to prevent classical fractional differential operators from exhibiting singularities as a result of the kernel. Recently, authors from diverse disciplines of mathematics constructed and explored mathematical models employing AB-fractional derivative by utilizing the non-singular kernel found in the Atangana–Baleanu fractional derivative operators.
The purpose of this work is to investigate the relationship between IL-10, anti-PD-L1, CD8+T cells, and cancer cells by means of the mathematical model configured with fractional analysis. Another objective is to comprehend how an immune system strengthened by components can thwart the development of cancer cells.
The interplay amongst CD8+T and CD4+T cells, cancer cells, and IL-2 with Caputo derivative was analyzed by [38]. Bringing IL-12 and anti-PD-L1 into consideration, a study concerning the reciprocal action of the immune system and cancerous cells was introduced by [39]. A vaccine model incorporating cancerous cells with IL-12 and IL-2 was perused by [46]. A mathematical model of the treatment modality combining radiation and anti-PD-L1 in the framework of cancer was established by [47]. Here, two significant radiobiological elements, namely, cell mending and cell reconstruction, are considered by the model given in [41]. The fact that the Caputo fractional derivative value and the coefficient of cancer cell multiplication are relatively more susceptible amongst the model elements was proven when the sensitivity analysis is studied. The co-infection model of cancer and hepatitis dynamics is investigated using Atangana–Baleanu in the Caputo sense [48].
The model, given by [49], which will be investigated with the fractional derivative in this article, is as follows:
where T, C, , Z denote CD8+T cells, cancer cells, IL-10, and anti-PD-L1, respectively. In Equation (1), which demonstrates the change in CD8+T cells with time, the term denotes the formation and death of T cells, respectively; the term denotes the growth of CD8+T cells in consequence of the interaction of CD8+T cells with the IL-10 and cancer cells. In Equation (2), which demonstrates the change in cancer cells with time, the term denotes the growth of cancer cells; and the term represents the decrease in cancer cells with the effect of the IL-10 and the anti-PD-L1. In Equation (3), which demonstrates the change in the IL-10 cytokine with time, the term denotes the decrease in the IL-10 cytokine. In Equation (4), which defines the anti-PD-L1 dynamic with time, the term models the decrease in the anti inhibitor thought to be existent in the dynamic.
The study is formed with the following section: basic tools and model in the Caputo sense are briefly mentioned in Section 2 and Section 3, respectively. Section 3 also underpins the existence and uniqueness of the solution and stability analysis for Equations (9)–(12). The system with the Caputo fractional derivative and ABC derivative are numerically explained to look over the whole effect in Section 4. Finally, the study concludes by discussing the attained results.
2. Basic Tools
This section provides a reminder of some fundamental definitions and properties.
Definition 1
([9]). Let , the Riemann–Liouville (RL) fractional derivative is given by
Definition 2
([9]). Let , the Caputo fractional derivative is given by
Definition 3.
The Sobolev space of order 1 in is given by
Let , , be a function. is a normalization function with and is of the following form
is the Mittag–Leffler function, defined by its series representation as
Definition 4.
The AB derivative in Caputo sense of f is given [10]
Definition 5.
The AB derivative in RL type of order α of f is given by [10]:
Definition 6.
The fractional integral associated to the AB fractional derivative is defined by [10]:
3. Model in Caputo Sense
In this section, generalization of the model given by Equations (1)–(4) with Caputo fractional derivative and ABC derivative, respectively, will be made by using a dimensional representation.
The original Caputo fractional order model is given by the following
and the original model with ABC sense is given by the following
where the initial conditions are , , , , and , . will be assumed in the following sections.
3.1. Stability Analysis of the Model in Caputo Fractional Derivative
The stability analysis will be given for the model, which is rearranged using the Caputo fractional derivative and the dimension so that the right–left sides have the same size.
