Fractal Analysis on Pore Structure and Modeling of Hydration of Magnesium Phosphate Cement Paste
Abstract
:1. Introduction
2. Methods and Algorithms
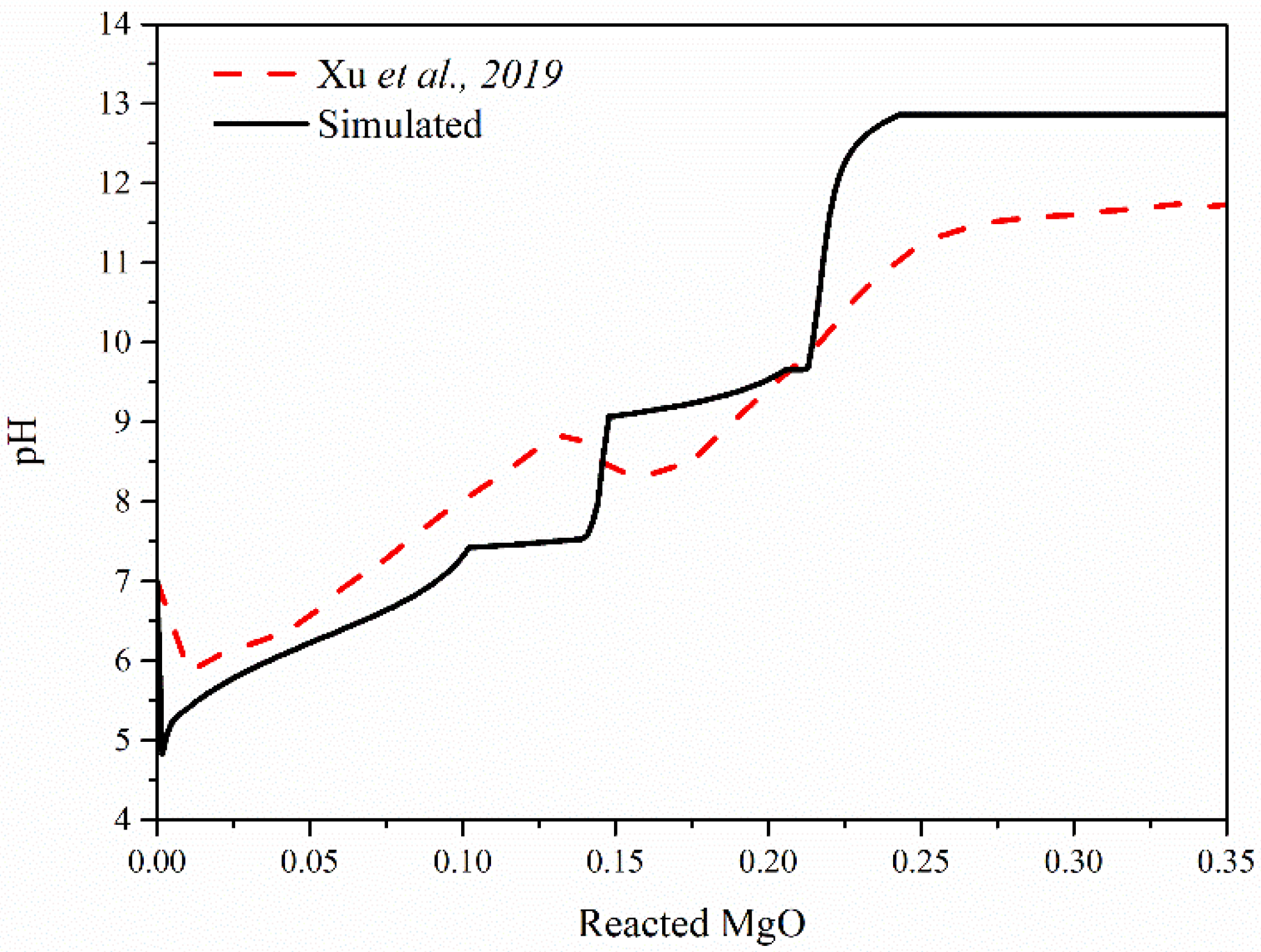
2.1. Establishment of a Thermodynamic Database of MPC
2.2. Hydration Algorithm
2.3. Construction of Reaction System
2.4. Fractal Analysis of Pore Structure in MKPC Paste
3. Simulation Results and Analysis of MKPC Hydration
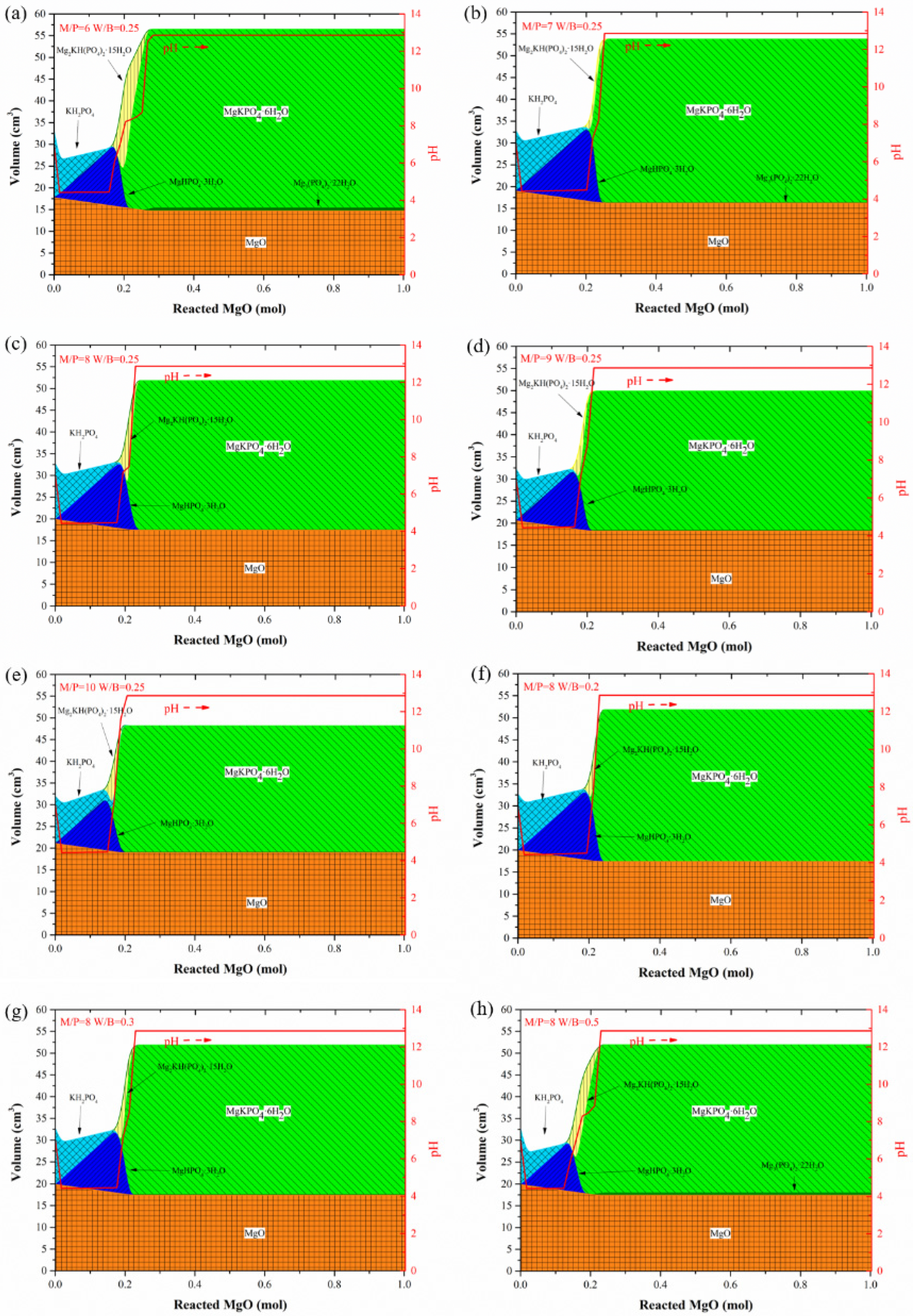
