On the Modeling of COVID-19 Transmission Dynamics with Two Strains: Insight through Caputo Fractional Derivative
Abstract
:1. Introduction
2. Basic Caputo Fractional-Order Preliminaries
3. The Model
4. Mathematical Analyses
4.1. Basic Properties of the Fractional Model
4.2. Existence and Uniqueness of Solutions
4.3. Model Equilibrium and the Basic Reproduction Number
4.3.1. Stability Analysis of the Equilibrium Point
4.3.2. Local Stability of DFE
4.3.3. Local Stability of the Endemic Equilibrium for Strain 1
- (i).
- .
- (ii).
- .
4.3.4. Local Stability of the Endemic Equilibrium for Strain 2
5. Numerical Simulations
5.1. Parameter Estimation
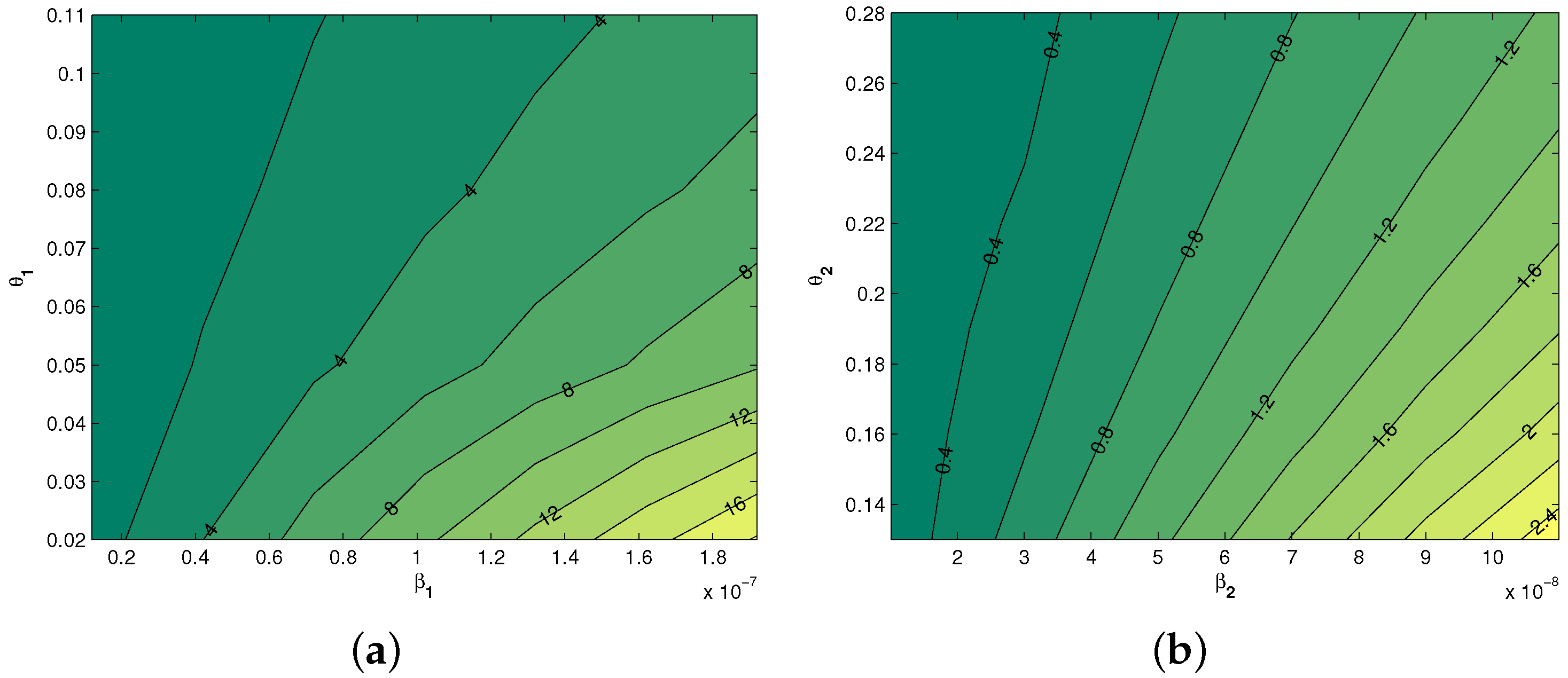
5.2. Local Sensitivity Analysis
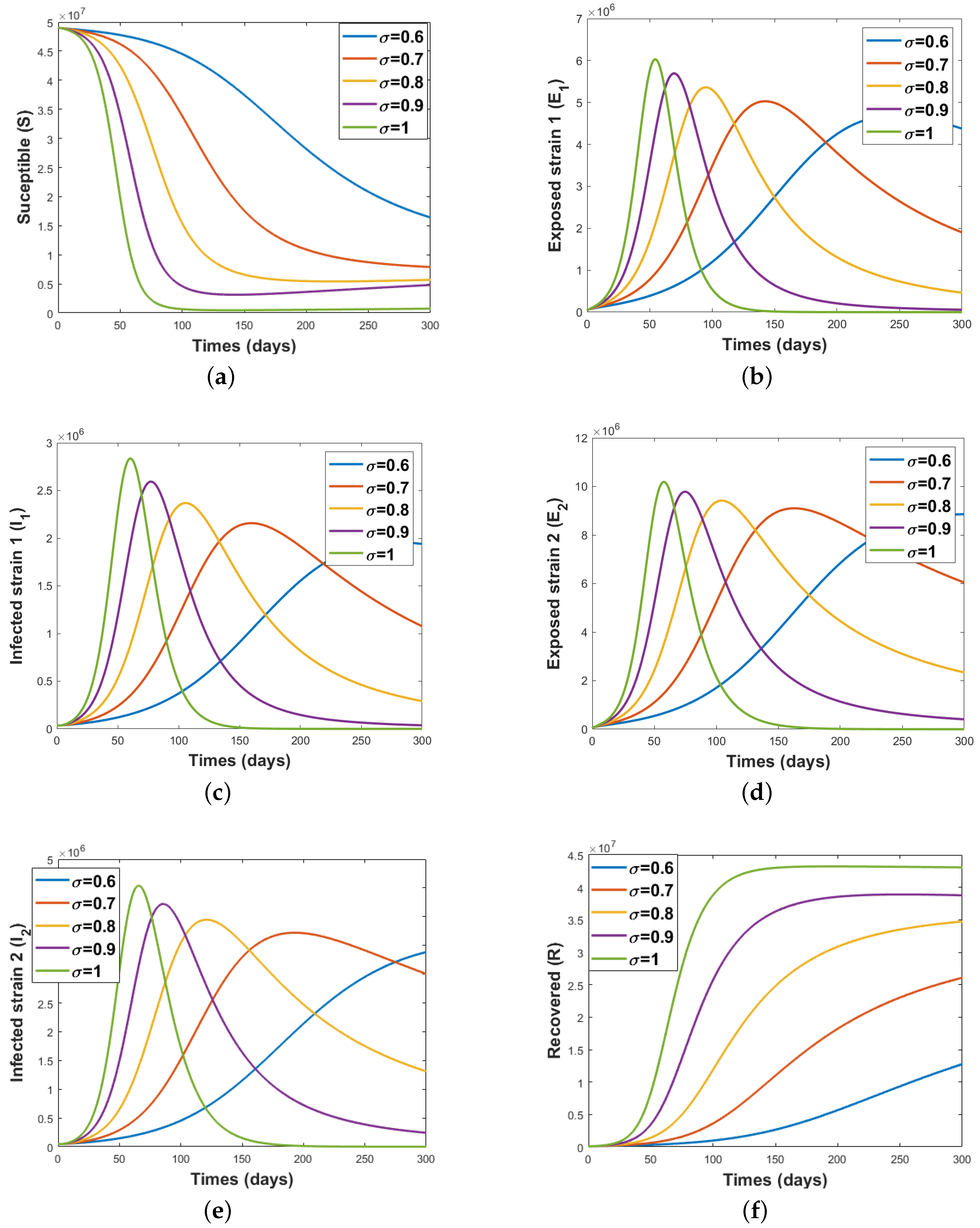
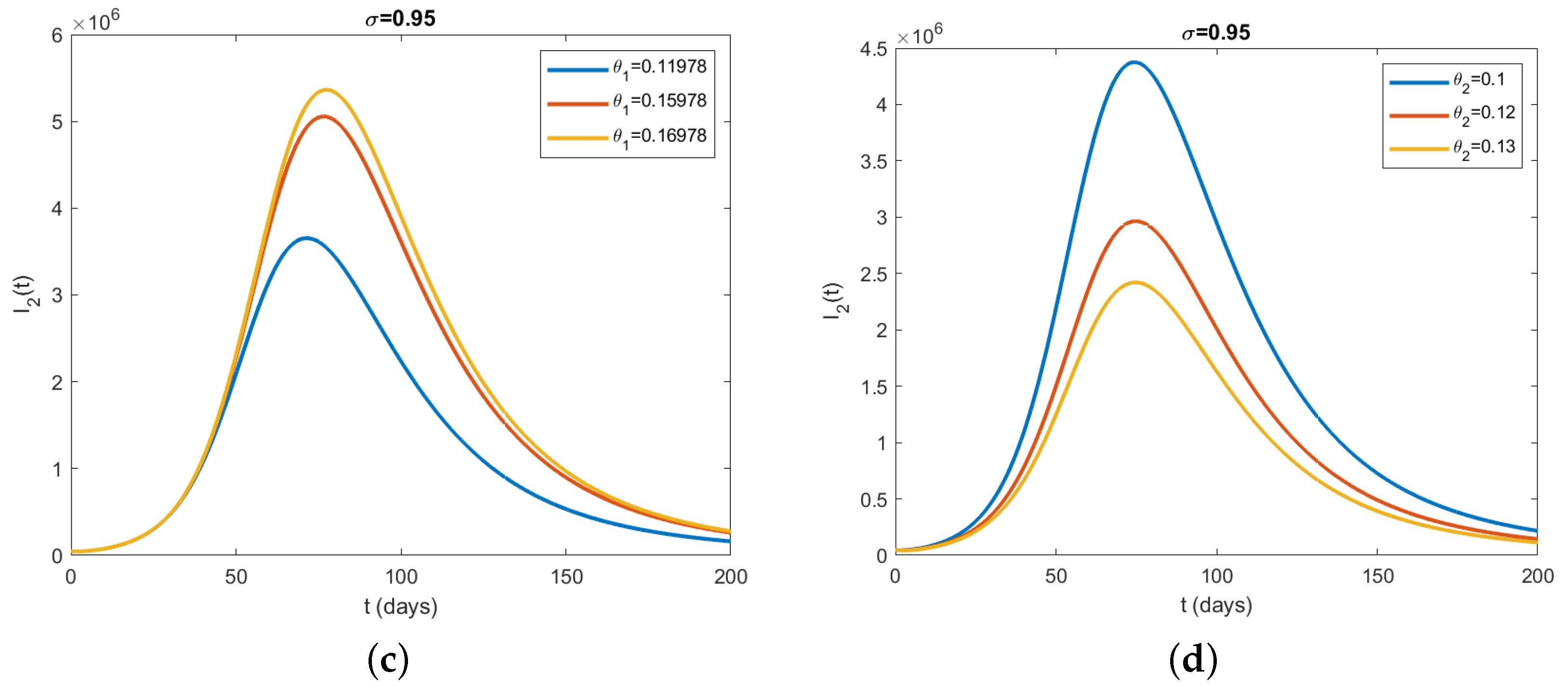
5.3. Simulation Results Using Caputo Operator