Let be the equilibrium points of Equations (9)–(12). The Jacobian matrix of this system is computed as follows:
As a result, the eigenvalues of the matrix produced by substituting the in the Jacobian matrix equilibrium point are determined to be as follows:
The eigenvalues of the matrix obtained in the same manner are as follows:
The stability of the system is dependent on the tumor’s growth rate when we utilize the values in Table 1, as shown from the 2nd components ( and ) of the eigenvalue vectors. Looking at the equation of and , the first of these values is positive, whereas the second is negative. As a result, we declare that is unstable, while is stable. In other words, for the eigenvalues derived from the solution of the characteristic equation of , for , is asymptotically stable since .
Using Table 1, it is seen that the tumor concentration at the two equilibria makes a significant difference. In terms of biology, the tumor-free equilibrium point exists when . In this instance, the tumor is successfully fought by the immune system, therefore, no therapy is required. Treatment is required at the other equilibrium point where is attained since the tumor concentration is nearly at its carrying capacity.
3.2. The Existence and Uniqueness of Solution in Caputo Fractional Derivative
Let positive parameters, the initial conditions , , , where for system given by Equations (9)–(12). Hence the system is the following:
where and
Definition 7
([12,13,14]). is the class of continuous functions on the interval and the class of continuous column vector Y(t) and its components , , , . The norm of is displayed by a
when we say ,
Definition 8.
The initial value problem in Equation (17) has a solution as if
- where × ; are positive constants.
- Y(t) is satisfied in Equation (17).
Theorem 1.
There is only unique solution to the initial value problem Equation (17).
Proof.
By using the properties of fractional calculus and Equation (17),
Using ,
Let . Hence,
Later,
We acquire
If K is chosen below , obtained such that
Thus, Equation (18) is said to have a unique solution Through F, shown by Equation (19), which appears to have a fixed point. Then, the following is obtained by using Equation (18)
Hence, the following is obtained:
and
4. Comparing Numerical Results of Model with Caputo Derivate and ABC Derivative
Figure 1a–d, Figure 2a–d and Figure 3a–d are drawn from the solution of the Equations (9)–(12). The solution is composed by the use of Caputo fractional derivative and the Equations (13)–(16), which is formed by the use of ABC derivative for fractional order . The ABM method is used to obtain the solution to Equations (9)–(12) and the predictor–corrector method is used to find the solution to Equations (13)–(16). Moreover, the system’s tumor burden has been attempted to be taken into account when choosing the fractional order.
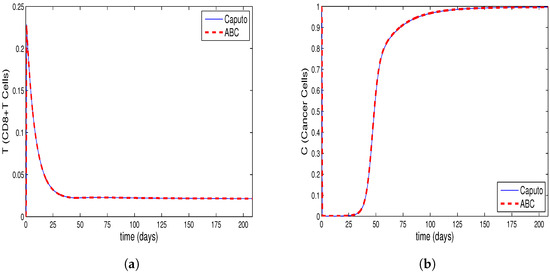
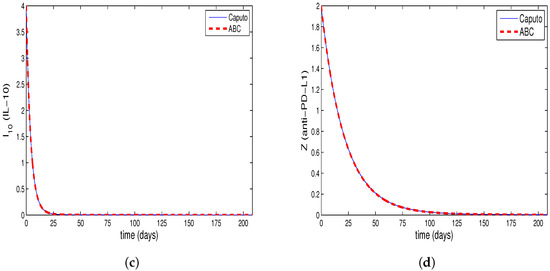
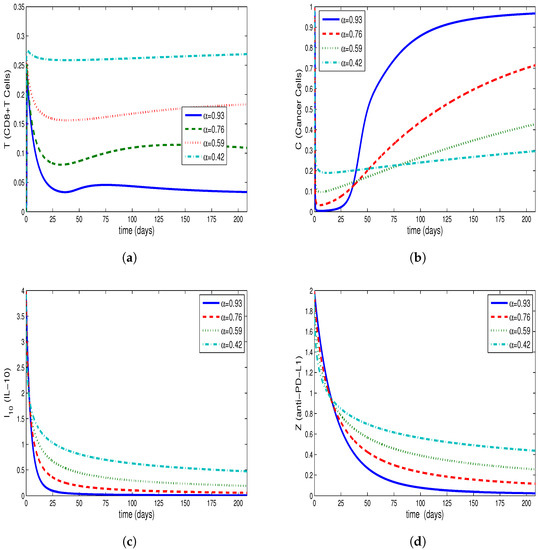
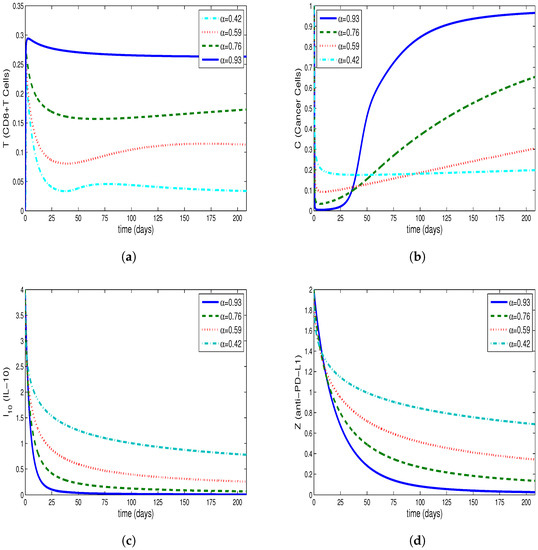