3.1. Influence of M/P and W/B Ratios on Hydration Products in MKPC Pastes
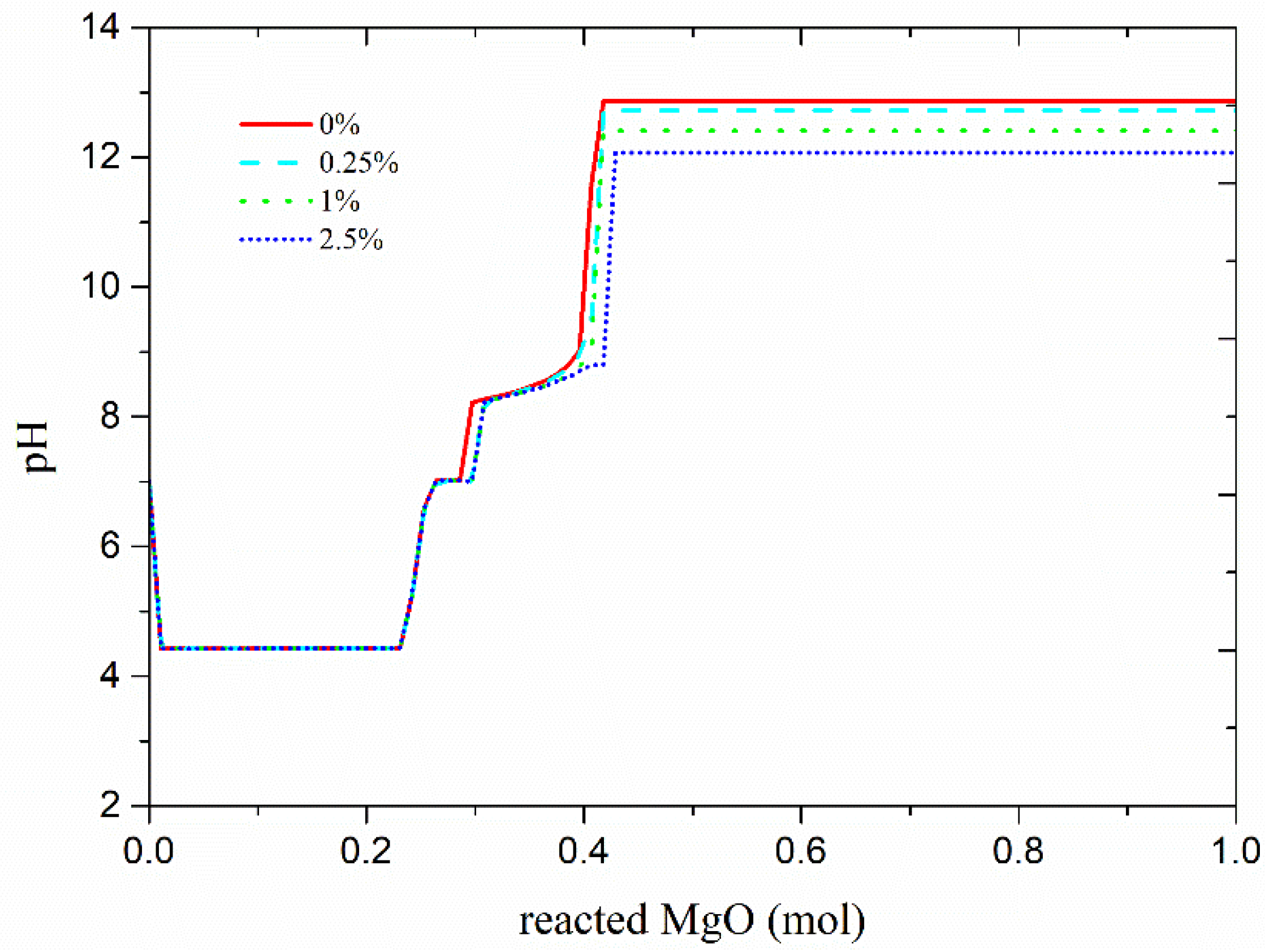
3.2. Influence of Boric Acid on Hydration Products and pH in MKPC Pastes
3.3. Pore structure of MKPC Pastes
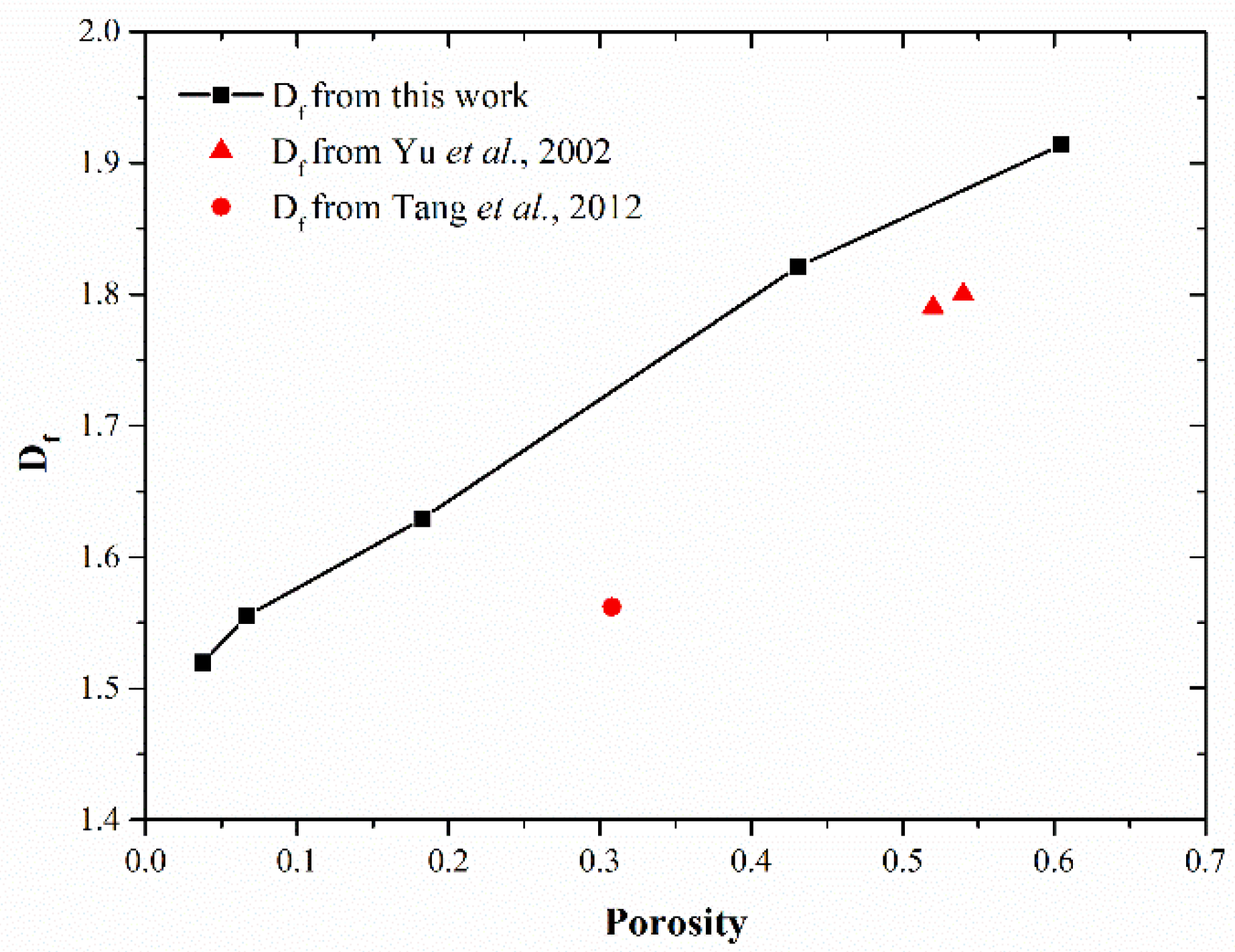
3.4. Analysis of Fractal Features of MKPC Pastes
4. Simulation Results and Analysis of MAPC Hydration
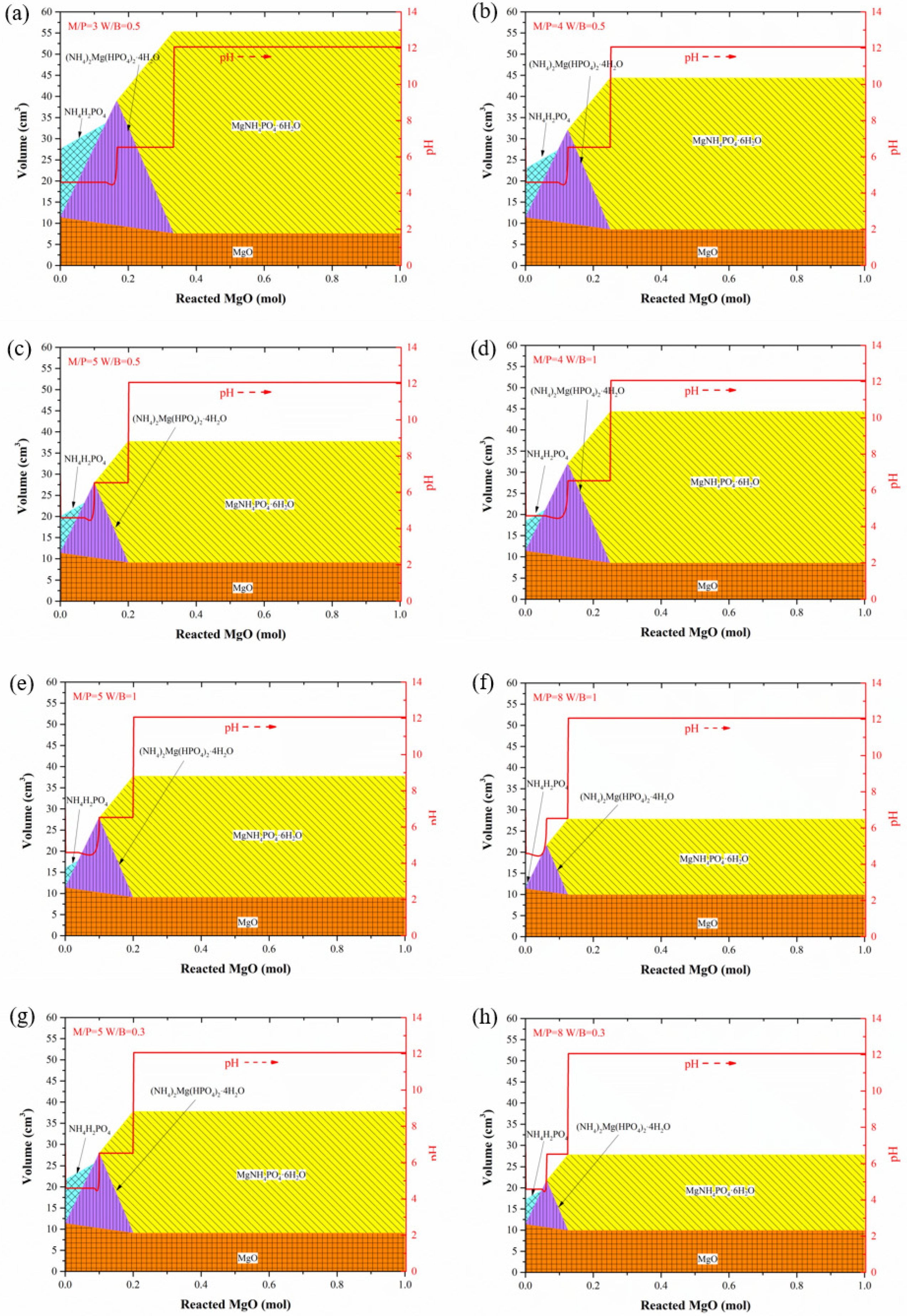
4.1. Influence of M/P and W/B Ratios on Hydration Products in MAPC Pastes
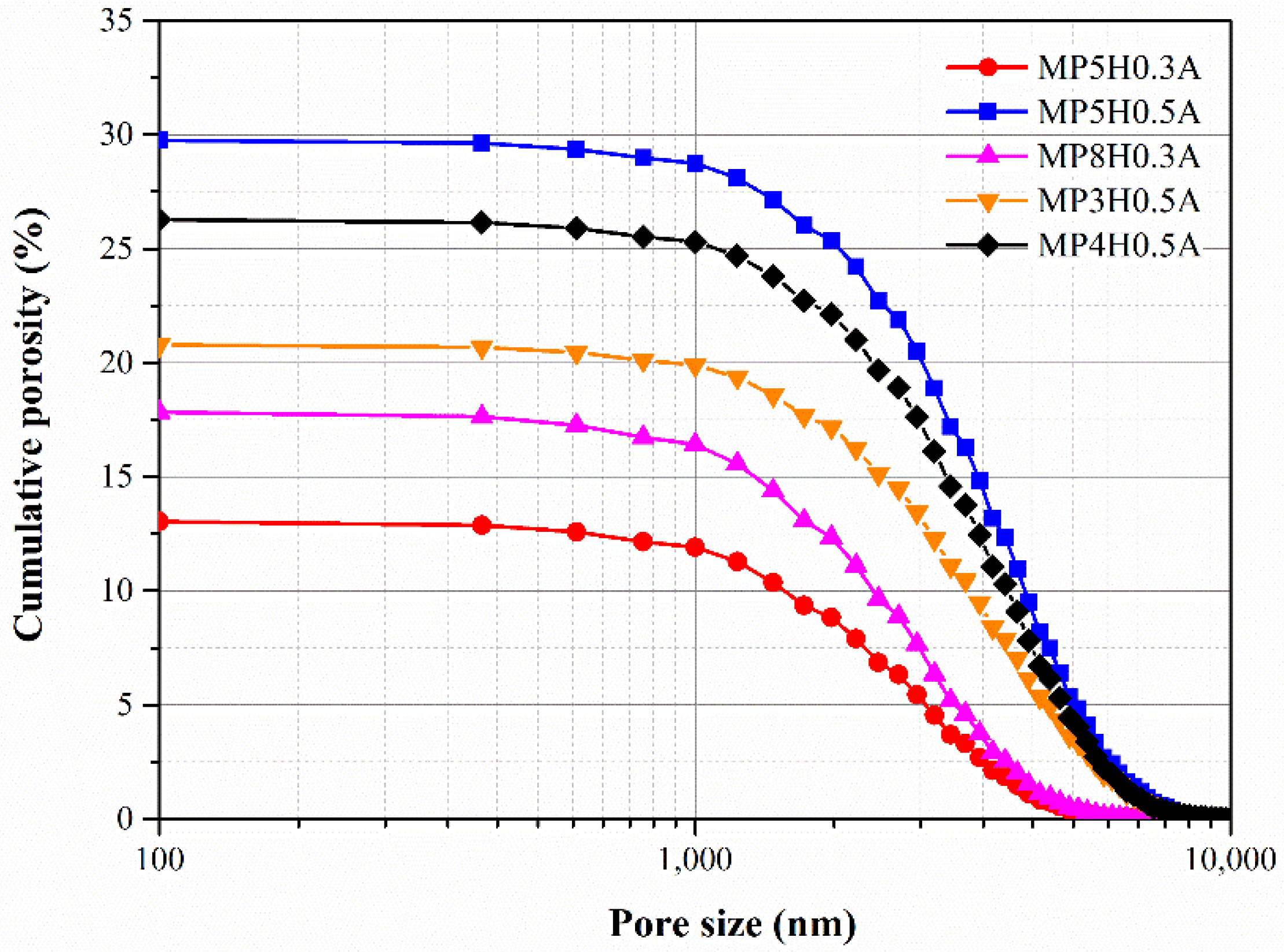
4.2. Pore Structure of MAPC Pastes
4.3. Comparison between MKPC System and MAPC System
5. Conclusions and Prospects
- (1)
- M/P and W/B ratios significantly affect the hydration products in MKPC pastes. In the hydration process of MKPC pastes, intermediate products, such as MgHPO4·3H2O and Mg2KH(PO4)2·15H2O, form first before the appearance of the main hydration product, K-struvite, and Mg2(PO4)3·22H2O is generated in a small amount together with K-struvite. Additionally, the increase in the M/P ratio inhibits the formation of K-struvite, and a low M/P ratio facilitates the formation of intermediate products. A high W/B ratio also causes the formation of intermediate products and accelerates the formation of K-struvite. As for MAPC pastes, M/P and W/B ratios play the same role in the influence of the main hydration product. With the hydration process, the intermediate product (NH4)2Mg(HPO4)2·4H2O is gradually replaced by struvite.