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 30 April 2022).
- Thakur, V.; dan Jain, A. COVID-19-Suicides: A Global Psychological Pandemic. Brain Behav. Immun. 2020, 88, 952–953. [Google Scholar] [CrossRef] [PubMed]
- CDC. Your Health. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/index.html (accessed on 28 April 2022).
- World Health Organisation. COVID-19 Weekly Epidemiological Update Edition 70. Available online: https://www.who.int/docs/defaultsource/coronaviruse/situationreports/20211214_weekly_epi_update_70.pdf?sfvrsn=ad19bf83_3 (accessed on 14 May 2022).
- Guruprasad, L. Human Coronavirus Spike Protein-Host Receptor Recognition. Prog. Biophys. Mol. Biol. 2021, 161, 39–53. [Google Scholar] [CrossRef]
- WHO. Media Statement: Knowing the Risks for COVID-19. Available online: https://www.who.int/indonesia/news/detail/08-03-2020-knowing-the-risk-for-covid-19 (accessed on 29 May 2022).
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell 2021, 184, 64–75. [Google Scholar]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827. [Google Scholar] [CrossRef] [PubMed]
- CDC. SARS-CoV-2 Variant Classifications and Definitions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 25 May 2022).
- World Health Organisation. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 25 April 2022).
- World Health Organisation. Network for Genomic Surveillance South Africa (NGS-SA). SARS-CoV-2 Sequencing. Available online: https://www.krisp.org.za/manuscripts/25Nov2021_B.1.1.529_Media.pdf (accessed on 25 September 2021).
- UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England: Technical Briefing 31. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042367/technical_briefing-31-10-december-2021.pdf (accessed on 10 March 2022).