The Figure 1a–d show that the tumor burden in the system has not diminished and that the cancer cells have reached their initial value. By synchronously examining Figure 2b and Figure 3b derived for tumor cells, along with Figure 2a and Figure 3a derived for CD8+T cells, it is observed that CD8+T cells peak at the point when the number of tumor cells is at minimum. After a time interval, CD8+T cells also show a decrease due to the decrease in the number of tumor cells; thus, the tumor cells, taking advantage of the deficiency of the immune system, start regrowing. This conclusion is in accordance with the fact that the patients whose immune systems are supressed have a greater risk of developing cancer. Furthermore, tumor congregation diminishes in intensity when the activity of the immune system is enhanced, mitigating the strain of tumors on the system.
As shown in Figure 2c,d and Figure 3c,d, the half-lives of the single-dose IL-10 cytokine and anti-PD-L1 inhibitor after being delivered to the system cause them to demonstrate decline up to a specific point. Moreover, these figures reveal that , which represents the situation where IL-10 and anti-PD-L1 remain at the highest level, is the lowest value amongst all the assessed values.
Additionally, for the relevant values of , the tumor cells decline by , and , respectively, for the system generalized by the Caputo derivative, and , and , respectively for the system generalized by the ABC derivative, compared to the initial value.
5. Concluding Remarks
Equations (1)–(4), generalized by the Caputo and ABC derivatives, are examined. It is seen that tumor cells show a reduction from to and from to , respectively. It is observed that the order representing the situation which elongates the time interval between the decreasing and regrowth of tumor cells is a fractional order . The obtained result is compatible with the fact that the system generalized by the fractional derivative is more appropriate for daily life issues involving memory and genetic factors.
Subsequent to the stability analysis of the system with the Caputo derivative, the existence–uniqueness problem concerning the solution of the system is scrutinized.
It is recognized that the number of cancer cells is decreased by in comparison with the initial level for the system given by Equations (9)–(12). The value of for the system given by Equations (13)–(16), for the situation where it declines by , is . Therefore, it is deduced that the CD8+T cells fight cancer cells more efficiently under the influence of IL-10 and anti-PD-L1 by means of the solutions of the systems generalized by Caputo and ABC derivative. We can state that the system with the ABC derivative puts up a more effective struggle compared to the system with the Caputo derivative.
In addition, it is observed that the order representing the situation, where the time interval until the cancer cells regrow to their initial value is elongated, is also of fractional order in these novel systems designed by fractional derivative. The obtained result evidences that a more efficient struggle is given against cancer in the event that the influence of the derivative on the system declines.