- (2)
- The addition of boric acid significantly reduces the pH of the reaction, inhibits the formation of K-struvite, as well as promotes the formation of intermediate products. The boric acid does not slow down the initial dissolution of MgO and KH2PO4, but together with slows down the formation of K-struvite and lowers the pH value of the solution.
- (3)
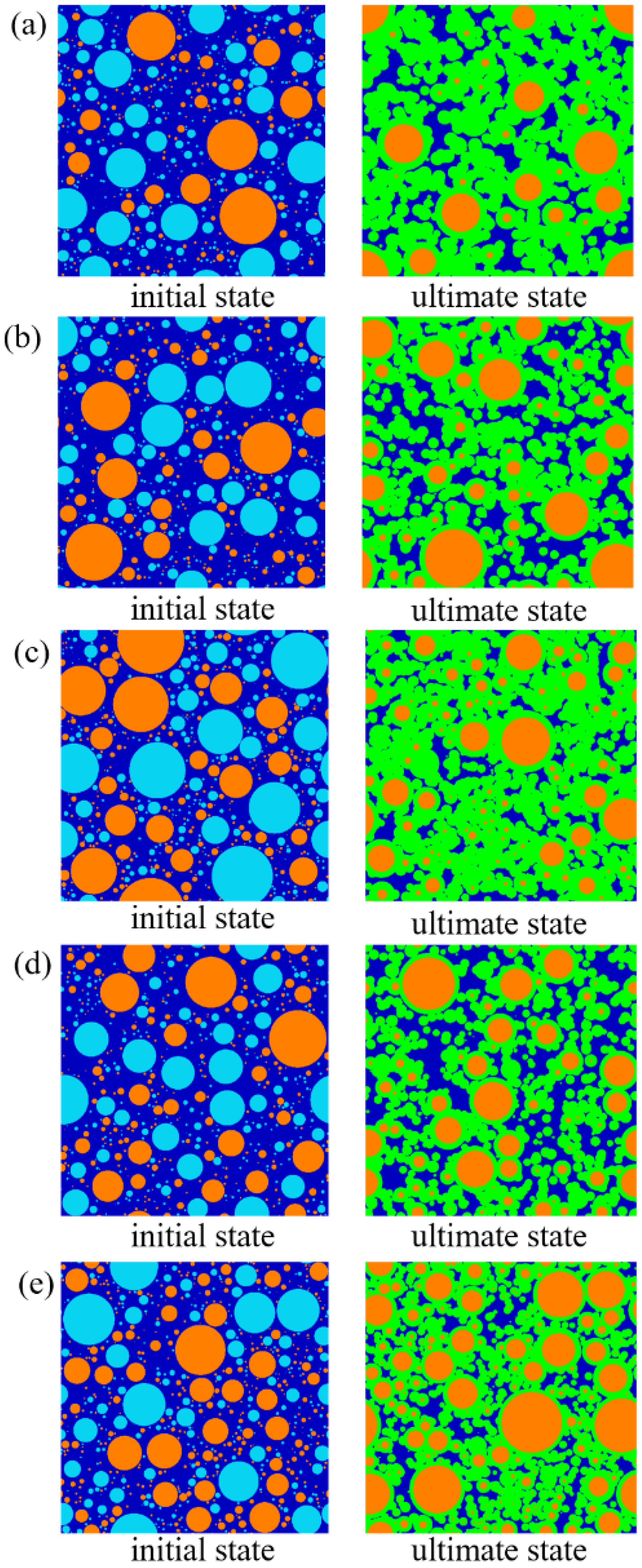
- From the initial and final hydration states of MKPC and MAPC pastes, it can be found that in the hydration process, the small MgO particles are entirely hydrated due to the quick reaction, while the large ones are covered by inner hydration products and outer hydration products. According to the porosity analysis conducted based on hydration state, the increase in M/P ratio in the paste leads to a large volume fraction of K-struvite/struvite, which is conductive to a small porosity of the paste. In contrast, a high W/B ratio increases the porosity of MPC pastes. With the increase of M/P and W/B ratios, there is a significant growth in the fraction of large pores. Additionally, fractal analysis in this work reveals that the Df of MKPC pastes is positively proportional to the porosity and small M/P ratios, and small W/B ratios are beneficial for reducing the Df of MKPC pastes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, D.M. New strong cement materials: Chemically bonded ceramics. Science 1987, 235, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Chau, C.K.; Li, Z. Property evaluation of magnesium phosphate cement mortar as patch repair material. Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Liu, C.; Zhang, B.; Chen, H.; Guo, H.; Zhong, G.; Qu, W.; Jiang, S.; Huang, H. Evaluation of inherent toxicology and biocompatibility of magnesium phosphate bone cement. Colloids Surf. B Biointerfaces 2010, 76, 496–504. [Google Scholar] [CrossRef]
- Xu, K.; Li, J.; Zheng, M.; Zhang, C.; Xie, T.; Wang, C. The precipitation of magnesium potassium phosphate hexahydrate for P and K recovery from synthetic urine. Water Res. 2015, 80, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farag, M.M.; Park, E.K.; Lim, J.; Yun, H.-S. A simultaneous process of 3D magnesium phosphate scaffold fabrication and bioactive substance loading for hard tissue regeneration. Mater. Sci. Eng. C 2014, 36, 252–260. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Zhao, X.; Zheng, S. Evaporation and cracked soda soil improved by fly ash from recycled materials. Land. Degrad. Dev. 2021, 32, 2823–2832. [Google Scholar] [CrossRef]
- Yang, B.; Li, D.; Yuan, S.; Jin, L. Role of biochar from corn straw in influencing crack propagation and evaporation in sodic soils. Catena 2021, 204, 105457. [Google Scholar] [CrossRef]
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium phosphate cement systems for hard tissue applications: A Review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Liu, J.; Pei, H.; Li, Z. Effects of water content, magnesia-to-phosphate molar ratio and age on pore structure, strength and permeability of magnesium potassium phosphate cement paste. Mater. Des. 2014, 64, 497–502. [Google Scholar] [CrossRef]
- Wagh, A.S.; Jeong, S.Y. Chemically bonded phosphate ceramics: I, a dissolution model of formation. J. Am. Ceram. Soc. 2003, 86, 1838–1844. [Google Scholar] [CrossRef]
- Wang, A.; Fan, X.; Li, J.; Ding, C. Curing behavior and structure of magnesium phosphate chemically bonded ceramics with different MgO to KH2PO4 ratios. Int. J. Appl. Ceram. Technol. 2015, 12, 1124–1130. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate based magnesium phosphate cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Leemann, A.; Winnefeld, F. Reaction mechanism of magnesium potassium phosphate cement with high magnesium-to-phosphate ratio. Cem. Concr. Res. 2018, 108, 140–151. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Li, Z. Influence of magnesia-to-phosphate molar ratio on microstructures, mechanical properties and thermal conductivity of magnesium potassium phosphate cement paste with large water-to-solid ratio. Cem. Concr. Res. 2015, 68, 1–9. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Kaufmann, J.; Lothenbach, B. Influence of magnesium-to-phosphate ratio and water-to-cement ratio on hydration and properties of magnesium potassium phosphate cements. Cem. Concr. Res. 2019, 123, 105781. [Google Scholar] [CrossRef]