- Wang, X.; Tang, T.; Cao, L.; Aihara, K.; Guo, Q. Inferring key epidemiological parameters and transmission dynamics of COVID-19 based on a modified SEIR model. Math. Model. Nat. Phenom. 2020, 15, 74. [Google Scholar] [CrossRef]
- Zeb, A.; Alzahrani, E.; Erturk, V.S.; Zaman, G. Mathematical model for coronavirus disease 2019 (COVID-19) containing isolation class. BioMed Res. Int. 2020, 2020, 3452402. [Google Scholar] [CrossRef]
- Aldila, D. Analyzing the impact of the media campaign and rapid testing for COVID-19 as an optimal control problem in East Java, Indonesia. Chaos Solitons Fractals 2020, 141, 109953. [Google Scholar] [CrossRef]
- Khan, M.A.; Atangana, A.; Alzahrani, E.; Fatmawati. The dynamics of COVID-19 with quarantined and isolation. Adv. Differ. Equations 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Mishra, A.M.; Purohit, S.D.; Owolabi, K.M.; dan Sharma, Y.D. A Nonlinear Epidemiological Model Considering Asymtotic and Quarantine Classes for SARS-CoV-2 Virus. Chaos Solitons Fractals 2020, 138, 109953. [Google Scholar] [CrossRef]
- Aldila, D.; Ndii, M.Z.; Samiadji, B.M. Optimal control on COVID-19 eradication program in Indonesia under the effect of community awareness. Math. Biosci. Eng. 2020, 17, 6355–6389. [Google Scholar] [CrossRef]
- Alqahtani, R.T.; Ajbar, A. Study of Dynamics of a COVID-19 Model for Saudi Arabia with Vaccination Rate, Saturated Treatment Function and Saturated Incidence Rate. Mathematics 2021, 9, 3134. [Google Scholar] [CrossRef]
- Mushanyu, J.; Chukwu, W.; Nyabadza, F.; Muchatibaya, G. Modelling the potential role of super spreaders on COVID-19 transmission dynamics. Int. J. Math. Model. Numer. Optim. 2022, 12, 191–209. [Google Scholar]
- Rezapour, S.; Mohammadi, H.; Samei, M.E. SEIR epidemic model for COVID-19 transmission by Caputo derivative of fractional order. Adv. Differ. Equ. 2020, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.A.; Yavuz, M.; Qureshi, S.; Zu, J.; Townley, S. Modeling and analysis of COVID-19 epidemics with treatment in fractional derivatives using real data from Pakistan. Eur. Phys. J. Plus 2020, 135, 795. [Google Scholar] [CrossRef] [PubMed]
- Boudaoui, A.; El hadj Moussa, Y.; Hammouch, Z.; Ullah, S. A fractional-order model describing the dynamics of the novel coronavirus (COVID-19) with nonsingular kernel. Chaos Solitons Fractals 2021, 146, 110859. [Google Scholar] [CrossRef]
- Chukwu, C.W.; Fatmawati. Modelling fractional-order dynamics of COVID-19 with environmental transmission and vaccination: A case study of Indonesia. AIMS Math. 2022, 7, 4416–4438. [Google Scholar] [CrossRef]
- Li, X.P.; DarAssi, M.H.; Khan, M.A.; Chukwu, C.W.; Alshahrani, M.Y.; Al Shahrani, M.; Riaz, M.B. Assessing the potential impact of COVID-19 Omicron variant: Insight through a fractional piecewise model. Results Phys. 2022, 38, 105652. [Google Scholar] [CrossRef]
- Bonyah, E.; Juga, M.; Fatmawati. Fractional dynamics of coronavirus with comorbidity via Caputo-Fabrizio derivative. Commun. Math. Biol. Neurosci. 2022, 2022, 12. [Google Scholar]
- Gao, W.; Veeresha, P.; Cattani, C.; Baishya, C.; Baskonus, H.M. Modified Predictor–Corrector Method for the Numerical Solution of a Fractional-Order SIR Model with 2019-nCoV. Fractal Fract. 2022, 6, 92. [Google Scholar] [CrossRef]
- Qureshi, S.; Bonyah, E.; Shaikh, A.A. Classical and contemporary fractional operators for modeling diarrhea transmission dynamics under real statistical data. Phys. Stat. Mech. Its Appl. 2022, 535, 122496. [Google Scholar] [CrossRef]
- Bonyah, E.; Juga, M.L.; Chukwu, C.W.; Fatmawati. A fractional order dengue fever model in the context of protected travelers. Alex. Eng. J. 2022, 61, 927–936. [Google Scholar] [CrossRef]
- Bonyah, E.; Chukwu, C.W.; Juga, M.; Fatmawati. Modeling fractional order dynamics of Syphilis via Mittag-Leffler law. AIMS Math. 2021, 6, 8367–8389. [Google Scholar] [CrossRef]
- Ade León, U.A.P.; Avila-Vales, E.; Huang, K.L. Modeling COVID-19 dynamic using a two-strain model with vaccination. Chaos Solitons Fractals 2022, 157, 111927. [Google Scholar] [CrossRef] [PubMed]