Table 2 and Table 3 show the cell concentrations of different cells in the same time interval for the systems generalized by Caputo or ABC derivatives. According to Table 2 and Table 3, the IL-10 and anti-PD-L1 concentrations are higher for ABC derivatives, although the CD8+T cells remain on the same level of concentration synchronously for both of the generalized systems. This leads to a striking result, demonstrating that the cancer cells decrease by a higher rate in comparison with their initial value in ABC as against Caputo derivative. High tumor burden in the systems given by Equations (9)–(12) and Equations (13)–(16) can be associated with a fractional order close to 1 via Figure 1a–d, Figure 2a–d and Figure 3a–d.

Table 2.
The concentration of variables of system at for order Caputo fractional derivative.

Table 3.
The concentration of variables of system at for order ABC derivative.
The system given by Equations (9)–(12) and Equations (13)–(16), which have been analyzed using the fractional derivative, also demonstrate that IL-10 and anti-PD-L1, which are given in a single dose, have a significant impact on the elimination of cancer cells. When a specific protocol is followed with repeated dose applications and treatments, the effectiveness of the drug is accepted.
Author Contributions
Conceptualization, E.U.; methodology, N.Ö.; software, E.U.; validation, N.Ö.; formal analysis, N.Ö.; investigation, N.Ö.; resources, E.U. and N.Ö.; writing—original draft preparation, E.U. and N.Ö.; writing—review and editing, E.U. and N.Ö.; visualization, E.U. and N.Ö.; supervision, N.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fife, B.T.; Pauken, K.E.; Eagar, T.N.; Obu, T.; Wu, J.; Tang, Q.; Bluestone, J.A. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 2009, 10, 1185–1192. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Birnbaum, D.; Mamessier, E. The PD1/PDL1 axis, a promising therapeutic target in aggressive breast cancers. OncoImmunology 2016, 5, e1085148. [Google Scholar] [CrossRef]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Daster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.H.; Wu, V.; Bilardello, M.; Mar, E.; Oft, M.; Van Vlasselaer, P.; Mumm, J.B. The potentiation of IFN-γ and induction of cytotoxic proteins by pegylated IL-10 in human CD8 T cells. J. Interferon Cytokine Res. 2015, 35, 948–955. [Google Scholar] [CrossRef]
- Naing, A.; Papadopoulos, K.P.; Autio, K.A.; Ott, P.A.; Patel, M.R.; Wong, D.J.; Falchook, G.S.; Pant, S.; Whiteside, M.; Rasco, D.R.; et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J. Clin. Oncol. 2016, 34, 3562–3569. [Google Scholar] [CrossRef]
- Sun, H.; Gutierrez, P.; Jackson, M.J.; Kundu, N.; Fulton, A.M. Essential role of nitric oxide and interferon-gamma for tumor immunotherapy with interleukin-10. J. Immunother. 2010, 23, 208–214. [Google Scholar] [CrossRef]
- Podlunby, I. Fractional Differantial Equations; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Atangana, A.; Baleanu, D. New fractional derivatives with non-local and non-singular kernel: Theory and applications to heat transfer model. Therm. Sci. 2016, 370, 763–769. [Google Scholar] [CrossRef]
- Piccoli, B.; Castiglione, F. Optimal vaccine scheduling in cancer immunotherapy. Phys. A 2006, 370, 672–680. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Sayed, A.M.A.; El-Saka, H.A.A. Equilibrium points, stability and numerical solutions of fractional-order predator-pey and rabies models. J. Math. Anal. Appl. 2007, 325, 542–553. [Google Scholar] [CrossRef]