- Lahalle, H.; Cau Dit Coumes, C.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c ratio on the hydration process of a magnesium phosphate cement and on its retardation by boric acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Lahalle, H.; Cau Dit Coumes, C.; Mesbah, A.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Investigation of magnesium phosphate cement hydration in diluted suspension and its retardation by boric acid. Cem. Concr. Res. 2016, 87, 77–86. [Google Scholar] [CrossRef]
- Sugama, T.; Kukacka, L. Magnesium monophosphate cements derived from diammonium phosphate solutions. Cem. Concr. Res. 1983, 13, 407–416. [Google Scholar] [CrossRef]
- Abdelrazig, B.; Sharp, J. A discussion of the papers on magnesia-phosphate cements by T. Sugama and LE Kukacka. Cem. Concr. Res. 1985, 15, 921–922. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Z.; Chen, Y.; Ni, H.; Zhao, Z.; Chen, W.; Zhao, J. Mechanical and microstructural properties of recycling granite residual soil reinforced with glass fiber and liquid-modified polyvinyl alcohol polymer. Chemosphere 2022, 286, 131652. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B. Potential to design magnesium potassium phosphate cement paste based on an optimal magnesia-to-phosphate ratio. Mater. Des. 2017, 118, 81–88. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, M.; Wang, Y.; Zhai, L.; Luo, Q.; Zhang, X. Full 3D displacement measuring system for 3D displacement field of soil around a laterally loaded pile in transparent soil. Int. J. Geomech. 2019, 19, 04019028. [Google Scholar] [CrossRef]
- Véhel, J.L.; Lutton, E.; Tricot, C. Fractals in Engineering: From Theory to Industrial Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yang, B.; Liu, Y. Application of fractals to evaluate fractures of rock due to mining. Fractal Fract. 2022, 6, 96. [Google Scholar] [CrossRef]
- Fisher, Y. Fractal Image Compression: Theory and Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Jin, S.; Zhang, J.; Han, S. Fractal analysis of relation between strength and pore structure of hardened mortar. Constr. Build. Mater. 2017, 135, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, F.; Ding, W.; Zhu, L. Comparative study on damage process of concrete subjected to uniaxial tensile and compression loads based on CT test and improved differential box counting method. Constr. Build. Mater. 2021, 285, 122693. [Google Scholar] [CrossRef]
- Lothenbach, B.; Wieland, E. A thermodynamic approach to the hydration of sulphate-resisting Portland cement. Waste Manag. 2006, 26, 706–719. [Google Scholar] [CrossRef]
- Leisinger, S.M.; Lothenbach, B.; Le Saout, G.; Johnson, C.A. Thermodynamic modeling of solid solutions between monosulfate and monochromate 3CaO·Al2O3·Ca[(CrO4)x(SO4)1−x]·nH2O. Cem. Concr. Res. 2012, 42, 158–165. [Google Scholar] [CrossRef]
- Lothenbach, B.; Zajac, M. Application of thermodynamic modelling to hydrated cements. Cem. Concr. Res. 2019, 123, 105779. [Google Scholar] [CrossRef]
- Lothenbach, B.; Xu, B.; Winnefeld, F. Thermodynamic data for magnesium (potassium) phosphates. Appl. Geochem. 2019, 111, 104450. [Google Scholar] [CrossRef]
- Myers, R.J.; Lothenbach, B.; Bernal, S.A.; Provis, J.L. Thermodynamic modelling of alkali-activated slag cements. Appl. Geochem. 2015, 61, 233–247. [Google Scholar] [CrossRef]
- Wagner, T.; Kulik, D.A.; Hingerl, F.F.; Dmytrieva, S.V. Gem-selektor geochemical modeling package: Tsolmod library and data interface for multicomponent phase models. Can. Mineral. 2012, 50, 1173–1195. [Google Scholar] [CrossRef]
- Bethke, C.M.; Yeakel, S. The Geochemist’s Workbench. Release 10.0. GWB Essentials Guide; LLC: Champagin, IL, USA, 2014. [Google Scholar]
- Helgeson, H.C. Summary and critique of the thermodynamic properties of rock-forming minerals. Am. J. Sci. 1978, 278, 1–229. [Google Scholar]
- Thoenen, T.; Berner, U.; Curti, E.; Hummel, W.; Pearson, F.J. The Nagra/PSI Thermochemical Data Base. Geochim. Cosmochim. Acta 2002, 66, A771. [Google Scholar]
- Blanc, P.; Bourbon, X.; Lassin, A.; Gaucher, E.C. Chemical model for cement-based materials: Thermodynamic data assessment for phases other than C–S–H. Cem. Concr. Res. 2010, 40, 1360–1374. [Google Scholar] [CrossRef]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1991.