- Arruda, E.F.; Das, S.S.; Dias, C.M.; Pastore, D.H. Modelling and optimal control of multi strain epidemics, with application to COVID-19. PLoS ONE 2021, 16, e0257512. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Zou, Z.; Liu, Y.; Li, X.; Zhuang, G.; Shen, M.; Zhang, L. Evaluating the impact of SARS-CoV-2 variants on the COVID-19 epidemic and social restoration in the United States: A mathematical modelling study. Front. Public Health 2021, 9, 2067. [Google Scholar] [CrossRef]
- González-Parra, G.; Arenas, A.J. Qualitative analysis of a mathematical model with presymptomatic individuals and two SARS-CoV-2 variants. Comput. Appl. Math. 2021, 40, 1–25. [Google Scholar] [CrossRef]
- Layton, A.T.; Sadria, M. Understanding the dynamics of SARS-CoV-2 variants of concern in Ontario, Canada: A modeling study. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Podlubny, I. Fractional Differential Equations: An Introduction to Fractional Derivatives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Vargas-De-Le´on, C. Volterra-type Lyapunov functions for fractional-order epidemic systems. Commun. Nonlinear Sci. Numer. Simul. 2015, 24, 75–85. [Google Scholar] [CrossRef]
- Van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Bull. Math. Biol. 2008, 70, 29–48. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Sayed, A.M.A.; El-Saka, H.A.A. On some Routh-Hurwitz conditions for fractional order differential equations and their applications in Lorenz, Rossler, Chua and Chen systems. Phys. Lett. 2006, 1, 1–4. [Google Scholar] [CrossRef]
- Chitnis, N.; Hyman, J.M.; Cushing, J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 2008, 70, 1272–1296. [Google Scholar] [CrossRef] [PubMed]
- Diethelm, K.; Freed, A.D. The FracPECE subroutine for the numerical solution of differential equations of fractional order. Forsch. Und Wiss. Rechn. 1999, 57–71. [Google Scholar]



| Symbol | Description | Units |
|---|---|---|
| Recruitment rate | ||
| Natural death rate | ||
| Transmission rate by | ||
| Transmission rate by | ||
| Transmission rate by | ||
| Transmission rate by | ||
| Progression rate from to | ||
| Progression rate from to | ||
| Death rate due to strain 1 | ||
| Death rate due to strain 2 | ||
| Recovery rate of infected with strain 1 | ||
| Recovery rate of infected with strain 2 |
| Parameters | Value | Source |
|---|---|---|
| 1930 | [15] | |
| [15] | ||
| [21] | ||
| [15] | ||
| [15] | ||
| [15] | ||
| [15] | ||
| Assumed | ||
| Assumed | ||
| Assumed | ||
| Assumed | ||
| Assumed |
| Parameter | Sensitivity Index () | Parameter | Sensitivity Index () |
|---|---|---|---|
| 0.9999 | 1 | ||
| −1 | −1 | ||
| 0.0031 | 0.0041 | ||
| 0.9969 | 0.9959 | ||
| −0.0026 | −0.0034 | ||
| −0.0966 | −0.1245 | ||
| −0.9 | −0.871 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatmawati; Yuliani, E.; Alfiniyah, C.; Juga, M.L.; Chukwu, C.W. On the Modeling of COVID-19 Transmission Dynamics with Two Strains: Insight through Caputo Fractional Derivative. Fractal Fract. 2022, 6, 346. https://doi.org/10.3390/fractalfract6070346
Fatmawati, Yuliani E, Alfiniyah C, Juga ML, Chukwu CW. On the Modeling of COVID-19 Transmission Dynamics with Two Strains: Insight through Caputo Fractional Derivative. Fractal and Fractional. 2022; 6(7):346. https://doi.org/10.3390/fractalfract6070346
Chicago/Turabian StyleFatmawati, Endang Yuliani, Cicik Alfiniyah, Maureen L. Juga, and Chidozie W. Chukwu. 2022. "On the Modeling of COVID-19 Transmission Dynamics with Two Strains: Insight through Caputo Fractional Derivative" Fractal and Fractional 6, no. 7: 346. https://doi.org/10.3390/fractalfract6070346
APA StyleFatmawati, Yuliani, E., Alfiniyah, C., Juga, M. L., & Chukwu, C. W. (2022). On the Modeling of COVID-19 Transmission Dynamics with Two Strains: Insight through Caputo Fractional Derivative. Fractal and Fractional, 6(7), 346. https://doi.org/10.3390/fractalfract6070346