- Bozkurt, F. Stability Analysis of a Fractional-Order Differential Equation System of a GBM-IS Interaction Depending on the Density. Appl. Math. Inf. Sci. 2014, 8, 1021–1028. [Google Scholar] [CrossRef]
- El-Sayeda, A.M.A.; El-Mesiryb, A.E.M.; El-Sakab, H.A.A. On the fractional-order logistic equation. Appl. Math. 2007, 20, 817–823. [Google Scholar] [CrossRef]
- Kilbas, A.A.; Srivastava, H.M.; Trujillo, J.J. Theory and Applications of Fractional Differential Equations; Elsevier: New York, NY, USA, 2005. [Google Scholar]
- Iqbal, S.A.; Hafez, G.; Chu, Y.M.; Park, C. Dynamical analysis of nonautonomous RLC circuit with the absence and presence of Atangana-Baleanu fractional derivative. JAAC 2022, 12, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.M.; Yassen, M.F.; Ahmad, I.; Pongsakorn, S.; Khan, M.A. A fractional Sars-Cov-2 model with Atangana-Baleanu derivative: Application to fourth wave. Fractals 2022, 30, 2240210. [Google Scholar] [CrossRef]
- Khan, D.; Ali, G.; Khan, A.; Khan, I.; Chu, Y.M.; Nisar, K.S. A New Idea of Fractal-Fractional Derivative with Power Law Kernel for Free Convection Heat Transfer in a Channel Flow between Two Static Upright Parallel Plates. Comput. Mater. Contin. 2020, 65, 1237–1251. [Google Scholar] [CrossRef]
- Nigmatullin, R.R.; Baleanu, D. Is it possible to derive Newtonian equations of motion with memory? Int. J. Theor. Phys. 2010, 49, 701–708. [Google Scholar] [CrossRef]
- Pinto, C.M.A.; Machado, J.A.T. Fractional model for malaria transmission under control strategies. Comput. Math. Appl. 2013, 66, 908–916. [Google Scholar] [CrossRef]
- Baleanu, D. About fractional quantization and fractional variational principles. Commun. Nonlin. Sci. 2009, 14, 2520–2523. [Google Scholar] [CrossRef]
- Pinto, C.M.A.; Machado, J.A.T. Complex order van der Pol oscillator. Nonlinear Dyn. 2010, 65, 247–254. [Google Scholar] [CrossRef]
- Machado, J.A.T.; Ozdemir, N.; Baleanu, D. Mathematical Modelling and Optimization of Engineering Problems; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Hristov, J. Magnetic field diffusion in ferromagnetic materials: Fractional calculus approaches. Int. J. Optim. Control Theor. Appl. 2021, 11, 1–5. [Google Scholar] [CrossRef]
- Uçar, S. Analysis of hepatitis B disease with fractal–fractional Caputo derivative using real data from Turkey. J. Comput. Appl. Math. 2023, 419, 114692. [Google Scholar] [CrossRef]
- Uçar, S. Existence and Uniqueness Results for a Smoking Model with Determination and Education in the Frame of Non-Singular Derivatives. Discrete Contin. Dyn. Syst. Ser. S 2021, 14, 2571–2589. [Google Scholar] [CrossRef]
- Din, A.; Li, Y.; Yusuf, A.; Ali, A.I. Caputo Type Fractional Operator Applied to Hepatitis B System. Fractals 2021, 10, 2240023. [Google Scholar] [CrossRef]
- Ghanbari, B.; Kumar, S. A study on fractional predator-prey-pathogen model with Mittag-Leffler kernel-based operators. Numer. Methods Partial. Differ. Equ. 2020. [Google Scholar] [CrossRef]
- Joshi, H.; Jha, B.K. Chaos of calcium diffusion in Parkinson’s infectious disease model and treatment mechanism via Hilfer fractional derivative. Math. Model. Numer. Simul. Appl. 2021, 1, 84–94. [Google Scholar]
- Naik, P.A.; Eskandari, Z.; Yavuz, M.; Zu, J. Complex dynamics of a discrete-time Bazykin–Berezovskaya prey-predator model with a strong Allee effect. J. Comput. Appl. Math. 2022, 413, 114401. [Google Scholar] [CrossRef]
- Hammouch, Z.; Yavuz, M.; Özdemir, N. Numerical solutions and synchronization of a variable-order fractional chaotic system. Math. Model. Numer. Simul. Appl. 2021, 1, 11–23. [Google Scholar] [CrossRef]