- Speight, J.G. Lange’s Handbook of Chemistry; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Han, W.; Chen, H.; Li, X.; Zhang, T. Thermodynamic modeling of magnesium ammonium phosphate cement and stability of its hydration products. Cem. Concr. Res. 2020, 138, 106223. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Li, X.; Zhu, Z. Hydration behavior of magnesium potassium phosphate cement and stability analysis of its hydration products through thermodynamic modeling. Cem. Concr. Res. 2017, 98, 101–110. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Lu, Y.; Li, Z. Modeling magnesia-phosphate cement paste at the micro-scale. Mater. Lett. 2014, 125, 15–18. [Google Scholar] [CrossRef]
- Chau, C.K.; Qiao, F.; Li, Z. Microstructure of magnesium potassium phosphate cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- Góchez, R.; Wambaugh, J.; Rochner, B.; Kitchens, C.L. Kinetic study of the magnesium oxychloride cement cure reaction. J. Mater. Sci. 2017, 52, 7637–7646. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, H.; Zongjin, L.I.; Huajian, L.I. Simulation of the properties of MgO-MgfCl2-H2O system by thermodynamic method. Cem. Concr. Res. 2015, 68, 105–111. [Google Scholar] [CrossRef]
- Yu, P.; Duan, Y.H.; Chen, E.; Tang, S.W.; Wang, X.R. Microstructure-based fractal models for heat and mass transport properties of cement paste. Int. J. Heat Mass Tran. 2018, 126, 432–447. [Google Scholar] [CrossRef]
- Wang, R.; Singh, A.K.; Kolan, S.R.; Tsotsas, E. Fractal analysis of aggregates: Correlation between the 2D and 3D box-counting fractal dimension and power law fractal dimension. Chaos Solitons Fract. 2022, 160, 112246. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the influence of Mg/P ratio on the properties and durability of magnesium potassium phosphate cement pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Zhu, J.; Ao, Q.; Wang, J.; Yang, B.; Li, Y.N. Fractal dimension of pore-structure of porous metal materials made by stainless steel powder. Powder Technol. 2012, 217, 383–387. [Google Scholar] [CrossRef]
- Yu, B.; Cheng, P. A fractal permeability model for bi-dispersed porous media. Int. J. Heat Mass Trans. 2002, 45, 2983–2993. [Google Scholar] [CrossRef]
- Wang, A.-J.; Yuan, Z.-L.; Zhang, J.; Liu, L.-T.; Li, J.-M.; Liu, Z. Effect of raw material ratios on the compressive strength of magnesium potassium phosphate chemically bonded ceramics. Mater. Sci. Eng. C 2013, 33, 5058–5063. [Google Scholar] [CrossRef]
- Dong, P.; Ahmad, M.R.; Chen, B.; Munir, M.J.; Saleem Kazmi, S.M. Preparation and study of magnesium ammonium phosphate cement from waste lithium slag. J. Clean. Prod. 2021, 316, 128371. [Google Scholar] [CrossRef]





| Database | Content(s) | Software Support(s) | Reference |
|---|---|---|---|
| Thermoddem | Liquid ions, gas phases, and mineral phases | PHREEQC, Crunch, Geochemist′s workbench, Toughreact, Chess | [35] |
| PSI/Nagra | Solutes, actinides, and radioactive fission products in natural water | GEMS, PHREEQC | [36] |
| Blanc cement chemistry database | Detailed C-S-H data of different forms at different temperature | PHREEQC | [37] |
| MINTEQA2 V.4 database | A large number of complex ions and precipitation reactions | MINTEQ, Geochemist′S, WorkBench, PHREEQC | [38] |
| Ions and Phases | Reaction | Log K |
|---|---|---|
| 21.8 [37] | ||
| 0.278 | ||
| −18.5 [39] | ||
| −13.13 [40] | ||
| −36.3 | ||
| −10.62 [41] | ||
| 1.41 [41] | ||
| −29.54 [41] | ||
| −23.1 [41] | ||
| −9.24 [17] | ||
| −7.528 | ||
| −16.13 | ||
| −7.84 | ||
| 2 | −10.88 |
| Solid | Molar Mass (g/mol) | Molar Volume (cm3/mol) |
|---|---|---|
| MgO | 40 | 11.3 |
| KH2PO4 | 136 | 58.2 |
| NH4H2PO4 | 115 | 63.8 |
| MgHPO4·3H2O | 174 | 82.9 |
| Mg2KH(PO4)2·15H2O | 549 | 303.2 |
| MgKPO4·6H2O | 266 | 142.5 |
| MgNH4PO4·6H2O | 245 | 143.5 |
| (NH4)2Mg(HPO4)2·4H2O | 324 | 177.3 |
| Paste | MgO/KH2PO4 Molar Ratio | W/B Mass Ratio |
|---|---|---|
| MP8H0.2K | 8 | 0.2 |
| MP8H0.3K | 8 | 0.25 |
| MP8H0.5K | 8 | 0.5 |
| MP6H0.25K | 6 | 0.25 |
| MP7H0.25K | 7 | 0.25 |
| MP8H0.25K | 8 | 0.25 |
| MP9H0.25K | 9 | 0.25 |
| MP10H0.25K | 10 | 0.25 |
| Paste | Boric Acid to Solid Mass Ratio (%) | MgO (g) | KH2PO4 (g) | Water (g) | Boric Acid (g) |
|---|---|---|---|---|---|
| MP2.7B0 | 0 | 44.4 | 55.6 | 100 | 0 |
| MP2.7B0.25 | 0.25 | 44.4 | 55.6 | 100 | 0.25 |
| MP2.7B1 | 1 | 44.4 | 55.6 | 100 | 1 |
| MP2.7B2.5 | 2.5 | 44.4 | 55.6 | 100 | 2.5 |
| Paste | MgO/NH4H2PO4 Molar Ratio | W/B Mass Ratio |
|---|---|---|
| MP3H0.5A | 3 | 0.5 |
| MP4H0.5A | 4 | 0.5 |
| MP4H1A | 4 | 1 |
| MP5H0.3A | 5 | 0.3 |
| MP5H0.5A | 5 | 0.5 |
| MP5H1A | 5 | 1 |
| MP8H0.3A | 8 | 0.3 |
| MP8H1A | 8 | 1 |
| Stage | Reacted MgO (mol) | pH | Molarity | |||||
|---|---|---|---|---|---|---|---|---|
| Stage I | 0–0.231 | 4.4 | - | - | - | - | - | - |
| Stage II | 0.231–0.264 | 4.4–7 | increased | increased | increased | increased | increased | decreased |
| Stage III | 0.264–0.297 | 7 | - | - | - | - | - | - |
| Stage IV | 0.297–0.363 | 7–8.5 | increased | increased | - | - | increased | decreased |
| Stage V | 0.363–0.418 | 8.5–12.7 | - | decreased | decreased | decreased | increased | decreased |
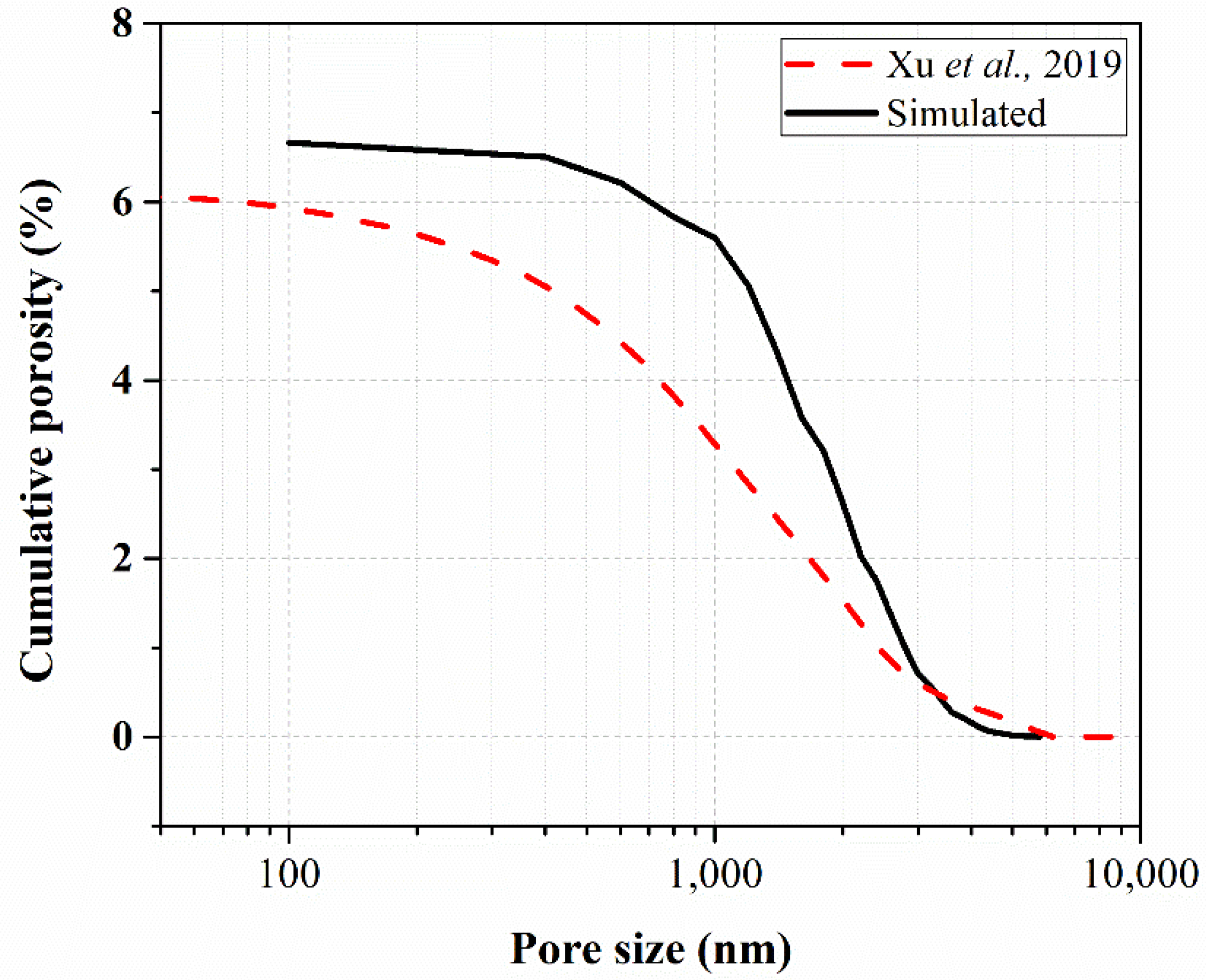
| Paste | Porosity by Simulation (%) | Porosity by Experiment (%) | Difference (%) |
|---|---|---|---|
| MP6H0.25K | 3.77 | - | - |
| MP6H0.2K | - | 5.46 [48] | - |
| MP8H0.25K | 6.66 | 6.06 [15] | 9.9 |
| MP8H0.5K | 37.67 | 40.24 [21] | 6.4 |
| MP10H0.25K | 18.31 | - | - |
| MP10H0.5K | - | 42.69 [21] | - |
| MP4H0.5A | 26.15 | - | - |
| MP5H0.3A | 12.88 | - | - |
| MP5H0.5A | 29.76 | - | - |
| MP4H0.22A | - | 6.18 [52] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Tang, S.; Huang, J.; Tang, C.; Wang, L.; Liu, Y. Fractal Analysis on Pore Structure and Modeling of Hydration of Magnesium Phosphate Cement Paste. Fractal Fract. 2022, 6, 337. https://doi.org/10.3390/fractalfract6060337
Peng Y, Tang S, Huang J, Tang C, Wang L, Liu Y. Fractal Analysis on Pore Structure and Modeling of Hydration of Magnesium Phosphate Cement Paste. Fractal and Fractional. 2022; 6(6):337. https://doi.org/10.3390/fractalfract6060337
Chicago/Turabian StylePeng, Yuxiang, Shengwen Tang, Jiasheng Huang, Can Tang, Lei Wang, and Yufei Liu. 2022. "Fractal Analysis on Pore Structure and Modeling of Hydration of Magnesium Phosphate Cement Paste" Fractal and Fractional 6, no. 6: 337. https://doi.org/10.3390/fractalfract6060337
APA StylePeng, Y., Tang, S., Huang, J., Tang, C., Wang, L., & Liu, Y. (2022). Fractal Analysis on Pore Structure and Modeling of Hydration of Magnesium Phosphate Cement Paste. Fractal and Fractional, 6(6), 337. https://doi.org/10.3390/fractalfract6060337