- Evirgen, F. Transmission of Nipah virus dynamics under Caputo fractional derivative. J. Comput. Appl. Math. 2023, 418, 114654. [Google Scholar] [CrossRef]
- Shafik, M.; Abbas, M.; Abdullah, M.A.; Majeed, A.; Abdeljawad, T.; Alqudah, M. Numerical solutions of time fractional Burgers’ equation involving Atangana–Baleanu derivative via cubic B-spline functions. Results Phys. 2022, 34, 105244. [Google Scholar] [CrossRef]
- Koca, I. Analysis of rubella disease model with non-local and non-singular fractional derivatives. Int. J. Optim. Control Theor. Appl. 2018, 8, 17–25. [Google Scholar] [CrossRef]
- Hamou, A.A.; Rasul, R.R.; Hammouch, Z.; Ozdemir, N. Analysis of rubella disease model with non-local and non-singular fractional derivatives. Comput. Appl. Math. 2022, 41, 1–33. [Google Scholar]
- Özdemir, N.; Uçar, E. Investigating of an immune system-cancer mathematical model with Mittag-Leffler kernel. AIMS Math. 2020, 5, 1519–1531. [Google Scholar] [CrossRef]
- Dokuyucu, M.A.; Celik, E.; Bulut, H.; Baskonus, H.M. Cancer treatment model with the Caputo-Fabrizio fractional derivative. Eur. Phys. J. Plus 2018, 133, 1–6. [Google Scholar] [CrossRef]
- Uçar, E.; Özdemir, N.; Altun, E. Fractional order model of immune cells influenced by cancer cells. Math. Model. Nat. Phenom. 2019, 14, 308. [Google Scholar] [CrossRef]
- Uçar, E.; Özdemir, N. A fractional model of cancer-immune system with Caputo and Caputo-Fabrizio derivatives. Eur. Phys. J. Plus 2021, 136, 1–17. [Google Scholar] [CrossRef]
- Naim, M.; Yassine, S.; Nwar, Z. Stability characterization of a fractional-order viral system with the non-cytolytic immune assumption. Math. Model. Numer. Simul. Appl. 2022, 2, 164–176. [Google Scholar] [CrossRef]
- Farayola, M.F.; Shafie, S.; Siam, F.M.; Khan, I. Mathematical modeling of radiotherapy cancer treatment using Caputo fractional derivative. Comput. Methods Programs Biomed. 2020, 188, 105306. [Google Scholar] [CrossRef]
- Sheergojri, A.R.; Iqbal, P.; Agarwal, P.; Ozdemir, N. Uncertainty-based Gompertz growth model for tumor population and its numerical analysis. Int. J. Optim. Control Theor. Appl. 2022, 12, 137–150. [Google Scholar] [CrossRef]
- Magin, R.L. Fractional calculus models of complex dynamics in biological tissues. Comput. Math. Appl. 2010, 59, 1586–1593. [Google Scholar] [CrossRef]
- Balzotti, C.; D’Ovidio, M.; Loret, P. Fractional SIS epidemic models. Fractal Fract. 2020, 4, 44. [Google Scholar] [CrossRef]
- Traver, J.E.; Nuevo-Gallardo, C.; Tejado, I.; Fernández-Portales, J.; Ortega-Morán, J.F.; Pagador, J.B.; Vinagre, B.M. Cardiovascular Circulatory System and Left Carotid Model: A Fractional Approach to Disease Modeling. Fractal Fract. 2022, 6, 64. [Google Scholar] [CrossRef]
- Lai, X.; Friedman, A. Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model. PLoS ONE 2017, 12, e0178479. [Google Scholar] [CrossRef]
- Lai, X.; Friedman, A. Mathematical modeling of cancer treatment with radiation and PD-L1 inhibitor. Sci. China Math. 2020, 63, 465–484. [Google Scholar] [CrossRef]
- Bonyah, E.; Zarin, R.; Wati, F. Mathematical modeling of cancer and hepatitis co-dynamics with non-local and non-singular kernel. Commun. Math. Biol. Neurosci. 2020, 2020, 91. [Google Scholar]
- Ucar, E.; Ozdemir, N.; Altun, E. Qualitative analysis and numerical simulations of new model describing cancer. J. Comput. Appl. 2023, 422, 114899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).